Abstract
sPLA2-IIa is an enzyme at high concentration in tears that has been known as an innate barrier of the ocular surface against microbial infection. sPLA2-IIa and other enzymes in the same protein family are known to hydrolyze fatty acids resulting in the generation of free arachidonic acid (AA) and lysophospholipids, which are the precursors of pro-inflammatory lipid mediators, such as PGE2. sPLA2-IIa has been shown to be an inflammatory mediator in non-ocular inflammatory diseases such as rheumatoid arthritis (RA). It was also found to be increased in the tears of the patients with dry eye disease, chronic blepharitis and contact lens intolerance. However, the role of sPLA2-IIa in chronic ocular surface inflammation has yet to be determined.
In the current study, we examined the potential role of sPLA2-IIa in inflammation of ocular surface diseases. Our results show that the activity of sPLA2-IIa was significantly increased in tears from dry eye disease patients compared with that from normal subjects. Also, sPLA2-IIa stimulated the production of PGE2 in ocular surface epithelial cell cultures. The stimulating effect was markedly enhanced when the cells or tissues were pre-compromised with TNF-α, IL-1β or desiccation. Furthermore, sPLA2-IIa stimulated inflammatory cytokine production in the ocular surface epithelial cell cultures in vitro. To our knowledge, this is the first report regarding the role of sPLA2-IIa as an inflammatory mediator in ocular surface inflammation. These findings indicate that sPLA2-IIa may play an important role in chronic ocular surface inflammation, especially when the ocular surface is compromised.
Keywords: sPLA2-IIa, PGE2, cytokines, dry eye disease, compromised ocular surface
1. Introduction
The secretory form of phosphalipase A2 group 2a (sPLA2-IIa) belongs to a family of enzymes that generates precursors of pro-inflammatory lipid mediators such as free arachidonic acid (AA) and lysophospholipids (Dennis et al., 1994; Fijneman and Cormier, 2008; Mayer et al., 1993). Human sPLA2-IIa, a gene product of PLA2G2A at chromosomal 1p35-1p36 region (Praml et al., 1995), has been reported to be in high concentration in tears (Nevalainen et al., 1994; Qu et al., 1998; Saari et al., 2001). The enzyme appears to be secreted by both the lacrimal glands and the goblet cells of conjunctival epithelia (Nevalainen, et al., 1994; Aho, et al., 1996; Turner et al., 2007). In normal subjects, the concentration of sPLA2-IIa in tears is 54.5+/-33.9 μg/ml, one of the highest levels of sPLA2-IIa reported in any normal human secretions (Saari et al., 2001). Since its bactericidal role has been well appreciated through direct catalytic activity of the enzyme on the gram-positive bacterial wall, the presence of sPLA2-IIa has been considered as an innate immune barrier of the ocular surface against microbial infection (Buckland et al., 2000; Girgis et al., 2003; Nevalainen et al., 2008; Qu et al., 1998). Accumulated evidence indicates that the non-catalytic activity of sPLA2-IIa also plays an important role in modulating the pathogenesis of many inflammatory-related diseases and cancers (Adibhatla et al., 2007; 2008; Fijneman and Cormier, 2008; Lambeau and Gelb, 2008; Menschikowski, et al 2006; 2008). The internalized signaling of sPLA2-IIa is proposed to be mediated through the interactions between sPLA2-IIa and its membrane-bound specific receptors, such as the M-type receptor in rodents and αVβ3/α4β1 integrins in humans (Lambeau, et al., 1999; Saegusa, et al., 2008). Furthermore, the physiological role in clearance of anionic pathological cell debris from the inflammatory response has recently been established, which is independent of the catalytic activity of sPLA2-IIa (Birts, et al., 2008).
sPLA2-IIa has been shown to play an important role in many non-ocular inflammatory diseases. Increased levels of sPLA2-IIa in the inflamed tissue and/or serum have been detected in rheumatoid arthritis (RA) (Bidgood et al., 2000; Cirino et al., 1994; Hara et al., 1989; Stefanski et al., 1986), inflammatory bowel diseases (IBD) (Lilja et al., 1995; Minami et al., 1994), acute chest syndrome (Styles et al., 1996), asthma (Bowton et al., 1997) and septic shock (Cai et al., 1999). Among these inflammatory diseases with high levels of sPLA2-IIa, RA has been intensively studied. The synovial fluids from arthritic joints of patients with RA contain high catalytic activity of sPLA2-IIa (Pruzanski, et al., 1994). Injection of human recombinant sPLA2-IIa into the joints of rabbits elicits a dramatic inflammatory and arthritogenic response, suggesting that sPLA2-IIa in synovial fluid plays an exacerbating role in chronic inflammatory conditions such as RA (Bomalaski et al., 1991). Correlations have been found in patients with RA between the serum sPLA2-IIa concentration and clinical markers of diseases such as swollen joints, elevated platelet count, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) and depressed hemoglobin concentration (Lin et al., 1996). Given the pivotal role of sPLA2-IIa in inflammatory diseases, inhibition of sPLA2-IIa may have therapeutic effect by blocking the formation of a wide variety of secondary inflammatory mediators. To support this notion, clinical trial studies have indeed shown that multiple markers of RA have been significantly improved at the earlier stage of the treatment with sPLA2-IIa inhibitors (Bradley et al., 2005; Reid, 2005).
However, to our knowledge no mechanistic study has been carried out to investigate the inflammatory mediator role of sPLA2-IIa on the ocular surface, despite the concentration or activity of sPLA2-IIa in tears being reported significantly changed in patients with external inflammatory diseases, such as dry eye disease (Aho et al., 2002), post PRK laser surgery (Aho et al., 2003), chronic blepharitis (Song et al., 1999), ocular rosacea (Kari et al., 2005) and atopic blepharoconjunctivitis (Peuravuori et al., 2004). These clinical documentations imply that there may be an association of tear sPLA2-IIa with inflammatory diseases of the ocular surface. Yet, little research has been conducted to examine it. In this study, we examined the potential role of sPLA2-IIa in the inflammation of ocular surface disease. Our results show that sPLA2-IIa activity was significantly higher in tears from dry eye disease patients than that from normal subjects. sPLA2-IIa stimulated a mild production of PGE2 and inflammatory cytokines/chemokines in normal ocular surface epithelial corneal and conjunctival cell cultures; whereas the stimulating effect was significantly amplified when the cells or tissues were pre-compromised with TNF-α, IL-1β, or desiccation. These findings indicate that sPLA2-IIa may be an important amplifier of chronic ocular surface inflammation, especially when the ocular surface is compromised.
2. Material & methods
2.1. Patient information
Ten patients with dry eye disease (nine women and one man), ages from 27 to 65 years, were evaluated. IRB approved informed consent was obtained from all participants after explanation of the study. All patients had typical signs and symptoms of dry eye disease and the diagnosis of dry eye disease was confirmed with decreased tear production by Schirmers test, positive ocular surface vital dye staining and shortened tear break-up time (TBUT). Ten age-matched normal subjects were recruited as controls. None of the control subjects wore contact lenses and all of them revealed normal findings in a routine eye examination. An additional 5 healthy volunteers were recruited for methodology development and pilot experiments.
2.2. Tear collection
A non-stimulated tear sample was collected using disposable 5 μl micro capillaries (Microcaps 5 μl, Drummond Scientific, Broomall, PA, USA). Tears from both eyes were collected. In the pilot experiment, approximately 20 μl of tears were collected from each of the five healthy volunteers and were pooled for assays. In the experiment comparing dry eye patients with normal subjects, 5 μl of tears were obtained from each patient, pooled from both eyes when necessary. The samples were gathered from the marginal tear strip of the lower lid near the lateral canthus, with care being taken not to irritate the conjunctiva, cornea, or lid margin. Tears were immediately transferred into Eppendorf tubes, placed on dry ice and kept at −70°C until analyzed.
