Abstract
Epilepsy is one of the most common serious neurological conditions, and 30 to 40% of people with epilepsy have seizures that are not controlled by medication. Patients are considered to have refractory epilepsy if disabling seizures continue despite appropriate trials of two antiseizure drugs, either alone or in combination. At this point, patients should be referred to multidisciplinary epilepsy centers that perform specialized diagnostic testing to first determine whether they are, in fact, pharmacoresistant, and then, if so, offer alternative treatments. Apparent pharmacoresistance can result from a variety of situations, including noncompliance, seizures that are not epileptic, misdiagnosis of the seizure type or epilepsy syndrome, inappropriate use of medication, and lifestyle issues. For patients who are pharmacoresistant, surgical treatment offers the best opportunity for complete freedom from seizures. Surgically remediable epilepsy syndromes have been identified, but patients with more complicated epilepsy can also benefit from surgical treatment and require more specialized evaluation, including intracranial EEG monitoring. For patients who are not surgical candidates, or who are unwilling to consider surgery, a variety of other alternative treatments can be considered, including peripheral or central neurostimulation, ketogenic diet, and complementary and alternative approaches. When such alternative treatments are not appropriate or effective, quality of life can still be greatly improved by the psychological and social support services offered by multidisciplinary epilepsy centers. A major obstacle remains the fact that only a small proportion of patients with refractory epilepsy are referred for expert evaluation and treatment.
Keywords: Complementary and alternative medicine, diagnostic approaches, epilepsy surgery, ketogenic diet, neurostimulation, refractory epilepsy
Burden of Refractory Epilepsy
Epilepsy is one of the most common serious neurological conditions, accounting for 1% of the global burden of disease, based on disability-adjusted life years, the number of person years lost due to disability and premature death.[1] This is equivalent to breast cancer in women and lung cancer in men. Among primary disorders of the brain, it is equivalent to depression and other affective disorders, Alzheimer's disease and other dementias, and substance abuse. Ten percent of the world's population will have at least one seizure during their lifetime and one-third of these will develop epilepsy at any given time.[2] One percent of the world's population has active epilepsy. In countries where adequate diagnosis and treatment are available, 30-40% of people with epilepsy have seizure that are uncontrolled by medication,[3] which accounts for 80% of the cost of epilepsy in the United States.[4] Medically refractory epilepsy, therefore, is a major health concern not only for patients and their families, but for society.
Treatment objectives for epilepsy are no seizures, and no side effects, as soon as possible. Early effective interventions provide the best opportunity to prevent adverse psychological and social consequences of recurrent seizures, progressive deficits that lead to irreversible disability, and premature death. In particular, seizures that interfere with school, work, and interpersonal relationships, during adolescence and early adulthood, prevent the acquisition of vocational and social skills necessary to live independently. When available treatments with the potential to eliminate disabling seizures are delayed (for instance, in the United States the average time from onset of epilepsy to surgery is 22 years[5]), patients who do become seizure free often cannot be rehabilitated and remain dependent on their families and society. Early identification of pharmacoresistant epilepsy and institution of alternative treatments can prevent a lifetime of disability.
