Abstract
Introduction
Melanoma microsatellitosis is classified as stage IIIB/C disease and is associated with a poor prognosis. Prognostic factors within this group, however, have not been well characterized.
Methods
We performed a retrospective analysis of 1621 patients undergoing sentinel lymph node (SLN) biopsy at our institution (1996–2011) to compare patients with (n=98) and patients without (n=1523) microsatellites. Univariate and multivariate logistic and Cox regression analyses were used to identify factors associated with SLN positivity and melanoma-specific survival (MSS) in patients with microsatellites.
Results
Patients with microsatellites were older and had lesions with higher Clark level and greater thickness that more frequently had mitoses, ulceration, and lymphovascular invasion (LVI) (all p<0.0001). In microsatellite patients, the SLN positivity rate was 43%. Lesional ulceration (OR=2.9, 95% CI: 1.5–8.6), absent tumor infiltrating lymphocytes (OR=2.8, 95% CI: 1.1–7.1), and LVI (OR=3.3, 95% CI: 1.7–10.0) were significantly associated with SLN positivity by multivariate analysis. With a median follow up of 4.5 years in survivors, ulceration (HR=3.4, 95% CI: 1.5–7.8) and >1 metastatic LN (HR=2.7, 95% CI: 1.1–6.6) were significantly associated with decreased MSS by multivariate analysis. In patients without these prognostic factors, the 5-year MSS was 90% (n=49), compared to 50% (n=23) among patients with ulceration only, 51% (n=12) in those with >1 metastatic LN only, or 25% in those with both (n=14, p<0.01).
Discussion
Microsatellitosis was frequently associated with multiple adverse pathologic features. In the absence of ulceration and >1 metastatic LN, however, the outcome for patients with microsatellites compared favorably to stage IIIB patients overall.
Introduction
Stage III melanoma is comprised of diverse clinical and pathologic entities, including lymph node metastases, in-transit metastases, and macroscopic or microscopic satellitosis.1 The heterogeneity of this stage is reflected in the wide range of patient outcomes with 5-year survival ranging from 78% for stage IIIA to 40% for stage IIIC.1, 2 The independent prognostic value of nodal status in primary melanoma patients is well established,1, 3 and there is ample evidence that the presence of in-transit disease is associated with a poor prognosis as well.4–6 Microscopic satellites surrounding the primary tumor are currently classified in the 7th edition of the AJCC staging system with in-transit disease as stage IIIB or, if concurrent nodal metastases or ulceration of the primary tumor are present, as IIIC.7 The rarity of microsatellites, however, has limited further risk stratification of this patient group.
Microscopic satellites were first described by Day et al. in 1981 as being associated with poor disease-free survival.8 Since that time a number of studies have found the presence of microscopic satellites to be associated with decreased disease-free survival9–13 and overall survival.9, 13 They were thus included with macroscopic satellites and in-transit lesions in stage III in the 1997 staging system.14 This inclusion was heavily influenced by work from Leon et al., who found microsatellites to be associated with a significantly worse overall survival compared to a non-microsatellite cohort matched on multiple adverse features of the primary tumor such as thickness and mitotic rate. Survival was 37% vs. 65% at 10 years in patients with absent and present microsatellites, respectively.9 In a more recent study, Kimsey et al. described a 34% 5-year survival for their patients with microsatellites.15 Overall, these survival figures are consistent with stage IIIB/C patients.
The role in prognostication for sentinel lymph node biopsy (SLNB) in patients with microsatellites is controversial, given the high baseline risk for distant metastasis. Multiple studies have identified a high rate of nodal metastases in patients with microsatellites,9, 12, 15, 16 but few have addressed the role of SLNB in these patients.15 One study of patients with microsatellites found SLN status had a profound influence on 5-year disease-free survival (60% for node negative versus 0% for node positive patients) but was underpowered to perform a multivariate analysis for prognostic factors and did not address the impact of nodal status on overall survival.15
Studies addressing microsatellites as an independent, poor prognostic factor in patients with primary melanoma have been limited by relatively small sample sizes and therefore been underpowered to sufficiently identify factors associated with survival. Here, we examined a large cohort of patients with microsatellitosis who underwent SLNB to study the prognostic utility of SLNB in this cohort and to identify factors that may further risk-stratify this group. We hypothesized that patients with microsatellites frequently present with multiple adverse prognostic factors but there is, as for Stage III patients overall, a significant heterogeneity of survival within this subgroup.
Methods
Between 1995–2011, 2097 SLNBs were performed at our institution. Patients with multiple primary lesions, macroscopic satellites, in-transit disease, or unknown microsatellite status were excluded from the analysis (n=476), leaving 1621 patients with known microsatellite status and evaluable clinicopathologic data. All variables were more frequently unknown in excluded patients. Additionally, ulceration (12% vs. 16%) and regression (13% vs. 20%) were less commonly present in excluded patients. SLNB was performed routinely on patients with clinically negative nodes and a primary melanoma >1mm in thickness. SLNB in patients with thin (<1mm) melanoma was selectively performed based upon factors thought to be associated with increased SLN metastasis. The presence of microsatellites was generally considered to be a high-risk feature for patients with thin melanoma.