2.3. Measurement of sPLA2 activity in tears
The sPLA2-IIa activity in tear samples were measured by an EIA kit from Cayman Chemical Co. (Ann Arbor, MI) using the 1, 2-dithio analog of diheptenoyl phosphatidylcholine (DTNB) as a substrate according to the manufacture's instructions (Reynolds et al., 1992). In brief, 10 μl DTNB, 5 μl assay buffer and 10 μl sample were added in each well of a 96 well plate. Standard bee venom sPLA2 was used as positive control and the assay buffer alone was used as negative control. All samples and controls were tested in triplicate. The reaction was initiated by adding 200 μl substrate to all the wells and the result was measured immediately using a microplate reader (Molecular Devices, Sunnyvale, CA) at 414 nm absorbance at various time points. sPLA2-IIa activity was calculated according to manufacturer's instructions.
2.4. Animals and Reagents
Wild type BALB/C mice at 8-12 weeks of age were purchased from The Jackson Laboratory (Bar Harbor, ME) and housed in a specific pathogen-free condition until use. The use of the animals conformed to the ARCO Statement of the Use of Animals in Ophthalmic and Vision Research. Recombinant human sPLA2-IIa was purchased from BioVendor (Candler, NC). sPLA2 from bee venom was purchased from Sigma (St. Louis, MO). Human TNF-α was purchased from PeproTech (Rocky Hill, NJ). The TNF-α or sPLA2-IIa stock solutions were made according to the manufacturer's recommendation. Sterile phosphate-buffered saline containing 0.1% bovine serum albumin was used to prepare stock solutions of 50 ng/μl TNF-α and 50 μg/μl sPLA2-IIa.
2.5. Cell culture
Human conjunctival cell line, Wong-Kilbourne derivative of Chang's conjunctiva, clone 1-5c-4, American Type Culture Collection (ATCC, Manassas, VA) certified number CCL-20.2, was cultured under standard conditions (moist atmosphere of 5% CO2 at 37°C) in 75cm2 bottles in Dulbecco's modified Eagle Medium (DMEM) with L-glutamine substitute (GlutaMAX-I; Invitrogen) containing 10% fetal calf serum (FCS), 4.5 g/L glucose, 1% kanamycin, and 50 μg/ml amoxicillin. Although this cell line was reported by Lavappa in 1978 to have contaiminated with HeLa cell markers, it's still been widely used for in vitro studies on the ocular surface. Recently (Brasnu, et al., 2008), a comparative in vitro study on the cytotoxic effects of benzalkonium chloride confirmed the equal supports by both the Chang's cell line and the IOBA-NHC, another widely used human conjunctival epithelium cell line (Diebold, et al., 2003). The SV40 immortalized human corneal epithelial cell line, SV40-HCECs (Araki-Sasaki K, 1995), kindly provided by Dr. Mario Wolosin, was maintained in DMEM/HamF12 (1:1) supplemented with 5% fetal bovine serum (HyClone, Logan, UT), 5 μg/ml insulin, 0.1 μg/ml cholera toxin (Sigma-Aldrich, St. Louis, MO), 10 ng/ml recombinant human epidermal growth factor (hEGF; BD Biosciences, San Jose, CA) and 0.5% dimethyl sulfoxide (DMSO) in 95% air and 5% CO2 at 37°C. At the beginning of the experiment, cells were seeded in 24 or 96 well plates and the treatments were given when cells reached 80% confluency. At the end of the experiment, culture media were harvested for the PGE2 assay and cells were procured for RNA isolation.
2.6. Measurement of PGE2
PGE2 was measured with a monoclonal enzyme immunoassay (EIA) kit (Cayman Chemical Co, Ann Arbor, MI). Each EIA kit contains PGE2 standard, PGE2 monoclonal antibody, AChE tracer, Ellman's reagent and a mouse anti-rabbit IgG coated 96-well strip plate. The PGE2 standards were serially diluted to ranges of 7.8–1000 pg/ml and the assay procedures were followed by the manufacturer's instructions. Culture medium from each sample was removed and centrifuged for 7 min at 3,000 rpm to pellet any dislodged cells, and then diluted from 1:50 to 1:500 (determined with a pilot experiment) with the EIA buffer provided by the kit. An aliquot (50 μl) from each sample was placed in a 96-well plate in triplicates and 200 μl Ellaman's reagent was added to each well for 90 minutes and the absorbance at 414 nm was recorded with a microplate reader (Molecular Devices, Sunnyvale, CA).
2.7. RNA isolation from tissue culture samples
Total RNA was extracted from conjunctival cells with RNeasy from Qiagen (Valencia, CA) as described by the manufacturer. The integrity of total RNA was determined by formaldehyde denaturing RNA gel electrophoresis before proceeding to microarray and real-time PCR analysis. RNA samples were treated with deoxyribonuclease I at 25°C for 10 minutes, and then reverse transcribed at 42°C for 1 hour using random primer and the SuperScript II RT kit (Gibco).
2.8. Microarrays and data analysis
Relative mRNA expression of inflammatory cytokines and chemokines was analyzed with a pathway-specific cRNA microarray (SuperArray Bioscience Corp, Frederick, MD). In brief, cRNA was transcribed, amplified and labeled with Biotin-16-UTP using the TrueLabeling-AMP 2.0 kit (SuperArray). The Biotin-labeled cRNA samples were then hybridized overnight to the human inflammatory cytokine GEArray membranes. After incubation with streptavidin-AP conjugate, the array image was developed with CDP-Star chemiluminescent substrate and recorded with X-ray film. Web-based GEASuite analyzing software was used to extract GEArray probe signals from the image and analyze gene expression profiles. Background-corrected signals were normalized to the average intensity of signals from the controlled genes by using the analyzing software.
2.9. Quantitative real-time RT-PCR
cDNA equivalent to 50 ng of total RNA was boiled for 3 minutes and quenched on ice before amplification. PCR was performed in glass capillaries on a LightCycler (Roche Diagnostics, Mannheim, Germany) with the QuantiTect SYBR Green PCR Kit (Qiagen, Valencia, CA) and including a negative control without template and a calibrator. The PCR reactions were cycled 45 times after initial denaturation (95°C, 15 min) and then denatured at 94°C for 15s, annealed at 56°C for 20s, and extended at 72°C for 15s with temperature transition rates of 20°C/s. After PCR amplification, melting temperature curve analysis was performed. The PCR products were cooled to 65°C and then slowly heated to 95 °C at a rate of 0.2°C/s. Fluorescence signals were measured once in each cycle at melting temperature -3°C to eliminate fluorescence from SYBR Green I binding to primer-dimers, which was several degrees (>5°C) less than the melting temperatures of the specific primer pairs. The primers were designed to be intron-spanning if possible, and the product sizes are: 190 bp, 130 bp and 278 bp for β-actin, CCL25 and CCL5, respectively.
Primers for β-actin (official gene symbol: ATCB, GenBank accession number is NM_001101):
Sense 5′-GGTGGGCATGGGTCAGAAGGATT-3′
antisense 5′- CCTCGGTCAGCAGCAC -3′
Primers for CCL25 (official gene symbol: CCL25, GenBank accession number is NM_005624):
Sense 5′-CCATCAGCAGCAGTAAGAGG-3′
Antisense 5′-CTGTAGGGCGACGGTTTTAT-3′
Primers for CCL5 (officail gene symbol: CCL5, GenBank accession number is NM_002985):
sense 5′-CCATGAAGGTCTCCGCGGCAG-3′
antisense 5′-CCTAGCTCATCTCCAAAGAG-3′
2.10. Reproducibility and statistical analysis
Experiments were repeated at least twice, usually more than three times. Results were highly reproducible. Figures show pooled data from repeated experiments or representative experiments as indicated. Student's t test was used to compare the means of two treatments performed under the same experimental condition. Two mean values were considered significantly different when P < 0.05.