Diagnosis of Pharmacoresistance
The term “pharmacoresistant epilepsy” can no longer be taken literally, as there are now so many antiseizure drugs that it would take a lifetime to try all of them alone and in combination in any given patient. The best predictor of pharmacoresistance is failure of the first antiseizure drug, due to efficacy and not intolerance. Given failure of one appropriate drug trial, only 11% of patients eventually become seizure free, while only 3% become seizure free after failure of two appropriate antiseizure drug trials.[6] There are likely many reasons for pharmacoresistance and research to elucidate underlying mechanisms is important for the future development of more effective treatments.[7]
A simple-minded approach to the triage of epilepsies is that there are benign epilepsy syndromes, which are easily treated, and severe epilepsies, which are not. Of the latter, there are conditions that are remediable but require specialized diagnostic and therapeutic approaches, and conditions that are not remediable and require supportive care. Early distinction between the two is critical in maximizing quality of life. In most cases, the distinction can only be made by physicians and other healthcare workers trained in the diagnosis and treatment of epilepsy, at specialized epilepsy centers with facilities and support services not available in the community. Full-service epilepsy centers are staffed by a multidisciplinary team consisting of neurologists/epileptologists, clinical neurophysiologists, neuropsychologists, neuroradiologists, neurosurgeons, psychiatrists, social workers, and nurses skilled in the management of epileptic seizures and their consequences.[8]
Apparent pharmacotherapy failure does not necessarily mean that standard antiseizure drugs will not work. Alternative causes include noncompliance, seizures that are not epileptic, misdiagnosis of the seizure type or epilepsy syndrome, inappropriate use of medications such as inadequate doses or drug-drug interactions, and lifestyle issues such as alcohol binging, drug abuse, stress, and sleep deprivation. Differential diagnosis can usually be achieved with specialized approaches available at epilepsy centers, which often requires inpatient video-electroencephalogram (EEG) monitoring to capture the habitual events and characterize their electroclinical features. These approaches can permit recognition of nonepileptic seizures and their causes, diagnosis of specific seizure types, and epilepsy syndromes, and determinations of which patients who are truly pharmacoresistant might be candidates for surgical therapy. In addition, epilepsy centers have the ability to utilize specialized pharmacologic approaches, including enrollment in clinical trials of experimental antiseizure drugs, to provide alternative treatments other than surgery, such as vagus nerve stimulation (VNS) and the ketogenic diet, and to evaluate and address psychological and social problems resulting from uncontrolled seizures. Usually, patients seek help not because they are having seizures, but because their seizures are interfering with their lives. It is, therefore, necessary not only to direct therapy towards the epileptic seizures, but also each individual patient's predicament, in order to maximize quality of life.
Concerning the diagnosis of drug-resistant epilepsy, the International League against Epilepsy (ILAE) has proposed, as a testable hypothesis: “That drug-resistant epilepsy is defined as failure of adequate trials of two tolerated, appropriately chosen, and used antiepileptic drug schedules (whether as monotherapies or in combination) to achieve sustained seizure freedom”.[9] Once this diagnosis is made, a number of options can be considered. It is most important to first determine whether the patient may be a candidate for surgical treatment, because this is the only therapeutic approach with the potential to cure epilepsy. Depending on the type of epilepsy and the surgical procedure, most patients experience elimination, or at least a reduction, in disabling seizures, and significant improvement in quality of life. For patients who are not surgical candidates, stimulation approaches have outcomes similar to those brought about by additional medication trials, but with different side effect profiles. Ketogenic diet, particularly in young children, can eliminate seizures, and various complementary and alternative treatments offer additional opportunities to reduce the seizure burden and improve quality of life.
Surgical Treatment
Surgically remediable epilepsy syndromes are conditions with a known pathophysiology and a predictable natural history that includes unresponsiveness to pharmacotherapy and progressive features, such as developmental delay in infants and young children, or interictal behavioral disorders, most commonly depression.[10] Patients with surgically remediable epilepsy syndromes are the most cost-effective surgical candidates, because presurgical evaluation can be performed noninvasively in most cases; by definition there is a 70-90% chance of complete elimination of disabling seizures, and disabling psychological and social consequences can be avoided or reversed, but only if surgical intervention is early. Mesial temporal lobe epilepsy (MTLE) is the prototype of a surgically remediable epilepsy syndrome.[11] Other surgically remediable epilepsy syndromes include focal epilepsies due to discrete resectable structural lesions, epilepsies due to diffuse hemispheric disturbances, such as hemimegencephaly, Rasmussen's encephalitis, Sturge-Weber syndrome, and large porencephalic cysts, and gelastic seizures with hypothalamic hamartomas, because seizures originate within this alien tissue.[12]
MTLE is the most common form of epilepsy in adolescents and adults, the most medically refractory, and the most easily treated surgically. Most patients with MTLE have hippocampal sclerosis,[13] although other lesions within the hippocampus, or in neocortical areas that preferentially project to mesial temporal structures, can produce the same characteristic limbic seizures. Features of MTLE with hippocampal sclerosis that should prompt early referral for epilepsy surgery are shown in [Table 1].