Clinical variables analyzed included age, sex, and anatomic site. Pathologic variables of the primary melanoma were determined on H&E stained sections and included thickness, Clark level, mitoses, tumor infiltrating lymphocytes (TIL), regression, ulceration, lymphovascular invasion (LVI), and microsatellitosis. Consistent with staging criteria, microsatellitosis was defined as 1 or more discontinuous nests of melanoma cells at least 0.3mm in diameter and separated from the primary lesion by >0.05mm of normal dermis or subcutaneous tissue.1, 8 The additional pathologic variables were defined as previously reported.17 Nodal status was categorized by tumor involvement of SLNs, non-SLNs, and by total number of positive nodes (the sum of positive sentinel and, when available, positive non-SLN nodes). The following categorical variables were used in the analyses: age (≤40, 41–65, and >65 years), Clark level (II-III or IV-V/unknown), thickness (0.01–1, 1.01–2, 2.01–4, and >4mm), TIL (present/unknown or absent), and mitoses (present/unknown or absent), regression (present or absent/unknown in the radial growth phase), ulceration (present or absent/unknown), and lymphovascular invasion (LVI, present or absent/unknown).
SLNB was performed using the standard technique as previously described.18 All SLN specimens were reviewed by specialized surgical pathologists or dermatopathologists at the Hospital of the University of Pennsylvania. Lymph node specimens were stained for S100 and HMB45 as previously described.18 Recurrences were defined clinically or by definitive pathologic diagnosis. Melanoma-specific survival (MSS) was calculated from the date of definitive surgical excision of the primary lesion to the date of death from melanoma. The majority of patients were followed at the University of Pennsylvania for subsequent melanoma care. Phone calls were made in an attempt to contact each of the patients who was lost to follow up.
Descriptive statistics were computed. Predictors of SLN positivity were evaluated using logistic regression analyses. Prognostic factors associated with MSS were evaluated using Cox regression analyses. Kaplan-Meier survival curves were used to compute five-year MSS survival rates with standard errors computed using Greenwood’s formula. The log rank test was used to compare survival curves.19, 20 In the data used for the models presented in Tables 2 and 3, missing data values were imputed using the most frequent category observed in the study cohort assuming the missing values were missing at random.21 A p-value of less than 0.05 was considered statistically significant. Analysis was performed using STATA 12.0/IC statistical software (StataCorp, College Station, TX).22 This study was approved by the University of Pennsylvania Institutional Review Board.
Table 2.
Factors Associated with SLN Positivity in Patients with Microsatellites (n=98)
| Characteristic | SLN Negative (N=56) |
SLN Positive (N=42) |
Univariate | Multivariate | ||
|---|---|---|---|---|---|---|
| N (%) | N (%) | OR | p- value |
OR | p- value |
|
| Age | 0.950 | |||||
| ≤40 | 4 (7) | 3 (7) | -- | -- | -- | |
| 41–65 | 24 (43) | 19 (45) | 1.1 | -- | -- | |
| >65 | 28 (50) | 20 (48) | 1.0 | -- | -- | |
| Sex | 0.500 | |||||
| Female | 21 (38) | 13 (31) | -- | -- | -- | |
| Male | 35 (63) | 29 (69) | 1.3 | -- | -- | |
| Anatomic Site | 0.474 | |||||
| Extremity | 27 (48) | 17 (41) | -- | -- | -- | |
| Trunk | 24 (43) | 18 (43) | 1.2 | -- | -- | |
| Head/Neck | 5 (9) | 7 (17) | 2.2 | -- | -- | |
| Histology | 0.115 | |||||
| Superficial Spreading | 19 (34) | 19 (45) | -- | -- | -- | |
| Nodular | 15 (27) | 14 (33) | 0.9 | -- | -- | |
| Other/ALM/LMM | 12 (21) | 2 (5) | 0.2 | -- | -- | |
| Unclassified/Unk | 10 (18) | 7 (17) | 0.6 | -- | -- | |
| Clark Level | 1 | |||||
| II-III | 3 (5) | 3 (7) | -- | -- | -- | |
| IV-V/UNK | 53 (95) | 39 (93) | 0.7 | -- | -- | |
| Thickness | 0.053 | |||||
| 0.01–1 | 5 (9) | 2 (5) | -- | -- | -- | |
| 1.01–2 | 15 (27) | 5 (12) | 0.8 | -- | -- | |
| 2.01–4 | 20 (36) | 12 (29) | 1.5 | -- | -- | |
| >4 | 16 (29) | 23 (55) | 3.6 | -- | -- | |
| Mitoses | 0.133a | |||||
| Absent | 4 (7) | 0 (0) | -- | -- | -- | |
| Present/Unk | 52 (93) | 42 (100) | -- | -- | -- | |
| TIL | 0.025 | |||||
| Absent | 10 (18) | 16 (38) | -- | 2.8 | 0.041 | |
| Present/Unk | 46 (82) | 26 (62) | 0.4 | -- | -- | |
| Regression | 0.485 | |||||
| Absent/Unk | 42 (75) | 34 (81) | -- | -- | -- | |
| Present | 14 (25) | 8 (19) | 0.7 | -- | -- | |
| Ulceration | 0.003 | |||||
| Absent/Unk | 42 (75) | 19 (45) | -- | -- | -- | |
| Present | 14 (25) | 23 (55) | 3.6 | 2.9 | 0.022 | |
| LVI | 0.002 | |||||
| Absent/Unk | 45 (80) | 21 (50) | -- | -- | -- | |
| Present | 11 (20) | 21 (50) | 4.1 | 3.3 | 0.015 | |
Using Fisher exact test. Mitoses omitted from univariate/multivariate analysis because it was present in 95% of patients.