3. Results
3.1. The activity of sPLA2 from dry eye patients' tears was significantly increased
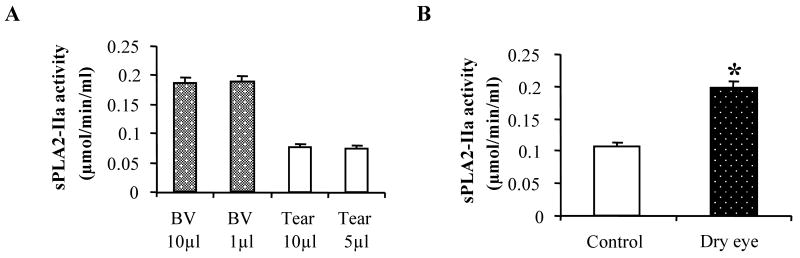
Since tear production is greatly reduced in dry eye patients and the commercial sPLA2 activity kit is designed for using 10 μl of tears as a sample, we first carried out studies to establish whether the sPLA2-IIa activity measured using a smaller volume of tears is as accurate as using a 10 μl tear sample. In these studies, we collected and pooled fresh tears from 5 healthy volunteers and used 5 or 10 μl of tears per sample for sPLA2-IIa activity measurement. 1 or 10 μl of bee venom sPLA2 standards were used as control. At least 3 measurements for each volume were conducted. As shown in Figure 1 A, sPLA2-IIa activities for bee venom were 0.186 ± 0.041 μmol/min/ml using 10 μl of venom and 0.189 ± 0.051 μmol/min/ml using 1 μl of venom. Tear sPLA2-IIa activities were 0.078 ± 0.048 μmol/min/ml using 10 μl of tears and 0.075 ± 0.047 μmol/min/ml using 5 μl of tears. There was no significant difference in sPLA2-IIa activity when measured with different sample volumes in this assay. The values of sPLA2-IIa activity were also within one standard deviation of the mean value in another study using 10 tear samples from normal subjects, which suggests that even with a smaller volume of human tears, this method is sensitive enough to measure the sPLA2-IIa activity.
Figure 1. The sPLA2-IIa activity in tear samples.
A: sPLA2-IIa activity in a small volume of human tears. The sPLA2-IIa activities in 5 or 10 μl human tears from healthy subjects were measured using the EIA assay (open bars). The sPLA2-IIa activities in 1 and 10 μl bee venom were also measured as controls (closed bars). The results are presented as mean values ± SD from two independent experiments of triplicate measurements.
B: sPLA2-IIa activity in the tears from dry eye patients and normal subjects. sPLA2-IIa activity in 5 μl tear samples from 10 dry eye patients and 10 age-matched healthy subjects were measured using the EIA assay. The sPLA2-IIa activity in dry eye patients (closed bar) is significantly higher than that in normal subjects (open bar). *P < 0.05 significantly different from the control.
We further compared the sPLA2-IIa activity in 10 dry eye patients and 10 age-matched healthy subjects. Tear samples (5 μl from each patient) were collected and assayed as described above. As shown in Figure 1B, tear sPLA2-IIa activities from dry eye patients (0.198 ± 0.053 μmol/min/ml) were significantly higher compared to those from normal subjects (0.107 ± 0.046 μmol/min/ml), p= 0.021. This result is consistent with the previous report of sPLA2 activity in the tears from dry eye patients (Aho et al., 2002).
3.2. sPLA2-IIa stimulates PGE2 production in conjunctival epithelial cells
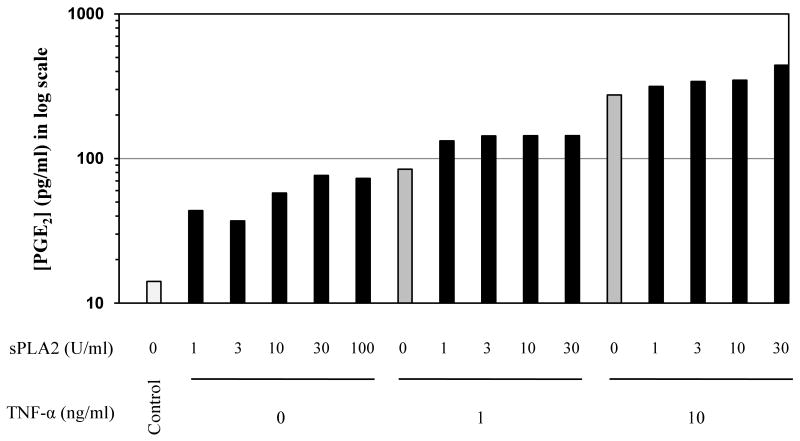
Prostaglandin E2 (PGE2) is a known inflammatory mediator that contributes to pain and vessel dilation during inflammation. Half of the patients with chronic conjunctival complaints exhibit high levels of PGE2 (Gluud et al., 1985). sPLA2-IIa has been shown to amplify TNF-α-induced PGE2 production by cultured rheumatoid synovial fibroblasts (Pruzanski, et al., 1992; Bidgood, et al., 2000). To determine whether sPLA2-IIa and TNF-α have effects on PGE2 production in conjunctival cells, we treated the cells with increasing amounts of sPLA2-IIa in the presence or absence of 10 ng/ml TNF-α. As shown in Figure 2, either sPLA2-IIa or TNF-α alone stimulated a mild to moderate level of PGE2 production in conjunctival cells. However, when cells were exposed to both sPLA2-IIa and TNF-α, greater PGE2 production was observed in a sPLA2-IIa dose-dependent manner. In unstimulated conjunctival cells, PGE2 levels ranged from 80 to 341 pg/ml. Different amounts of sPLA2-IIa alone only stimulated 0 to 4 fold increases in PGE2 production. TNF-α alone stimulated about an 18 fold increase in PGE2 production. However, the combination of sPLA2-IIa and TNF-α resulted in a strong synergistic response that led to a 23-30 fold increase in PGE2 production (Figure 2).
Figure 2. The effect of sPLA2-IIa and TNF-α on PGE2 production by conjunctival cells.
Conjunctival cells were treated with increased concentrations of sPLA2-IIa both in the presence or absence of TNF-α (0, 1 or 10 ng/ml). The PGE2 production was measured with a monoclonal enzyme immunoassay (EIA) kit. The PGE2 concentration was presented as logarithm scale to cover the broad range between control and treatment groups. Data are the average of two independent experiments with triplicate measurements.
These results suggest that, although sPLA2-IIa alone does not cause significant production of pro-inflammatory mediators in normal conjunctival epithelial cells, it causes a marked increase in pro-inflammatory mediator production when the cells are compromised by exposure to TNF-α.
3.3. sPLA2-IIa stimulates PGE2 production in mouse conjunctival organ culture
To further investigate the effect of sPLA2-IIa on PGE2 production, we conducted studies using a more physiological model, mouse conjunctival organ culture (Figure 3A). Previous work has demonstrated that the fresh isolated conjunctival tissue gradually releases the pre-made sPLA2-IIa into the culture medium and reaches an equilibration level in 30 to 40 minutes (Wolosin JM, data not shown). In this study, we substituted bee venom sPLA2 for recombinant human sPLA2-IIa for the cost considerations. Our preliminary assays indicate that ten units per ml of bee venom sPLA2 were equivalent to 10 to 20 μg/ml recombinant human sPLA2-IIa in terms of stimulation of PGE2 production in conjunctival cells (data not shown). As shown in Figure 3A, the fresh isolated conjunctival tissues were cultured in SHEM medium for one hour equilibration, changed to new medium with the indicated amounts of sPLA2-IIa or TNF-α alone, or sPLA2-IIa plus TNF-α. The tissues were further incubated for an additional 4 hours at 37°C and the media were collected and used for the PGE2 measurement.