Table 1.
The syndrome of mesial temporal lobe epilepsy

Surgical therapy can eliminate disabling seizures completely in appropriately chosen patients.[14,15] Multiple types of surgical interventions are now offered [Table 2], depending on the type of epileptic seizures and their presumed underlying causes. Standardized resections include anterior temporal resections and amygdalohippocampectomy for MTLE,[16] and hemispherectomy or hemispherotomy, for patients, usually infants and young children, with diffuse epileptogenic regions limited to one hemisphere.[17] Presurgical evaluation requires demonstration that epileptic seizures are originating within the standard area of resection.
Table 2.
Common surgical procedures for epilepsy

Neocortical resections are always tailored, in which case the presurgical evaluation must not only localize, but also determine the extent of the epileptogenic region. This is defined as the area of brain necessary and sufficient to generate habitual seizures and is, therefore, the smallest amount of tissue that can be removed in order to achieve a seizure-free outcome.[18] There are no diagnostic tests that reliably determine the extent of the epileptogenic region, and this is approximated using a number of different tests of function and structure, including inpatient video-EEG monitoring, neuroimaging, and neuropsychological evaluation [Table 3].
Table 3.
Definition of abnormal brain areas
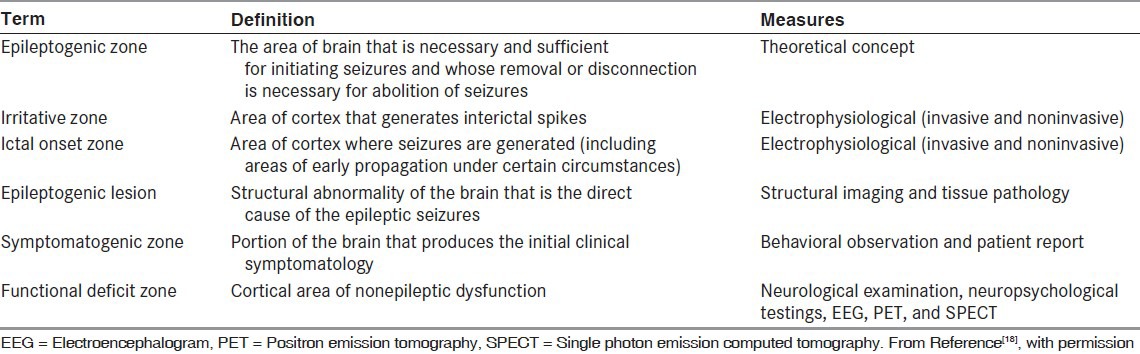
Lesionectomies without removal of cortical margins are performed when adjacent tissue cannot be damaged, and in the case of hypothalamic hamartoma where seizures actually originate within the tumor.[19] Multiple subpial transection (MST) is a technique that can be used in cortical areas with essential functions, such as motor or language cortex, to reduce epileptogenicity without producing serious deficits.[20] Presurgical evaluation is the same as that for tailored resection.
Corpus callosotomy is a disconnection procedure effective particularly against drop attacks.[21] Presurgical evaluation need only demonstrate that the patient is not a candidate for another, more definitive localized surgical procedure. Corpus callosotomy is performed rarely today because VNS may be equally effective against drop attacks in most patients.
Stereotactic ablative surgery can be performed with noninvasive stereotactic radiotherapy, also called gamma knife surgery (GKS).[22] This produces edema, which can have serious side effects and the beneficial effects take months to a year or more. A more recent laser ablative approach requires an intracranial probe, but the results are more immediate.[23] These treatments are particularly useful in patients who have medical contraindications to surgery.