Abbreviations: odds ratio (OR), acral lentiginous melanoma (ALM), lentigo maligna melanoma (LMM), tumor infiltrating lymphocytes (TIL), lymphovascular invasion (LVI)
Table 3.
Patient and Tumor Characteristics Associated with MSS in Patients with Microsatellites (n=98)
| Characteristic | Univariate | Multivariate Reduced |
||
|---|---|---|---|---|
| HR | p-value | HR | p-value | |
| Age | 0.765 | |||
| ≤40 | -- | -- | -- | |
| 41–65 | 1.096 | -- | -- | |
| >65 | 1.417 | -- | -- | |
| Sex | 0.347 | |||
| Male | -- | -- | -- | |
| Female | 0.689 | -- | -- | |
| Anatomic Site | 0.729 | |||
| Extremity | -- | -- | -- | |
| Trunk | 1.254 | -- | -- | |
| Head/Neck | 0.812 | -- | -- | |
| Histology | 0.243 | |||
| Superficial Spreading | -- | -- | -- | |
| Nodular | 1.233 | -- | -- | |
| Other/ALM/LMM | 0.774 | -- | -- | |
| Unclassified/Unk | 0.231 | -- | -- | |
| Clark Level | 0.414 | |||
| II-III | -- | -- | -- | |
| IV-V/Unk | 0.586 | -- | -- | |
| Thickness | 0.479 | |||
| 0.01–1 | -- | -- | -- | |
| 1.01–2 | 1.441 | -- | -- | |
| 2.01–4 | 2.609 | -- | -- | |
| >4 | 2.760 | -- | -- | |
| Mitoses | 0.602 | |||
| Absent | -- | -- | -- | |
| Present/Unk | 0.560 | -- | -- | |
| TIL | 0.464 | |||
| Absent | -- | -- | -- | |
| Present/Unk | 0.741 | -- | -- | |
| Regression | 0.835 | |||
| Absent/Unk | -- | -- | -- | |
| Present | 1.096 | -- | -- | |
| Ulceration | 0.002 | |||
| Absent/Unk | -- | -- | -- | |
| Present | 3.353 | 3.409 | 0.004 | |
| LVI | 0.026 | |||
| Absent/Unk | -- | -- | -- | |
| Present | 2.370 | 1.700 | 0.184 | |
| Total Number of Positive LNs | ||||
| Zero | -- | -- | -- | |
| One | 1.780 | 0.253 | 0.965 | 0.947 |
| >1 | 3.760 | 0.002 | 2.749 | 0.024 |
Abbreviations: hazard ratio (HR), acral lentiginous melanoma (ALM), lentigo maligna melanoma (LMM), tumor infiltrating lymphocytes (TIL), lymphovascular invasion (LVI)
Results
Comparison of Patients with and without Microsatellitosis
Of 1621 included patients undergoing SLNB, 98 (6%) were found to have microsatellitosis. Patients with microsatellites were older than those without microsatellites (49% vs. 26% >65 years old, p<0.0001). Microsatellites were associated with a number of additional aggressive features in the primary tumor including: elevated Clark level (92% vs. 67% level IV/V), increased thickness (40% vs. 8% >4mm), mitoses (94% vs. 76% present), ulceration (38% vs. 15% present), and LVI (33% vs. 5% present, p<0.0001 for each). Nodal metastasis was significantly more frequent in patients with microsatellites compared to those without: the SLN positivity rates were 43% vs. 11%, the non-SLN positivity rates were 25% vs. 16% (in those undergoing completion lymphadenectomy) and the rates of >1 node positive were 27% vs. 4% (p<0.0001 for each). Table 1. After a positive SLNB, completion lymphadenectomy was performed in 90% of patients with and 86% of patients without microsatellitosis.
Table 1.