Figure 3. PGE2 production in mouse conjunctival organ culture.
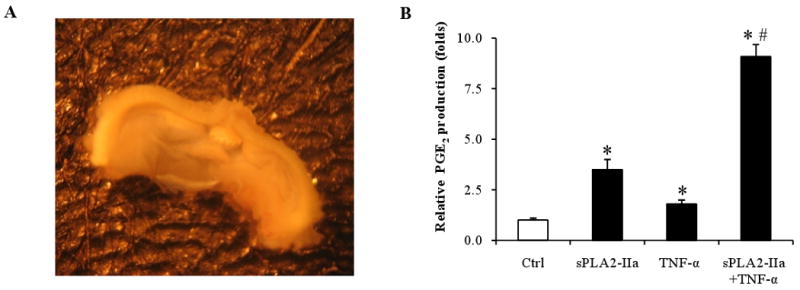
A: Conjunctival tissue culture, which shows a piece of isolated mouse conjunctiva in SHEM culture medium.
B: sPLA2-IIa stimulates PGE2 production in mouse conjunctiva. The isolated conjunctival tissue was cultured in SHEM medium for one hour for equilibration and then 10 μg/ml sPLA2-IIa or 10 ng/ml TNF-α, or 10 μg/ml sPLA2-IIa plus 10 ng/ml TNF-α were added to the culture medium. After a further incubation (4 hours, 37°C), PGE2 production was measured using a PGE2 assay kit. The results are presented as mean values ± SD of triplicate measurements. *P < 0.05 significantly different from the control. # P < 0.05 significantly different from TNF-α alone.
As shown in Figure 3B, sPLA2-IIa alone stimulated PGE2 production 2-3 fold in the mouse conjunctival organ cultures, TNF-α alone also stimulated PGE2 production to a similar level as that of sPLA2-IIa; however, when both sPLA2-IIa and TNF-α were applied, the PGE2 production was dramatically stimulated up to 9 fold. These results from mouse conjunctival organ cultures further demonstrated that sPLA2-IIa caused a marked synergistic increase in PGE2 production only when the conjunctival epithelial organs were compromised by exposure to TNF-α.
3.4. sPLA2-IIa stimulates PGE2 production in compromised SV40-HCECs corneal epithelial cells
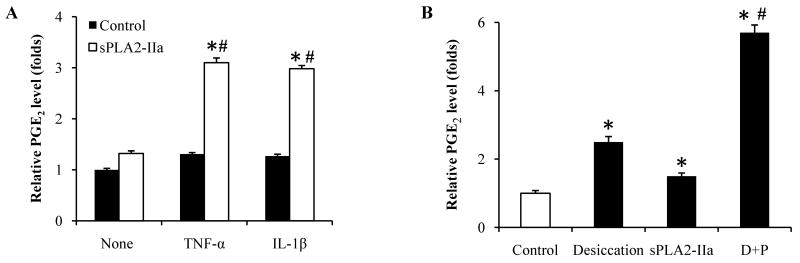
After investigating the effects of sPLA2-IIa on PGE2 production in TNF-α treated conjunctival cell and tissue cultures, we further examined the effects of sPLA2-IIa on PGE2 production in human corneal epithelial cells. In this study, SV40-HCECs cells (80% confluency) were first incubated with one of the pro-inflammatory cytokines (10 ng/ml IL-1β or 10 ng/ml TNF-α) for 20 hours, then changed to medium with or without 20 μg/ml sPLA2-IIa (equivalent to a lower range of sPLA2-IIa concentration in tears). After 24 hours of incubation, the medium was collected and PGE2 production was measured with the EIA assay. As shown in Figure 4A, cytokine pre-treatment alone or sPLA2-IIa alone had little effect on PGE2 production in SV40-HCECs cells; however, when the cells were pre-treated with cytokines and then exposed to sPLA2-IIa, the PGE2 production was significantly stimulated. We also examined an additional compromising inducer, desiccation, which approximates human dry eye disease using a modified method by Matsuo (2001). In brief, after 80-95% confluency was reached, the culture medium was completely aspirated and the cell cultures were left to dry for 60 minutes under the culture hood at room temperature and normal humidity. Fresh medium was added with or without the addition of 10 μl/ml sPLA2-IIa and incubated for 24 hours. Our results showed that desiccation alone caused moderately increased PGE2 production. However when sPLA2-IIa was added to the desiccation-compromised cells, PGE2 production was markedly elevated (Figure 4B).
Figure 4. sPLA2-IIa stimulates PGE2 production in compromised corneal epithelial cells.
A: SV40-HCECs cells were pre-incubated without or with either 10 ng/ml TNF-α or IL-1β alone for 20 hours (controls, black bars) and then treated with 10 μg/ml sPLA2-IIa for an additional 24 hours (open bars). PGE2 production was measured before and after sPLA2-IIa treatment. The results are presented as mean values ± SD from two independent experiments with triplicate measurements. *P < 0.05 significantly different from the control. # P < 0.05 significantly different from TNF-α or IL-1β alone.
B: SV40-HCECs cells without any treatment (first open bar), or exposed to desiccating for one hour (second black bar), were treated with 10 μg/ml sPLA2-IIa for 24 hours respectively (third and forth black bars). PGE2 production was measured from the four groups and the results presented as mean values ± SD of two independent experiments with triplicate measurements. *P < 0.05 significantly different from the control. # P < 0.05 significantly different from desiccating alone.
These data suggest that sPLA-IIa can significantly stimulate the PGE2 production in compromised corneal cells compared to normal corneal cells, and sPLA2-IIa may be a potential mediator by which desiccation contributes to chronic ocular surface inflammatory diseases, for example, DED.
3.5. sPLA2-IIa stimulates inflammatory cytokine production in conjunctival cells
Since studies have shown that sPLA2-IIa not only produces lipid mediators, but also stimulates production of inflammatory cytokines or chemokines through its non-hydrolytic activity (Touqui, et al., 2001), we further explored the effect of sPLA2-IIa on the mRNA expression of inflammatory cytokines and chemokines in conjunctival cells. In this study, human conjunctival epithelial cells were seeded in a 24-well plate and treated with: placebo, sPLA2-IIa alone, TNF-α alone or sPLA2-IIa plus TNF-α. After 24 hours, the cells were harvested and the RNA was isolated. The levels of inflammatory cytokine/chemokine production were detected by pathway-specific microarray (SuperArray) technology. Figure 5A shows the hybridization images on X-ray films for scanning. Figure 5 B shows the scanning results from Figure 5A after normalization with the internal controls. As shown in Figure 5B, sPLA2-IIa alone moderately stimulated the production of inflammatory cytokines and chemokines (CCL5, CCL25, IL-1β and IL-6), similar results were drawn from the low concentration of TNF-α treatment as well. However, combination of sPLA2-IIa and TNF-α resulted in a marked synergistic effect on the production of CCL5 and CCL25. Since SuperArray is a semi-quantitative assay, a quantitative real time PCR on CCL5 and CCL25 was further performed. As shown in Figure 5C, the expression of CCL5 and CCL25 mRNA were significantly increased upon the addition of sPLA2-IIa and TNF-α together. These results were consistent with our microarray results and further confirmed that sPLA2-IIa is an inflammatory mediator that can induce the inflammatory cytokine/chemokine production.
Figure 5. sPLA2-IIa stimulates inflammatory cytokine production in conjunctival cells.