Stimulation
Patients who are not candidates for surgical treatment may benefit from VNS, which has a therapeutic profile similar to that of new antiseizure drugs; 50% of patients experience 50% reduction in seizures, but a different side effect profile, and patients rarely become seizure free.[24] Trigeminal nerve stimulation (TNS) is an experimental alternative approach that does not require surgical implantation of the simulating device.[25] Electrodes can be applied to the skin of the forehead for 8-12h daily. Deep brain stimulation (DBS) of the anterior nucleus of the thalamus is now approved in many countries but remains experimental in others.[26] Another direct brain stimulation approach, still under review, is response cortical stimulation (RCS), which involves a device imbedded in the skull that detects seizure onset and delivers stimulation directly to the site(s) of seizure generation to abort the ictal event.[27] This approach requires localization of the epileptogenic region.
Ketogenic diet
The ketogenic diet can be highly effective in children with severe seizures who are not candidates for surgery.[28] Ketosis is instituted in the hospital and the diet can be maintained from months to years. Changes in the diet over the years have made it more palatable. Up to 50% of children experience seizure control and benefits may persist after the diet is terminated. Rigid dietary restrictions may not be necessary, and some patients achieve benefit from less stringent carbohydrate restriction, such as with the Atkins diet.
Complementary and Alternative Medicine (CAM)
Various CAM therapies, particularly herbal remedies, are currently under investigation.[29] Ayurvedic medicine and other traditional approaches have value in cultures open to these treatments. Behavioral therapeutic approaches are being used, for instance those that help patients recognize and avoid their seizure triggers, as well as those that reduce stress, such as meditation.[30] Interventions that address stress, whether introduced by epilepsy or other causes, not only lessen the patient's predicament and improve their quality of life, but can also have a beneficial effect on seizure occurrence. Recognizing and avoiding seizure triggers can be particularly useful in reflex epilepsies,[31] for instance patching one eye or wearing colored glasses for photosensitive epilepsy, but most seizures are caused by triggers that are poorly understood. Their elucidation in individual patients could lead to personalized effective behavioral therapeutic paradigms in a much broader population of patients.
Future epilepsy therapies
Although the number of available antiseizure drugs has more than doubled in the past few decades, this has had little effect on the percentage of patients with epilepsy who remain pharmacoresistant. Many of the newer drugs have been designed to address fundamental neuronal mechanisms of epilepsy revealed by basic research, but often such “designer drugs” are not clinically effective or work for reasons other than initially anticipated. Some of the newer drugs also have unique mechanisms of action, but the spectrum of antiseizure effectiveness is similar to that of the older, standard drugs. There clearly is value in the fact that many of the newer drugs have better side effect profiles than older drugs and are easier to use, in part because several do not have drug-drug interactions; but there is a great need to identify new targets, specifically those that are responsible for pharmacoresistance.[32] Perhaps more importantly, there are no interventions that prevent the development of or cure epilepsy, and much research is now being focused on approaches to antiepileptogenesis.[33]
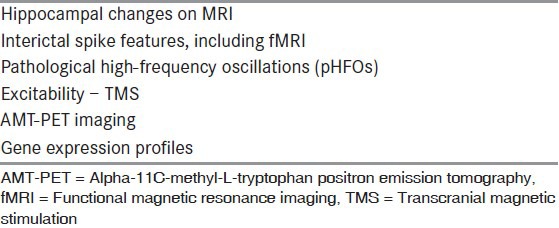
The discovery and validation of new antiseizure and antiepileptogenic interventions would be greatly advanced by the identification of reliable epilepsy biomarkers.[34] Biomarkers are measures of dynamic changes that indicate the presence of an epileptogenic process with a sufficiently high degree of reliability to warrant intervention. Biomarkers of epileptogenesis would identify the development and extension of tissue capable of generating spontaneous seizures, including the development of an epilepsy condition and the progression of epilepsy after the disease is established. Biomarkers of ictogenesis would identify tissue capable of generating spontaneous behavioral seizures. Potential targets for epilepsy biomarkers are shown in [Table 4] and potential epilepsy biomarkers are shown in [Table 5].
Table 4.