Characteristics of Patients with and without Microsatellitosis (n=1621)
| Characteristic | Satellites (n=98) |
No Satellites (n=1523) |
p-value |
|---|---|---|---|
| N (%) | N (%) | ||
| Age | <.0001 | ||
| ≤40 | 7 (7) | 302 (20) | |
| 41–65 | 43 (44) | 824 (54) | |
| >65 | 48 (49) | 397 (26) | |
| Sex | 0.109 | ||
| Male | 64 (65) | 869 (57) | |
| Female | 34 (35) | 654 (43) | |
| Anatomic Site | 0.823 | ||
| Extremity | 44 (45) | 647 (42) | |
| Trunk | 42 (43) | 702 (46) | |
| Head/Neck | 12 (12) | 174 (11) | |
| Histology | <.0001 | ||
| Superficial Spreading | 38 (39) | 925 (61) | |
| Nodular | 29 (30) | 326 (21) | |
| Other/ALM/LMM | 14 (14) | 151 (10) | |
| Unclassified/Unk | 17 (17) | 121 (8) | |
| Clark Level | <.0001 | ||
| II-III | 6 (6) | 483 (32) | |
| IV-V | 90 (92) | 1020 (67) | |
| Unknown | 2 (2) | 20 (1) | |
| Thickness | <.0001 | ||
| 0.01–1 | 7 (7) | 658 (43) | |
| 1.01–2 | 20 (20) | 490 (32) | |
| 2.01–4 | 32 (33) | 258 (17) | |
| >4 | 39 (40) | 117 (8) | |
| Mitoses | <.0001 | ||
| Absent | 4 (4) | 311 (20) | |
| Present | 92 (94) | 1163 (76) | |
| Unknown | 2 (2) | 49 (3) | |
| TIL | 0.654 | ||
| Absent | 26 (27) | 356 (23) | |
| Present | 67 (68) | 1105 (73) | |
| Unknown | 5 (5) | 62 (4) | |
| Regression | 0.799 | ||
| Absent | 66 (67) | 1067 (70) | |
| Present | 22 (22) | 300 (20) | |
| Unknown | 10 (10) | 156 (10) | |
| Ulceration | <.0001 | ||
| Absent | 57 (58) | 1246 (82) | |
| Present | 37 (38) | 224 (15) | |
| Unknown | 4 (4) | 53 (3) | |
| LVI | <.0001 | ||
| Absent | 64 (65) | 1359 (89) | |
| Present | 32 (33) | 70 (5) | |
| Unknown | 2 (2) | 94 (6) | |
| SLN Status | <.0001 | ||
| Negative | 56 (57) | 1345 (89) | |
| Positive | 42 (43) | 173 (11) | |
| Non-SLN Status | <.0001 | ||
| Negative | 81 (83) | 1474 (97) | |
| Positive | 13 (13) | 24 (2) | |
| Unknowna | 4 (4) | 25 (2) | |
| Total Number of Positive LNs | <.0001 | ||
| 0 | 56 (57) | 1320 (87) | |
| 1 | 19 (19) | 141 (9) | |
| >1 | 23 (23) | 62 (4) | |
Patients did not undergo completion lymph node dissection after positive SLNB.
Abbreviations: acral lentiginous melanoma (ALM), lentigo maligna melanoma (LMM), tumor infiltrating lymphocytes (TIL), lymphovascular invasion (LVI)
Predictors of SLN Positivity
In the overall cohort, nodular histology, elevated Clark level, increasing thickness, present mitoses, absent TIL, present ulceration, present LVI, and present microsatellites were all significantly associated with SLN positivity by univariate analysis (p<0.05 for each). In the multivariate analysis, thickness (OR=2.2 for 1.01–2mm, OR=4.8 for 2.01–4mm, OR=7.8 for >4mm lesions), present mitoses (OR=3.8), absent TIL (OR=1.7), present LVI (OR=2.8), and present microsatellites (OR=2.1) remained significantly associated with SLN positivity (p<0.005 for each, data not shown).
Among the 98 patients with present microsatellitosis, the SLN positivity rate was 43% (95% CI: 33–53%). Factors associated with SLN metastasis by univariate analysis in this subgroup were absent TIL (p=0.03), present ulceration (p=0.003), and present LVI (p=0.002). Table 2. In the multivariate analysis, absent TIL (OR=2.8), present ulceration (OR=2.9), and present LVI (OR=3.3) remained significantly associated with SLN positivity (p<0.05 for each). In patients with at least 2 of these adverse features (n=28), the SLN positivity rate was 75%. All 5 patients with three adverse features had a positive SLN. In patients with none of these three risk factors present (n=37), the SLN positivity rate remained high at 22% (95% CI: 11–37%).
In patients with microsatellitosis, 16 (16%) had multiple positive SLNs, and a positive non-SLN was identified in 13 patients (13%). Overall, 56 patients (57%) had a negative nodal evaluation, 19 patients (19%) had only a single node positive, and 23 patients (23%) had multiple positive nodes.