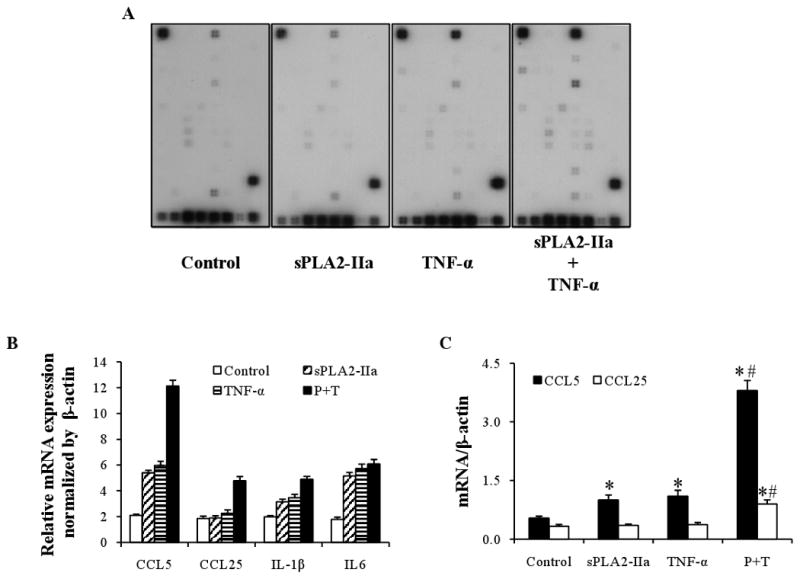
A: Array images. Inflammatory cytokine/chemokine production by human conjunctival epithelial cells was detected by a Pathway-Specific Microarray (SuperArray) assay. The cultures were treated 24 hours with placbo alone as control, sPLA2-IIa (10 μg/ml) or TNF-α (10 ng/ml) alone, or together. After treatments finished, total RNA was isolated and the cRNA was transcriptional labeled with Biotin-16-UTP, hybridized to Oligo GEArray membranes and then conjugated to AP-streptavidin. The signals were developed by addition of a CDP-Star substrate, and image results were detected by scanning.
B: Relative gene expression level of selected genes. Human conjunctival epithelial cells were treated with placebo, sPLA2-IIa alone, TNF-α alone or sPLA2-IIa plus TNF-α for 24 hours. The amount of inflammatory cytokine/chemokine production was then detected. Gene expression profiles were obtained through the analysis using GEAsuite software and the results are presented as mean values of the relative gene expression level.
C: Quantitative real time PCR on CCL5 and CCL25. Human conjunctival epithelial cells were treated with placebo, sPLA2-IIa alone, TNF-α alone or sPLA2-IIa plus TNF-α for 24 hours. cDNA corresponding to 50ng of total RNA was extracted and used for PCR on CCL5 and CCL25. The results are presented as mean values ± SD of two independent experiments with triplicate measurements. *P < 0.05 significantly different from the control. # P < 0.05 significantly different from TNF-α or IL-1β alone.
4. Discussion
In the current study, we specifically examined the role of sPLA2-IIa in ocular surface inflammation associated with dry eye disease. Our results from tear samples show that the sPLA2-IIa activity in the tears from dry eye patients is significantly higher compared to that from healthy subjects, in agreement with previous studies (Aho et al., 2002; Aho et al., 2003; Glasson et al., 2002; Song et al., 1999). Using in vitro tissue culture models, we also showed that sPLA2-IIa moderately stimulated PGE2 production in ocular surface epithelial cells, and the stimulation was significantly elevated when the cells were compromised. PGE2 has been well recognized as a pro-inflammatory mediator induced by various pro-inflammatory signals, and exerts its biological activities through interactions with membrane receptors such as EP1, EP2, EP3, and EP4 to modulate cytosolic concentrations of secondary signaling molecules, such as IP3, cAMP, and Ca++ (Coleman et al., 1994; Khan, et al 2008). A direct correlation has been characterized between inflammatory responses and PGE2 levels in the inflamed tissues (Pruzanski, et al., 1994). Cyclooxygenase 2 (COX2) is another key enzyme in converting arachidonic acid to PGE2 and other eicosanoids. COX-2 inhibitors can reduce PGE2 production and effectively reduce inflammatory disease progression (Osiri et al., 1999; Khan, et al 2008). Therefore, our results showing that sPLA2-IIa significantly stimulates PGE2 production in compromised ocular surface epithelial cell cultures strongly imply that sPLA2-IIa plays an important role in the inflammation of ocular surface disease. These results also imply that sPLA2-IIa causes pathogenic inflammation only when the eye surface is compromised by harmful agents such as UV exposure, contact lenses, some eye medications, etc. Accordingly, we propose that sPLA2-IIa has two major roles in the ocular surface: in the normal ocular surface, it serves as an innate barrier to protect the ocular surface from bacterial infections; when ocular surface cells are compromised, it amplifies the inflammatory process. Although the pathogenesis for dry eye disease is not well understood, abundant evidence from animal studies and clinical evaluations indicate that inflammation is an integral part of moderate to severe dry eye disease. Pro-inflammatory cytokines, chemokines and inflammatory T cells have all been shown to increase in the conjunctival and lacrimal tissue in both non-Sjögren's and Sjögren's dry eye patients as well as in animal models (Song et al., 2003; Pflugfelder et al.,1990; Solomon et al., 2001; Luo et al., 2004). Anti-inflammatory agents like corticosteroids have been shown to effectively alleviate symptoms and signs both experimentally and clinically (Marsh et al., 1999). Our results show an explicit role of sPLA2-IIa in the compromised ocular surface inflammation and open up a new opportunity for the treatment of ocular surface inflammation and the associated ocular tissue damage.
We also investigated the expression of inflammatory cytokines mediated by sPLA2-IIa by using a microarray assay. Our results showed that sPLA2-IIa only moderately stimulates inflammatory cytokine production in normal ocular surface epithelial cells. However when the cells are compromised, sPLA2-IIa can markedly stimulate the cytokine production. This further supports the notion that sPLA2-IIa may amplify ocular surface inflammation only when the surface is compromised. To our knowledge, this is the first study that investigates the inflammatory role of sPLA2-IIa-mediated cytokine expression in ocular surface disease. The mechanism by which sPLA2-IIa participates in inflammatory reactions has been suggested through its catalytic production of PGEs, and a catalytic-independent effect on inflammatory cytokine/chemokine production, as well as the interactions with cPLA2 (Murakami, et al., 1997). Studies showed that sPLA-IIa increases the affinity of apoptotic T cells binding to heparin sulfate proteoglycans (HSPG) (Boilard et al., 2003a), and the central core (rod domain) of vimentin (Boilard et al., 2003b). In multiple non-ocular cell models, sPLA2-IIa has been shown to cause IL-1β, TNF-α pre-activation as well as increased PGE2 production (Bidgood et al., 2000; Pruzanski et al., 1992; Jaulmes et al., 2006). sPLA2-IIa either alone or in cooperation with TNF-α and IL-1β activate dendritic cells (DC), by up-regulating the surface markers and increasing the migratory and immunostimulatory capacity of DCs; and DCs treated with sPLA2-IIa significantly enhance the production of IFN-γ, IL-2, TNF-α and IL-5 in a mixed lymphocyte reaction (Ramoner et al., 2005). This may explain why apoptotic or cytokine pre-sensitized cells are more susceptible to sPLA2-IIa: sPLA2-IIa binding with emerged anion phospholipid side chains from inside the facial layer or externalized cell skeletal proteins, or through activation of cPLA2 during early apoptosis (Atsumi et al., 1997; Wilson et al., 2000). More recently, using human K562 erythroleukemia cells and CHO cells, it has been shown that integrin αVβ3 and α4β1 may serve as receptors for sPLA2-IIa to mediate the pro-inflammatory action of sPLA2-IIa regardless of its catalytic activity, and their interaction may serve as a novel therapeutic target (Saegusa, et al., 2008). The release of large amounts of sPLA2-IIa is a major character of inflammation during atherosclerosis. The activity and transcriptional expression of sPLA2-IIa on rat vascular smooth muscle cells (VSMCs) under inflammatory conditions are regulated by the three peroxisome proliferator-activated receptors (PPARα, PPARβ and PPARγ) that are heterodimerized with RXRα, a retinoid X receptor α, and require either the interaction with a PPAR response element (PPRE) or the binding of BCL-6, a proto-oncogenic transcriptional repressor, to the sPLA2-IIa promoter region (Ravaux, et al., 2007). A few studies have also showed that TNF-α pre-treatment activated NF-kB, which is the gene transcription factor that stimulates sPLA2-IIa synthesis (Wu et al., 2003). Therefore, whether NF-kB is involved in the modulation of inflammatory ocular surface diseases will remain to be studied in the future. The interactions and signaling pathways involved in sPLA2-IIa regulation have been extensively reviewed (Fijneman and Cormier, 2008). Taken together, these results indicate that there exists a fine-tuned network to coordinate the expression and function of sPLA2-IIa with the production of inflammatory mediators, apoptosis and proliferation of cells. Therefore, studies on the mechanism of sPLA2-IIa's involvement in the inflammatory process on the unique structure/function of the ocular surface will provide new insights and knowledge and promising approaches for therapeutics to treat dry eye disease and other inflammatory processes.