Potential target mechanisms for biomarkers of epilepsy
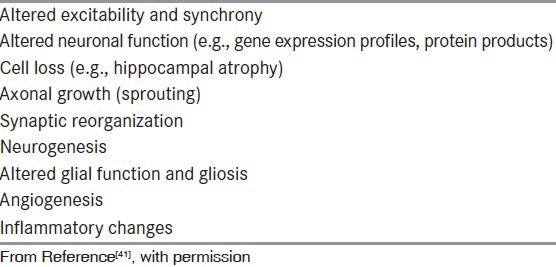
Table 5.
Potential biomarkers
Although considerable research is being carried out in the animal laboratory to discover potential antiepileptogenic compounds, the cost required to carry out clinical trials remains prohibitive, because: 1) The percentage of patients who develop epilepsy even after the most severe traumatic brain injury is sufficiently small that it would take very large populations of patients to obtain statistically significant results, and 2) epileptic seizures may appear 10 years or more after injury so prolonged trials would be necessary. A biomarker that could identify individuals at very high risk for epilepsy after a potentially epileptogenic insult would make it possible to enrich trial populations, and a biomarker that reliably identified the existence of epilepsy would make it possible to measure outcome quickly without waiting for spontaneous seizures to occur, thus reducing the cost of clinical trials to an affordable range.
Biomarkers of epileptogenesis would also help to identify patients requiring antiepileptogenic interventions once an antiepileptogenic agent is identified, and could be used to diagnose progression in patients in order to determine when to institute early aggressive treatment and prevent irreversible social and psychological disabling consequences of recurrent seizures.
Biomarkers of ictogenesis could predict who has epilepsy after a single seizure, in order to begin antiseizure drug treatment immediately and not wait for a second seizure, which could cause injury or death. It would also facilitate diagnosis of epilepsy in patients with equivocal events without the need for inpatient video-EEG monitoring. With such a biomarker, individual pharmacotherapy could be tailored to identify the best drug regimen in each individual patient immediately in place of the usual trial-and-error process that requires waiting to see if further seizures will occur. Biomarkers might also identify pharmacoresistance, which would aid in referring patients for early surgical therapy.
Biomarkers that localize epileptogenic brain tissue could be used to determine the extent of the epileptogenic region for surgical resection without the need for other, expensive presurgical evaluation. At the present time, there is increasing evidence that pathological high-frequency oscillations (pHFOs) could serve this purpose.[35,36] These EEG events appear to be more reliable than interictal EEG spikes and the site of ictal onset,[37,38] but so far can only be recorded with intracranial electrodes. Future research may make it possible for pHFOs to be recorded from scalp EEG, magnetoencephaolography, or simultaneous EEG with functional magnetic resonance imaging.
Reliable biomarkers would also facilitate the development of more cost-effective, rapid-throughput approaches for screening potential antiseizure and antiepileptogenic compounds and devices, facilitating new drug and device discovery.
Conclusions
A considerable proportion of the economic and social cost of epilepsy is due to patients with seizures that are not controlled by standard medical therapy. There are many approaches, however, to treat their refractory conditions. Surgical treatment for epilepsy remains arguably the most underutilized of all accepted medical treatments. Much could be achieved to reduce the global burden of epilepsy by early identification and referral of appropriate surgical candidates. MTLE is the prototype of a surgically remediable epilepsy syndrome, but other conditions include resectable structural lesions, diffuse disturbances in infants and young children limited to one hemisphere, and gelastic seizures with hypothalamic hamartomas. Alternative treatments other than epilepsy are also underutilized because patients with medically refractory epilepsy are too often not referred to epilepsy centers, or referred too late to prevent irreversible disability. There invariably will remain a significant number of patients who will not become seizure-free despite appropriate drug treatments and other alternative therapeutic interventions, and many of these will be compromised by their seizures. Supportive care at home or in assisted living facilities can help improve quality of life. Further intensive research is needed to elucidate basic mechanisms of epileptogenesis and seizure generation in order to devise more effective approaches to prevent the development of epilepsy, to control epileptic seizures, cure epilepsy after it has developed, and to avoid adverse consequences. Identification of reliable epilepsy biomarkers would greatly facilitate efforts to discover and validate new antiseizure and antiepileptogenic interventions, and permit individualized treatment regimens.[41]
Acknowledgments
Original research reported by the author was supported in part by Grants NS-02808, NS-15654, NS-33310, and NS-80181.