Recurrence Patterns and Survival in Patients with Microsatellitosis
With a median follow up time of 4.5 years in surviving patients, the 5-year overall and MSS for patients with microsatellitosis were 64% and 68% respectively. Forty-one patients (42%) experienced a disease recurrence. Thirteen patients (13%) had distant metastases alone at initial recurrence, 2 patients (2%) had simultaneous loco-regional and distant recurrence, and 26 patients (27%) had only loco-regional recurrence first. Loco-regional recurrences included subcutaneous disease in 8 patients, LN recurrence in 7 (4 of whom had undergone a negative SLNB), in-transit disease in 4, and multiple kinds in 7. The majority of patients who recurred died of disease (n=16); 7 remained alive with disease; 2 died of other causes, and a single patient with a local recurrence had no evidence of disease after resection with a follow up of 4.2 years.
In the univariate analysis, factors significantly associated with decreased MSS were ulceration (HR=3.4, 95% CI: 1.6–7.2, p=0.002), LVI (HR=2.4, 95% CI: 1.1–5, p=0.026), and total number of positive lymph nodes >1 (HR=3.8, 95% CI: 1.6–8.6, p=0.002). Table 3. In the multivariate analysis, LVI was strongly associated with >1 metastatic node, and thus only >1 metastatic node (HR=2.7, 95% CI: 1.1–6.6, p=0.02) and ulceration (HR=3.4, 95% CI: 1.5–7.8, p=0.004) remained significantly associated with decreased MSS.
The 5-year MSS for patients without ulceration (n=61) was 83% (95% CI: 68–91%) compared to 43% (95% CI: 25–61%) in those with ulceration (n=37, p<0.01). Figure 1A. The 5-year MSS for patients with no positive nodes (n=56) was 81% (95% CI: 65–90%) compared to 63% (95% CI: 35–82%), if 1 positive node was present (n=19) and 38% (95% CI: 13–62%) if more than one positive node was identified (n=23, p<0.01). Figure 1B. Patients with microsatellitosis were stratified into four risk groups based upon the presence or absence of ulceration and more than one positive node. Patients without ulceration and with ≤1 metastatic node (n=49) had a 5-year MSS of 90% (95% CI: 76–96%), which was higher than for patients with either ulceration alone (50%, 95% CI: 27–70%, n=23), >1 metastatic node alone (51%, 95% CI: 14–80%, n=12), or both (25%, 95% CI: 29–59%, n=14, p<0.01). Figure 2A. Among microsatellitosis patients, stage IIIB patients had an 87% (95% CI: 70–94%) 5-year MSS rate compared to a 45% (95% CI: 28–62%) rate for stage IIIC patients (p<0.01). Figure 2B.
Figure 1. MSS in Patients with Microsatellitosis by Individual Prognostic Factors.
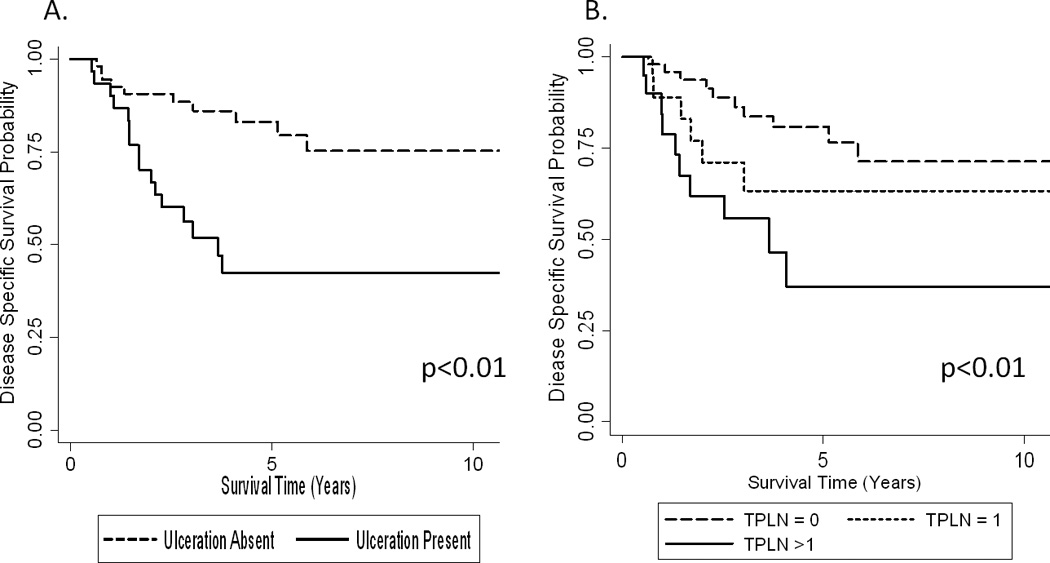
A) MSS stratified by ulceration status. Lesional ulceration absent (n=61) and present (n=37). B) MSS stratified by total positive lymph nodes (TPLN). No positive lymph nodes (n=56), one positive lymph node (n=19), and more than one positive lymph node (n=23). p-values presented for the log-rank test.