Our study also implies that a sPLA2-IIa inhibitor may be promising for treatment of inflammatory ocular surface diseases. Theoretically, inhibition of sPLA2-IIa may block the formation of a wide variety of secondary inflammatory mediators. Clinical trial studies using sPLA2-IIa inhibitors on RA and chronic inflammatory angiogenesis have shown significant improvement in multiple markers of the diseases after the treatment only at an earlier stage (Bradley et al., 2005; Reid 2005), but the improvement quickly decayed and reversed after 4 weeks of inhibitor administrations. This may be due to an inadequate level of inhibitor in the synovial fluids and surrounding tissues, implying that the time and methods to deliver the medication are very crucial for the success of applications of sPLA2-IIa inhibitors. Given the accessibility of the opened ocular surface, these problems may be less difficult to handle for eye diseases. Therefore, we think that sPLA2-IIa antagonists may have potential as a treatment for chronic ocular surface inflammation.
In summary, the current study showed the sPLA2-IIa activity is significantly increased in the tears of dry eye patients. sPLA2-IIa moderately stimulates PGE2 production in corneal and conjunctival epithelial cells, as well as in conjunctival organ culture. This stimulatory effect is markedly increased when the epithelial cells are pre-compromised. In addition, we also showed that sPLA2-IIa stimulates inflammatory cytokine production in ocular surface epithelial cells and this stimulation is also markedly enhanced when the cells are compromised. We have shown that sPLA2-IIa plays an important role in the inflammatory process of the ocular surface. Studies to address the molecular mechanism by which sPLA2-IIa acts as a mediator of ocular surface inflammation will be explored in the future.
Acknowledgments
This work is supported by NIH fund (1R34EY017626-01A2), Research to Prevent Blindness and the Martin and Toni Sosnoff Foundation. We would like to thank Tatiana Deveney, Morgan Massingale, Maithreyi Vallabhajosyula, OD and Dr. Arthur Guffanti for their comments and proof-reading.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adibhatla RM, Dempsy R, Hatcher JF. Integration of cytokine biology and lipid metabolism in stroke. Front Biosci. 2008;13:1250–1270. doi: 10.2741/2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adibhatla RM, Hatcher JF. Secretory phospholipase A2 IIA is up-regulated by TNF-alpha and IL-1alpha/beta after transient focal cerebral ischemia in rat. Brain Res. 2007;1134:199–205. doi: 10.1016/j.brainres.2006.11.080. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Aho HJ, Saari KM, Kallajoki M, Nevalainen TJ. Synthesis of group II phospholipase A2 and lysozyme in lacrimal glands. Invest Ophthalmol Vis Sci. 1996;37:1826–1832. [PubMed] [Google Scholar]
- Aho VV, Nevalainen TJ, Paavilainen V, Saari KM. Group IIA phospholipase A2 content of tears in patients with keratoconjunctivitis sicca. Graefes Arch Clin Exp Ophthalmol. 2002;240:521–523. doi: 10.1007/s00417-002-0477-8. [DOI] [PubMed] [Google Scholar]
- Aho VV, Holopainen JM, Tervo T, Moilanen JA, Nevalainen T, Saari KM. Group IIA phospholipase A(2) content in tears of patients having photorefractive keratectomy. J Cataract Refract Surg. 2003;29:2163–7. doi: 10.1016/s0886-3350(03)00419-x. [DOI] [PubMed] [Google Scholar]
- Araki-Sasaki K, Ohashi Y, Sasabe T, Hayashi K, Watanabe H, Tano Y, Handa H. An SV40-immortalized human corneal epithelial cell line and its characterization. Invest Ophthalmol Vis Sci. 1995;36:614–21. [PubMed] [Google Scholar]
- Atsumi G, Murakami M, Tajima M, Shimbara S, Hara N, Kudo I. The perturbed membrane of cells undergoing apoptosis is susceptible to type II secretory phospholipase A2 to liberate arachidonic acid. Biochim Biophys Acta. 1997;1349:43–54. doi: 10.1016/s0005-2760(97)00082-9. [DOI] [PubMed] [Google Scholar]
- Bidgood MJ, Jamal OS, Cunningham AM, Brooks PM, Scott KF. Type IIA secretory phospholipase A2 up-regulates cyclooxygenase-2 and amplifies cytokine-mediated prostaglandin production in human rheumatoid synoviocytes. J Immunol. 2000;165:2790–2797. doi: 10.4049/jimmunol.165.5.2790. [DOI] [PubMed] [Google Scholar]
- Birts CN, Barton CH, Wilton DC. A catalytically independent physiological function for human acute phase protein group IIA phospholipase A2: cellular uptake facilitates cell debris removal. J Biol Chem. 2008;283:5034–5045. doi: 10.1074/jbc.M708844200. [DOI] [PubMed] [Google Scholar]
- Boilard E, Bourgoin SG, Bernatchez C, Poubelle PE, Surette ME. Interaction of low molecular weight group IIA phospholipase A2 with apoptotic human T cells: role of heparan sulfate proteoglycans. FASEB J. 2003a;17:1068–1080. doi: 10.1096/fj.02-0938com. [DOI] [PubMed] [Google Scholar]
- Boilard E, Bourgoin SG, Bernatchez C, Surette ME. Identification of an autoantigen on the surface of apoptotic human T cells as a new protein interacting with inflammatory group IIA phospholipase A2. Blood. 2003b;102:2901–2909. doi: 10.1182/blood-2002-12-3702. [DOI] [PubMed] [Google Scholar]
- Bomalaski JS, Lawton P, Browning JL. Human extracellular recombinant phospholipase A2 induces an inflammatory response in rabbit joints. J Immunol. 1991;146:3904–3910. [PubMed] [Google Scholar]
- Bowton DL, Seeds MC, Fasano MB, Goldsmith B, Bass DA. Phospholipase A2 and arachidonate increase in bronchoalveolar lavage fluid after inhaled antigen challenge in asthmatics. Am J Respir Crit Care Med. 1997;155:421–425. doi: 10.1164/ajrccm.155.2.9032172. [DOI] [PubMed] [Google Scholar]
- Bradley JD, Dmitrienko AA, Kivitz AJ, Gluck OS, Weaver AL, Wiesenhutter C, Myers SL, Sides GD. A randomized, double-blinded, placebo-controlled clinical trial of LY333013, a selective inhibitor of group II secretory phospholipase A2, in the treatment of rheumatoid arthritis. J Rheumatol. 2005;32:417–423. [PubMed] [Google Scholar]
- Brasnu E, Brignole-Baudouin F, Riancho L, Warnet JM, Baudouin C. Comparative study on the cytotoxic effects of benzalkonium chloride on the Wong-Kilbourne derivative of Chang conjunctival and IOBA-NHC cell lines. Mol Vis. 2008;14:394–402. [PMC free article] [PubMed] [Google Scholar]