Footnotes
Source of Support: Original research reported by the author was supported in part by Grants NS-02808, NS-15654, NS-33310, and NS-80181
Conflict of Interest: Nil
References
- 1.Murray CJ, Lopez AD, editors. Geneva: World Health Organization; 1994. Global Comparative Assessment in the Health Sector; Disease Burden, Expenditures, and Intervention Packages. [Google Scholar]
- 2.Hesdorffer DC, Logroscino G, Benn EK, Katri N, Cascino G, Hauser WA. Estimating risk for developing epilepsy: A population-based study in Rochester, Minnesota. Neurology. 2011;76:23–7. doi: 10.1212/WNL.0b013e318204a36a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kobau R, Zahran H, Thurman DJ, Zack MM, Henry TR, Schachter SC, et al. Centers for Disease Control and Prevention (CDC). Epilepsy surveillance among adults-19 states, Behavioral Risk Factor Surveillance System, 2005. MMWR Surveill Summ. 2008;57:1–20. [PubMed] [Google Scholar]
- 4.Begley CE, Famulari M, Annegers JF, Lairson DR, Reynolds TF, Coan S, et al. The cost of epilepsy in the United States: An estimate from population-based clinical and survey data. Epilepsia. 2000;41:342–51. doi: 10.1111/j.1528-1157.2000.tb00166.x. [DOI] [PubMed] [Google Scholar]
- 5.Berg AT, Langfitt J, Shinnar S, Vickrey BG, Sperling MR, Walczak T, et al. How long does it take for partial epilepsy to become intractable? Neurology. 2003;60:186–90. doi: 10.1212/01.wnl.0000031792.89992.ec. [DOI] [PubMed] [Google Scholar]
- 6.Kwan P, Brodie MJ. Early identification of refractory epilepsy. New Engl J Med. 2000;342:314–9. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- 7.Pharmacoresistance in epilepsy. Proceedings of the 2nd Halifax International Epilepsy Conference & Retreat. September 21-22, 2012. Halifax, Nova Scotia, Canada. Epilepsia. 2013;54:1–85. [PubMed] [Google Scholar]
- 8.Epilepsy Centers. [Last accessed on 2014 Jan 24]. Available from: http://www.nacc-epilepsy.org .
- 9.Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Allen Hauser W, Mathern G, et al. Definition of drug resistant epilepsy: Consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51:1069–77. doi: 10.1111/j.1528-1167.2009.02397.x. [DOI] [PubMed] [Google Scholar]
- 10.Engel J., Jr Surgery for seizures. N Engl J Med. 1996;334:647–52. doi: 10.1056/NEJM199603073341008. [DOI] [PubMed] [Google Scholar]
- 11.Engel J, Jr, Williamson PD, Wieser HG. Mesial temporal lobe epilepsy with hippocampal sclerosis. In: Engel J Jr, Pedley TA, editors. Epilepsy: A Comprehensive Textbook. 2nd ed. Philadelphia: Lippincott-Raven; 2008. pp. 2479–86. [Google Scholar]
- 12.Parvizi J, Le S, Foster BL, Bourgeois B, Riviello JJ, Prenger E, et al. Gelastic epilepsy and hypothalamic hamartomas: Neuroanatomical analysis of brain lesions in 100 patients. Brain. 2011;134:2960–8. doi: 10.1093/brain/awr235. [DOI] [PubMed] [Google Scholar]
- 13.Mathern GW, Wilson CL, Beck H. Hippocampal sclerosis. In: Engel J Jr, Pedley TA, editors. Epilepsy: A Comprehensive Textbook. 2nd ed. Philadelphia: Lippincott-Raven; 2008. pp. 121–36. [Google Scholar]