Figure 2. MSS in Patients with Microsatellitosis by Combined Prognostic Factors or AJCC Stage.
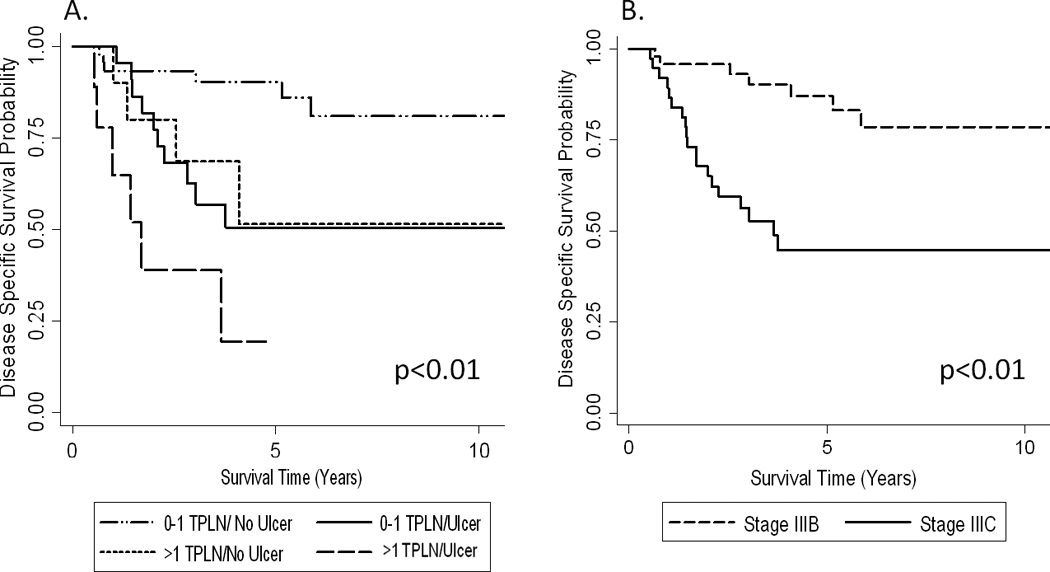
A) MSS was stratified by ulceration status and total number of positive lymph nodes (classified as 0–1 or >1 TPLN). 0–1 TPLN/no ulceration (n=49), 0–1 TPLN with ulceration (n=23), >1 TPLN/no ulceration (n=12), and >1 TPLN with ulceration (n=14). B) MSS stratified by stage of patient with microsatellitosis. Stage IIIB (n=39) and stage IIIC (n=59). p-values presented for the log-rank test.
Discussion
In a large cohort of patients undergoing SLNB for melanoma, microsatellitosis was identified as a rare (6%) pathologic feature associated with a generally poor prognosis. We found microsatellites to be associated with a number of other adverse features, including increased thickness, and frequent mitoses and LVI, yet microsatellitosis remained independently associated with SLN positivity in the whole cohort. Among patients with microsatellites, we identified a high rate of SLN positivity (43%), and a poor MSS (68% at 5-years). Further, ulceration of the primary tumor and metastasis to more than one lymph node were identified as independently associated with decreased MSS among patients with microsatellites.
The incidence of microsatellitosis in our cohort was similar to that reported in other recent studies.11–13, 15, 23 The SLN positivity rate associated with microsatellitosis, however, is lower than the 71% positivity reported by Kimsey et al.15 This likely reflects differences in the underlying patient populations and their management. Our institution has consistently considered microsatellitosis as a high risk feature even among thin melanomas in selection for SLNB. Therefore, nearly every patient with clinically localized disease and microsatellitosis would be offered a SLNB. As a result, the median thickness in our study was 3.2 mm compared to 5.4 mm in the Kimsey study. Additional variation in patient selection for SLNB may also have contributed to the differences observed in SLN positivity rates. Despite these differences, the SLN positivity rate in patients with microsatellites remained high irrespective of the presence of other adverse primary tumor features. Indeed, in patients with tumors ≤1mm thick the SLN positivity rate was 29%, albeit in a very limited sample (n=7).