- Buckland AG, Wilton DC. The antibacterial properties of secreted phospholipases A(2) Biochim Biophys Acta. 2000;1488:71–82. doi: 10.1016/s1388-1981(00)00111-6. [DOI] [PubMed] [Google Scholar]
- Cai TQ, Thieblemont N, Wong B, Thieringer R, Kennedy BP, Wright SD. Enhancement of leukocyte response to lipopolysaccharide by secretory group IIA phospholipase A2. J Leukoc Biol. 1999;65:750–756. doi: 10.1002/jlb.65.6.750. [DOI] [PubMed] [Google Scholar]
- Cirino G, Cicala C, Sorrentino L, Maiello FM, Browning JL. Recombinant secreted nonpancreatic phospholipase A2 induces a synovitis-like inflammation in the rat air pouch. J Rheumatol. 1994;21:824–829. [PubMed] [Google Scholar]
- Coleman RA, Smith WL, Narumiya S. International Union of Pharmacology classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacol Rev. 1994;46:205–229. [PubMed] [Google Scholar]
- Dennis EA. Diversity of group types, regulation, and function of phospholipase A2. J Biol Chem. 1994;6:13057–13060. [PubMed] [Google Scholar]
- Diebold Y, Calonge M, Enriquez de Salamanca A, Callejo S, Corrales RM, Saez V, Siemasko KF, Stern ME. Characterization of a spontaneously immortalized cell line (IOBA-NHC) from normal human conjunctiva. Invest Ophthalmol Vis Sci. 2003;44:4263–74. doi: 10.1167/iovs.03-0560. [DOI] [PubMed] [Google Scholar]
- Fijneman RJ, Cormier RT. The roles of sPLA2-IIA (Pla2g2a) in cancer of the small and large intestine. Front Biosci. 2008;13:4144–4174. doi: 10.2741/2998. [DOI] [PubMed] [Google Scholar]
- Girgis DO, Dajcs JJ, O'Callaghan RJ. Phospholipase A2 activity in normal and Staphylococcus aureus-infected rabbit eyes. Invest Ophthalmol Vis Sci. 2003;44:197–202. doi: 10.1167/iovs.02-0548. [DOI] [PubMed] [Google Scholar]
- Glasson M, Stapleton F, Willcox M. Lipid, lipase and lipocalin differences between tolerant and intolerant contact lens wearers. Curr Eye Res. 2002;25:227–35. doi: 10.1076/ceyr.25.4.227.13482. [DOI] [PubMed] [Google Scholar]
- Gluud BS, Jensen OL, Krogh E, Birgens HS. Prostaglandin E2 level in tears during postoperative inflammation of the eye. Acta Ophthalmol (Copenh) 1985;63:375–379. doi: 10.1111/j.1755-3768.1985.tb01547.x. [DOI] [PubMed] [Google Scholar]
- Hara S, Kudo I, Chang HW, Matsuta K, Miyamoto T, Inoue K. Purification and characterization of extracellular phospholipase A2 from human synovial fluid in rheumatoid arthritis. J Biochem. 1989;105:395–399. doi: 10.1093/oxfordjournals.jbchem.a122675. [DOI] [PubMed] [Google Scholar]
- Jaulmes A, Thierry S, Janvier B, Raymondjean M, Marechal V. Activation of sPLA2-IIA and PGE2 production by high mobility group protein B1 in vascular smooth muscle cells sensitized by IL-1beta. FASEB J. 2006;20:1727–1729. doi: 10.1096/fj.05-5514fje. [DOI] [PubMed] [Google Scholar]
- Kari O, Aho VV, Peltonen S, Saari JM, Kari M, Maatta M, Collan Y, Saari KM. Group IIA phospholipase A(2) concentration of tears in patients with ocular rosacea. Acta Ophthalmol Scand. 2005;83:483–486. doi: 10.1111/j.1600-0420.2005.00495.x. [DOI] [PubMed] [Google Scholar]
- Khan AH, Carson RJ, Nelson SM. Prostaglandins in labor--a translational approach. Front Biosci. 2008;13:5794–5809. doi: 10.2741/3117. [DOI] [PubMed] [Google Scholar]
- Lambeau G, Gelb MH. Biochemistry and physiology of mammalian secreted phospholipases A2. Annu Rev Biochem. 2008;77:495–520. doi: 10.1146/annurev.biochem.76.062405.154007. [DOI] [PubMed] [Google Scholar]
- Lambeau G, Lazdunski M. Receptors for a growing family of secreted phospholipases A2. Trends Pharmacol Sci. 1999;20:162–170. doi: 10.1016/s0165-6147(99)01300-0. [DOI] [PubMed] [Google Scholar]
- Lavappa KS. Survey of ATCC stocks of human cell lines for HeLa contamination. In Vitro. 1978;14:469–75. doi: 10.1007/BF02616110. [DOI] [PubMed] [Google Scholar]
- Lilja I, Smedh K, Olaison G, Sjodahl R, Tagesson C, Gustafson-Svard C. Phospholipase A2 gene expression and activity in histologically normal ileal mucosa and in Crohn's ileitis. Gut. 1995;37:380–385. doi: 10.1136/gut.37.3.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MK, Farewell V, Vadas P, Bookman AA, Keystone EC, Pruzanski W. Secretory phospholipase A2 as an index of disease activity in rheumatoid arthritis. Prospective double blind study of 212 patients. J Rheumatol. 1996;23:1162–1166. [PubMed] [Google Scholar]
- Luo L, Li DQ, Doshi A, Farley W, Corrales RM, Pflugfelder SC. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Invest Ophthalmol Vis Sci. 2004;45:4293–4301. doi: 10.1167/iovs.03-1145. [DOI] [PubMed] [Google Scholar]
- Marsh P, Pflugfelder SC. Topical nonpreserved methylprednisolone therapy for keratoconjunctivitis sicca in Sjogren syndrome. Ophthalmology. 1999;106:811–816. doi: 10.1016/S0161-6420(99)90171-9. [DOI] [PubMed] [Google Scholar]
- Matsuo T. Trehalose protects corneal epithelial cells from death by drying. Br J Ophthalmol. 2001;85:610–612. doi: 10.1136/bjo.85.5.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer RJ, Marshall LA. New insights on mammalian phospholipase A2(s); comparison of arachidonoyl-selective and -nonselective enzymes. FASEB J. 1993;7:339–48. doi: 10.1096/fasebj.7.2.8440410. [DOI] [PubMed] [Google Scholar]
- Menschikowski M, Hagelgans A, Gussakovsky E, Kostka H, Paley EL, Siegert G. Differential expression of secretory phospholipases A2 in normal and malignant prostate cell lines: regulation by cytokines, cell signaling pathways, and epigenetic mechanisms. Neoplasia. 2008;10:279–286. doi: 10.1593/neo.07965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menschikowski M, Hagelgans A, Siegert G. Secretory phospholipase A2 of group IIA: is it an offensive or a defensive player during atherosclerosis and other inflammatory diseases? Prostaglandins Other Lipid Mediat. 2006;79:1–33. doi: 10.1016/j.prostaglandins.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Minami T, Tojo H, Shinomura Y, Matsuzawa Y, Okamoto M. Increased group II phospholipase A2 in colonic mucosa of patients with Crohn's disease and ulcerative colitis. Gut. 1994;35:1593–1598. doi: 10.1136/gut.35.11.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M, Kuwata H, Amakasu Y, Shimbara S, Nakatani Y, Atsumi G, Kudo I. Prostaglandin E2 amplifies cytosolic phospholipase A2- and cyclooxygenase-2-dependent delayed prostaglandin E2 generation in mouse osteoblastic cells. Enhancement by secretory phospholipase A2. J Biol Chem. 1997;272:19891–19897. doi: 10.1074/jbc.272.32.19891. [DOI] [PubMed] [Google Scholar]