- 14.Engel J, Jr, McDermott MP, Wiebe S, Langfitt JT, Stern JM, Dewar S, et al. Early Randomized Surgical Epilepsy Trial (ERSET) Study Group. Early surgical therapy for drug-resistant temporal lobe epilepsy: A randomized trial. JAMA. 2012;307:922–30. doi: 10.1001/jama.2012.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wiebe S, Blume WT, Girvin JP, Eliasziw M Effectiveness and Efficiency of Surgery for Temporal Lobe Epilepsy Study Group. A randomized, controlled trial of surgery for temporal lobe epilepsy. N Engl J Med. 2001;345:311–8. doi: 10.1056/NEJM200108023450501. [DOI] [PubMed] [Google Scholar]
- 16.Leiphart J, Fried I. Mesial temporal lobe surgery and other lobar resections. In: Shorvon S, Perucca E, Engel J Jr, editors. The Treatment of Epilepsy. 3rd ed. West Sussex: Wiley-Blackwell; 2009. pp. 875–85. [Google Scholar]
- 17.Binder DK, Schramm J. Multilobar resections and hemispherectomy. In: Engel J Jr, Pedley TA, editors. Epilepsy: A Comprehensive Textbook. 2nd ed. Philadelphia: Lippincott-Raven; 2008. pp. 1879–89. [Google Scholar]
- 18.Lüders HO, Engel J, Jr, Munari C. General Principles. In: Engel J Jr, editor. Surgical Treatment of the Epilepsies. 2nd ed. New York: Raven Press; 1993. pp. 137–53. [Google Scholar]
- 19.Radhakrishnan K, Fried I, Cascino GD. Lesionectomy: Management of substrate-directed epilepsies. In: Engel J Jr, Pedley TA, editors. Epilepsy: A Comprehensive Textbook. 2nd ed. Philadelphia: Lippincott-Raven; 2008. pp. 1891–906. [Google Scholar]
- 20.Polkey CE, Smith MC. Multiple subpial transections and other interventions. In: Engel J Jr, Pedley TA, editors. Epilepsy: A Comprehensive Textbook. 2nd ed. Philadelphia: Lippincott-Raven; 2008. pp. 1921–8. [Google Scholar]
- 21.Roberts DW. Corpus callosotomy. In: Engel J Jr, Pedley TA, editors. Epilepsy: A Comprehensive Textbook. 2nd ed. Philadelphia: Lippincott-Raven; 2008. pp. 1907–13. [Google Scholar]
- 22.Quigg M, Ralston J, Barbaro N. Radio surgery for epilepsy: Clinical experience and potential antiepileptic mechanisms. Epilepsia. 2012;53:7–15. doi: 10.1111/j.1528-1167.2011.03339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Curry DJ, Gowda A, McNichols RJ, Wilfong AA. MR-guided stereotactic laser ablation of epileptogenic foci in children. Epilepsy Behav. 2012;24:408–14. doi: 10.1016/j.yebeh.2012.04.135. [DOI] [PubMed] [Google Scholar]
- 24.Schachter SC, Boon P. Vagus nerve stimulation. In: Engel J Jr, Pedley TA, editors. Epilepsy: A Comprehensive Textbook. 2nd ed. Philadelphia: Lippincott-Raven; 2008. pp. 1395–9. [Google Scholar]
- 25.DeGiorgio CM, Soss J, Cook IA, Markovic D, Gornbein J, Murray D, et al. Randomized controlled trial of trigeminal nerve stimulation for drug-resistant epilepsy. Neurology. 2013;80:786–91. doi: 10.1212/WNL.0b013e318285c11a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fisher R, Salanova V, Witt T, Worth R, Henry T, Gross R, et al. The SANTE Study Group. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51:899–908. doi: 10.1111/j.1528-1167.2010.02536.x. [DOI] [PubMed] [Google Scholar]
- 27.Morrell MJ RNS System in Epilepsy Study Group. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology. 2011;77:1295–304. doi: 10.1212/WNL.0b013e3182302056. [DOI] [PubMed] [Google Scholar]
- 28.Stafstrom CE, Vining EPG, Rho JM. Ketogenic diet. In: Engel J Jr, Pedley TA, editors. Epilepsy: A Comprehensive Textbook. 2nd ed. Philadelphia: Lippincott-Raven; 2008. pp. 1377–85. [Google Scholar]