Loco-regional recurrence with or without distant metastasis occurred in 29% of the overall cohort accounting for 68% of first recurrences. Microsatellitosis is hypothesized to be a metastatic event early in the spectrum of in-transit and nodal metastasis.23, 24 In-transit disease developed in a total of 9 patients (9%), which is not appreciably higher than has been observed after wide-local excision in all patients with melanoma.25–27 Likewise, 4 patients (4%) recurred in the regional nodal basin that had previously been found to be negative via SLNB, comparable to the 3.4% rate observed in MSLT-1.3
Consistent with the current staging system, we found ulceration status of the primary tumor and the total number of positive lymph nodes to impact on the MSS of patients with microsatellites. In the current study, 5-year MSS in patients with lesional ulceration versus none were 83% and 43% respectively; similarly, 5-year MSS was significantly poorer in patients with >1 nodal metastasis (38%) versus those with one positive node (63%) or those with none (81%). The importance of nodal involvement is consistent with other studies of patients with macroscopic satellite/in-transit disease.1, 28 Additionally, even in the presence of favorable prognostic features (e.g., thickness ≤1mm), the rate of SLN positivity associated with microsatellitosis is appreciable. Thus, SLNB appears to be an important staging and prognostic tool even in this already high risk cohort of patients. Further, it may provide clinical value in achieving regional control of disease.
Currently, the AJCC staging system accounts for the adverse prognostic value of nodal positivity or ulceration by classifying patients with microsatellites alone as stage IIIB (5-year survival of 59%), or stage IIIC (5-year survival of 40%) when either nodal metastases or ulceration are present.1 In the current study, patients with ≤1 metastatic LN and absent ulceration had a 5-year MSS of 90%, and this favorable group represented 50% of patients with microsatellitosis. Thus, although the staging system accurately reflects the negative prognostic features associated with microsatellitosis, in our cohort those patients who do not possess adverse features have a more favorable outcome than would be anticipated by their stage IIIB/C status. This heterogeneity in survival among patients with microsatellitosis mirrors that seen among patients with stage III disease. Indeed, the 90% 5-year MSS observed in our favorable cohort of patients with microsatellitosis is quite similar to the 87% MSS observed by Balch et al. in patients with a microscopically positive SLN and a non-ulcerated primary lesion ≤2mm in thickness.2
In summary, patients with microsatellitosis have a high prevalence of coincident aggressive features in their primary tumors as well as a high rate of nodal metastases. Thus, as a group, the overall prognosis is poor. Concordant with current staging, both ulceration and multiple metastatic nodes were independent adverse prognostic features in these patients. In the absence of these negative prognostic features, however, a subset of microsatellite patients demonstrate a considerably more favorable prognosis than their stage would suggest. Recognition of the favorable survival seen in this appreciable subset of patients with microsatellitosis can help guide clinicians in the counseling and follow-up for this group.
Footnotes
All authors have read and approved the manuscript.
The authors declare no funding or conflicts of interest
References
- 1.Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27(36):6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balch CM, Gershenwald JE, Soong SJ, et al. Multivariate analysis of prognostic factors among 2,313 patients with stage III melanoma: comparison of nodal micrometastases versus macrometastases. J Clin Oncol. 2010;28(14):2452–2459. doi: 10.1200/JCO.2009.27.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morton DL, Thompson JF, Cochran AJ, et al. Sentinel-node biopsy or nodal observation in melanoma. N Engl J Med. 2006;355(13):1307–1317. doi: 10.1056/NEJMoa060992. [DOI] [PubMed] [Google Scholar]
- 4.Hayes AJ, Clark MA, Harries M, Thomas JM. Management of in-transit metastases from cutaneous malignant melanoma. Br J Surg. 2004;91(6):673–682. doi: 10.1002/bjs.4610. [DOI] [PubMed] [Google Scholar]
- 5.Murali R, Moncrieff MD, Hong J, et al. The prognostic value of tumor mitotic rate and other clinicopathologic factors in patients with locoregional recurrences of melanoma. Ann Surg Oncol. 2010;17(11):2992–2999. doi: 10.1245/s10434-010-1078-0. [DOI] [PubMed] [Google Scholar]
- 6.Wong JH, Cagle LA, Kopald KH, et al. Natural history and selective management of in transit melanoma. J Surg Oncol. 1990;44(3):146–150. doi: 10.1002/jso.2930440305. [DOI] [PubMed] [Google Scholar]
- 7.Edge SBBDR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. (7th Edition) 2010;Vol. XV [Google Scholar]