- Nevalainen TJ, Aho HJ, Peuravuori H. Secretion of group 2 phospholipase A2 by lacrimal glands. Invest Ophthalmol Vis Sci. 1994;35:417–421. [PubMed] [Google Scholar]
- Nevalainen TJ, Graham GG, Scott KF. Antibacterial actions of secreted phospholipases A2. Review. Biochim Biophys Acta. 2008;1781:1–9. doi: 10.1016/j.bbalip.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Osiri M, Moreland LW. Specific cyclooxygenase 2 inhibitors: a new choice of nonsteroidal anti-inflammatory drug therapy. Arthritis Care Res. 1999;12:351–362. doi: 10.1002/1529-0131(199910)12:5<351::aid-art7>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Peuravuori H, Kari O, Peltonen S, Aho VV, Saari JM, Collan Y, Maatta M, Saari KM. Group IIA phospholipase A2 content of tears in patients with atopic blepharoconjunctivitis. Graefes Arch Clin Exp Ophthalmol. 2004;242:986–989. doi: 10.1007/s00417-004-0941-8. [DOI] [PubMed] [Google Scholar]
- Pflugfelder SC, Tseng SC, Pepose JS, Fletcher MA, Klimas N, Feuer W. Epstein-Barr virus infection and immunologic dysfunction in patients with aqueous tear deficiency. Ophthalmology. 1990;97:313–323. doi: 10.1016/s0161-6420(90)32595-2. [DOI] [PubMed] [Google Scholar]
- Praml C, Savelyeva L, Le Paslier D, Siracusa LD, Buchberg AM, Schwab M, Amler LC. Human homologue of a candidate for the Mom1 locus, the secretory type II phospholipase A2 (PLA2S-II), maps to 1p35-36.1/D1S199. Cancer Res. 1995;55:5504–6. [PubMed] [Google Scholar]
- Pruzanski W, Albin-Cook K, Laxer RM, MacMillan J, Stefanski E, Vadas P, Silverman ED. Phospholipase A2 in juvenile rheumatoid arthritis: correlation to disease type and activity. J Rheumatol. 1994;21:1951–1954. [PubMed] [Google Scholar]
- Pruzanski W, Scott K, Smith G, Rajkovic I, Stefanski E, Vadas P. Enzymatic activity and immunoreactivity of extracellular phospholipase A2 in inflammatory synovial fluids. Inflammation. 1992;16:451–457. doi: 10.1007/BF00918971. [DOI] [PubMed] [Google Scholar]
- Qu XD, Lehrer RI. Secretory phospholipase A2 is the principal bactericide for staphylococci and other gram-positive bacteria in human tears. Infect Immun. 1998;66:2791–2797. doi: 10.1128/iai.66.6.2791-2797.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravaux L, Denoyelle C, Monne C, Limon I, Raymondjean M, El Hadri K. Inhibition of interleukin-1beta-induced group IIA secretory phospholipase A2 expression by peroxisome proliferator-activated receptors (PPARs) in rat vascular smooth muscle cells: cooperation between PPARbeta and the proto-oncogene BCL-6. Mol Cell Biol. 2007;27:8374–8387. doi: 10.1128/MCB.00623-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramoner R, Putz T, Gander H, Rahm A, Bartsch G, Schaber C, Thurnher M. Dendritic-cell activation by secretory phospholipase A2. Blood. 2005;105:3583–7. doi: 10.1182/blood-2004-08-3001. [DOI] [PubMed] [Google Scholar]
- Reid RC. Inhibitors of secretory phospholipase A2 group IIA. Curr Med Chem. 2005;12:3011–3026. doi: 10.2174/092986705774462860. [DOI] [PubMed] [Google Scholar]
- Reynolds IJ. 1,5-(Diethylamino)piperidine, a novel spermidine analogue that more specifically activates the N-methyl-D-aspartate receptor-associated polyamine site. Mol Pharmacol. 1992;41:989–92. [PubMed] [Google Scholar]
- Saari KM, Aho V, Paavilainen V, Nevalainen TJ. Group II PLA(2) content of tears in normal subjects. Invest Ophthalmol Vis Sci. 2001;42:318–320. [PubMed] [Google Scholar]
- Saegusa J, Akakura N, Wu CY, Hoogland C, Ma Z, Lam KS, Liu FT, Takada YK, Takada Y. Pro-inflammatory secretory phospholipase A2 type IIA binds to integrins alphavbeta3 and alpha4beta1 and induces proliferation of monocytic cells in an integrin-dependent manner. J Biol Chem. 2008;283:26107–26115. doi: 10.1074/jbc.M804835200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon A, Rosenblatt M, Monroy D, Ji Z, Pflugfelder SC, Tseng SC. Suppression of interleukin 1alpha and interleukin 1beta in human limbal epithelial cells cultured on the amniotic membrane stromal matrix. Br J Ophthalmol. 2001;85:444–449. doi: 10.1136/bjo.85.4.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song CH, Choi JS, Kim DK, Kim JC. Enhanced secretory group II PLA2 activity in the tears of chronic blepharitis patients. Invest Ophthalmol Vis Sci. 1999;40:2744–2748. [PubMed] [Google Scholar]
- Song XJ, Li DQ, Farley W, Luo LH, Heuckeroth RO, Milbrandt J, Pflugfelder SC. Neurturin-deficient mice develop dry eye and keratoconjunctivitis sicca. Invest Ophthalmol Vis Sci. 2003;44:4223–4229. doi: 10.1167/iovs.02-1319. [DOI] [PubMed] [Google Scholar]
- Stefanski E, Pruzanski W, Sternby B, Vadas P. Purification of a soluble phospholipase A2 from synovial fluid in rheumatoid arthritis. J Biochem. 1986;100:1297–303. doi: 10.1093/oxfordjournals.jbchem.a121836. [DOI] [PubMed] [Google Scholar]
- Styles LA, Schalkwijk CG, Aarsman AJ, Vichinsky EP, Lubin BH, Kuypers FA. Phospholipase A2 levels in acute chest syndrome of sickle cell disease. Blood. 1996;87:2573–2578. [PubMed] [Google Scholar]
- Touqui L, Alaoui-El-Azher M. Mammalian secreted phospholipases A2 and their pathophysiological significance in inflammatory diseases. Curr Mol Med. 2001;1:739–754. doi: 10.2174/1566524013363258. [DOI] [PubMed] [Google Scholar]
- Turner HC, Budak MT, Akinci MA, Wolosin JM. Comparative analysis of human conjunctival and corneal epithelial gene expression with oligonucleotide microarrays. Invest Ophthalmol Vis Sci. 2007;48:2050–2061. doi: 10.1167/iovs.06-0998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson HA, Allred DV, O'Neill K, Bell JD. Activities and interactions among phospholipases A2 during thapsigargin-induced S49 cell death. Apoptosis. 2000;5:389–396. doi: 10.1023/a:1009647912056. [DOI] [PubMed] [Google Scholar]
- Wu YZ, Medjane S, Chabot S, Kubrusly FS, Raw I, Chignard M, Touqui L. Surfactant protein-A and phosphatidylglycerol suppress type IIA phospholipase A2 synthesis via nuclear factor-kappaB. Am J Respir Crit Care Med. 2003;168:692–699. doi: 10.1164/rccm.200304-467OC. [DOI] [PubMed] [Google Scholar]