- 29.Schachter SC, Acevedo C, Acevedo KA, Lai C-W, Diop AG. Complementary and alternative medical therapies. In: Engel J Jr, Pedley TA, editors. Epilepsy: A Comprehensive Textbook. 2nd ed. Philadelphia: Lippincott-Raven; 2008. pp. 1407–14. [Google Scholar]
- 30.Michaelis R, Schonfeld W, Elsas SM. Trigger self-control and seizure arrest in the Andrews/Reiter behavioral approach to epilepsy: A retrospective analysis of seizure frequency. Epilepsy Behav. 2012;23:266–71. doi: 10.1016/j.yebeh.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zifkin BG, Guerrini R, Plouin P. Reflex seizures. In: Engel J Jr, Pedley TA, editors. Epilepsy: A Comprehensive Textbook. 2nd ed. Philadelphia: Lippincott-Raven; 2008. pp. 2559–72. [Google Scholar]
- 32.Simonato M, Löscher W, Cole AJ, Dudek FE, Engel J, Jr, Kaminski RM, et al. Finding a better drug for epilepsy: Preclinical screening strategies and experimental trial design. Epilepsia. 2012;53:1860–7. doi: 10.1111/j.1528-1167.2012.03541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galanopoulou AS, Buckmaster PS, Staley KJ, Moshé SL, Perucca E, Engel J, Jr, et al. American Epilepsy Society Basic Science Committee And The International League Against Epilepsy Working Group On Recommendations For Preclinical Epilepsy Drug Discovery. Identification of new epilepsy treatments: Issues in preclinical methodology. Epilepsia. 2012;53:571–82. doi: 10.1111/j.1528-1167.2011.03391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Engel J., Jr Biomarkers in epilepsy. Biomark Med. 2011;5:529–664. doi: 10.2217/bmm.11.63. [DOI] [PubMed] [Google Scholar]
- 35.Staba RJ, Bragin A. High-frequency oscillations and other electrophysiological biomarkers of epilepsy: Underlying mechanisms. Biomark Med. 2011;5:545–56. doi: 10.2217/bmm.11.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Engel J, Jr, Bragin A, Staba R, Mody I. High-frequency oscillations: What's normal and what is not? Epilepsia. 2009;50:598–604. doi: 10.1111/j.1528-1167.2008.01917.x. [DOI] [PubMed] [Google Scholar]
- 37.Jacobs J, LeVan P, Chander R, Hall J, Dubeau F, Gotman J. Interictal high-frequency oscillations (80-500 Hz) are an indicator of seizure onset areas independent of spikes in the human epileptic brain. Epilepsia. 2008;49:1893–907. doi: 10.1111/j.1528-1167.2008.01656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jacobs J, Zijlmans M, Zelmann R, Chatillon CE, Hall J, Olivier A, et al. High-frequency electroencephalographic oscillations correlate with outcome of epilepsy surgery. Ann Neurol. 2010;67:209–20. doi: 10.1002/ana.21847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Engel J., Jr Update on surgical treatment of the epilepsies. Summary of the Second International Palm Desert Conference on the Surgical Treatment of the Epilepsies (1992) Neurology. 1993;43:1612–7. doi: 10.1212/wnl.43.8.1612. [DOI] [PubMed] [Google Scholar]
- 40.Engel J., Jr Why the doubt to cut it out? Epilepsy Curr. 2013;13:198–204. doi: 10.5698/1535-7597-13.5.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Engel J., Jr Biomarkers in epilepsy: Introduction. Biomark Med. 2011;5:537–44. doi: 10.2217/bmm.11.62. [DOI] [PubMed] [Google Scholar]