- 8.Day CL, Jr, Harrist TJ, Gorstein F, et al. Malignant melanoma. Prognostic significance of "microscopic satellites" in the reticular dermis and subcutaneous fat. Ann Surg. 1981;194(1):108–112. doi: 10.1097/00000658-198107000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leon P, Daly JM, Synnestvedt M, et al. The prognostic implications of microscopic satellites in patients with clinical stage I melanoma. Arch Surg. 1991;126(12):1461–1468. doi: 10.1001/archsurg.1991.01410360031006. [DOI] [PubMed] [Google Scholar]
- 10.Nagore E, Oliver V, Botella-Estrada R, et al. Prognostic factors in localized invasive cutaneous melanoma: high value of mitotic rate, vascular invasion and microscopic satellitosis. Melanoma Res. 2005;15(3):169–177. doi: 10.1097/00008390-200506000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Rao UN, Ibrahim J, Flaherty LE, et al. Implications of microscopic satellites of the primary and extracapsular lymph node spread in patients with high-risk melanoma: pathologic corollary of Eastern Cooperative Oncology Group Trial E1690. J Clin Oncol. 2002;20(8):2053–2057. doi: 10.1200/JCO.2002.08.024. [DOI] [PubMed] [Google Scholar]
- 12.Shaikh L, Sagebiel RW, Ferreira CM, et al. The role of microsatellites as a prognostic factor in primary malignant melanoma. Arch Dermatol. 2005;141(6):739–742. doi: 10.1001/archderm.141.6.739. [DOI] [PubMed] [Google Scholar]
- 13.Azimi F, Scolyer RA, Rumcheva P, et al. Tumor-infiltrating lymphocyte grade is an independent predictor of sentinel lymph node status and survival in patients with cutaneous melanoma. J Clin Oncol. 2012;30(21):2678–2683. doi: 10.1200/JCO.2011.37.8539. [DOI] [PubMed] [Google Scholar]
- 14.Buzaid AC, Ross MI, Balch CM, et al. Critical analysis of the current American Joint Committee on Cancer staging system for cutaneous melanoma and proposal of a new staging system. J Clin Oncol. 1997;15(3):1039–1051. doi: 10.1200/JCO.1997.15.3.1039. [DOI] [PubMed] [Google Scholar]
- 15.Kimsey TF, Cohen T, Patel A, et al. Microscopic satellitosis in patients with primary cutaneous melanoma: implications for nodal basin staging. Ann Surg Oncol. 2009;16(5):1176–1183. doi: 10.1245/s10434-009-0350-7. [DOI] [PubMed] [Google Scholar]
- 16.Harrist TJ, Rigel DS, Day CL, Jr, et al. "Microscopic satellites" are more highly associated with regional lymph node metastases than is primary melanoma thickness. Cancer. 1984;53(10):2183–2187. doi: 10.1002/1097-0142(19840515)53:10<2183::aid-cncr2820531029>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 17.Gimotty PA, Guerry D, Ming ME, et al. Thin primary cutaneous malignant melanoma: a prognostic tree for 10-year metastasis is more accurate than American Joint Committee on Cancer staging. J Clin Oncol. 2004;22(18):3668–3676. doi: 10.1200/JCO.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 18.Kesmodel SB, Karakousis GC, Botbyl JD, et al. Mitotic rate as a predictor of sentinel lymph node positivity in patients with thin melanomas. Ann Surg Oncol. 2005;12(6):449–458. doi: 10.1245/ASO.2005.04.027. [DOI] [PubMed] [Google Scholar]
- 19.Gordis L. Epidemiology. Saunders: Elsevier; 2009. [Google Scholar]
- 20.Rosner B. Fundamentals of Biostatistics. Seventh Ed. Brooks/Cole Cengage Learning; 2011. [Google Scholar]
- 21.Harrell FE. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York: Springer; 2001. [Google Scholar]
- 22.Juul SFM. An Introduction to Stata for Health Researchers. 3rd Ed. StataCorp LP; 2010. pp. 179–196. [Google Scholar]
- 23.Balch CM. Microscopic satellites around a primary melanoma: another piece of the puzzle in melanoma staging. Ann Surg Oncol. 2009;16(5):1092–1094. doi: 10.1245/s10434-009-0353-4. [DOI] [PubMed] [Google Scholar]
- 24.Balch CM, Soong S, Ross MI, et al. Long-term results of a multi-institutional randomized trial comparing prognostic factors and surgical results for intermediate thickness melanomas (1.0 to 4.0 mm). Intergroup Melanoma Surgical Trial. Ann Surg Oncol. 2000;7(2):87–97. doi: 10.1007/s10434-000-0087-9. [DOI] [PubMed] [Google Scholar]
- 25.Borgstein PJ, Meijer S, van Diest PJ. Are locoregional cutaneous metastases in melanoma predictable? Ann Surg Oncol. 1999;6(3):315–321. doi: 10.1007/s10434-999-0315-x. [DOI] [PubMed] [Google Scholar]
- 26.Cascinelli N, Bufalino R, Marolda R, et al. Regional non-nodal metastases of cutaneous melanoma. Eur J Surg Oncol. 1986;12(2):175–180. [PubMed] [Google Scholar]
- 27.Pawlik TM, Ross MI, Thompson JF, et al. The risk of in-transit melanoma metastasis depends on tumor biology and not the surgical approach to regional lymph nodes. J Clin Oncol. 2005;23(21):4588–4590. doi: 10.1200/JCO.2005.12.245. [DOI] [PubMed] [Google Scholar]
- 28.Weide B, Faller C, Buttner P, et al. Prognostic factors of melanoma patients with satellite or in-transit metastasis at the time of stage III diagnosis. PLoS One. 2013;8(4):e63137. doi: 10.1371/journal.pone.0063137. [DOI] [PMC free article] [PubMed] [Google Scholar]


