Abstract
Objective:
The anti-microbial efficacy of 2.5% sodium hypochlorite (SHC) and 0.2% chlorhexidine gluconate were compared with an experimental irrigant formulated from the Neem tree, Azadirachta indica A. Juss.
Materials and Methods:
A sample of 36 single rooted anterior teeth with periapical radiolucency and absence of response to vitality tests that required root canal treatment were selected for this study. The test irrigants and their combinations were assigned to five different groups and saline served as the control. Access cavities were prepared using an aseptic technique and samples collected for both anaerobic culture and Gram stained smears, followed by irrigation and sample collection again. The number of organisms were expressed in colony forming units/ml after 72 h of incubation; the smears were analyzed for their microbial loads and tissue clearance and assessed as per defined criteria.
Results:
Our results found the maximum reduction in microbial loads, when analyzed by culture method, with a combination of SHC and the experimental neem irrigant. Maximum tissue clearance on the Gram Stained smears was also found with the same combination.
Conclusion:
Neem irrigant has anti-microbial efficacy and can be considered for endodontic use.
Keywords: Antimicrobial activity, Azadirachta indica, chlorhexidine gluconate, sodium hypochlorite
INTRODUCTION
Root canal microflora is the foremost cause of periradicular pathosis. A complex root canal system anatomy makes elimination of micro-organisms difficult and irrigants are essential since canal instrumentation alone is unable to remove all tissue remnants and debris. The desirable properties of an irrigant thus include it's ability to flush loose debris, lubricate the dentinal walls, dissolve organic matter and possess antimicrobial properties.
Sodium hypochlorite (SHC) is the most commonly used endodontic irrigant with good antimicrobial efficacy[1] and tissue dissolution[2] but with concerns for vital peri-radicular tissues.[3] Chlorhexidine gluconate (CHX) is a broad spectrum anti-microbial bisbiguanide effective against bacteria and fungi.[4] It has lower toxicity than SHC[3] but lacks tissue dissolving property.[2]
The Neem (Azadirachta indica A. Juss) tree has been described for its value in traditional Indian medicinal texts. In recent years, the chemistry and structural diversity of over 135 compounds isolated from different parts of the neem tree has been documented.[5] The isoprenoid group of constituents of neem have anti-inflammatory,[6,7] anti-bacterial,[8,9] anti-fungal[10,11,12] and immunomodulatory properties.[13] Our in vitro research screened several neem-based products and found maximal anti-microbial properties with a leaf extract from the tree.[14] We thus aimed to evaluate the antimicrobial properties of this neem extract as an irrigant during root canal treatment and compared it with SHC and chlorhexidine irrigants. Combinations of the leaf extract with standard irrigants were also evaluated.
MATERIALS AND METHODS
An ethanolic leaf extract (ELE) of the neem tree, A. indica A. Juss, was prepared by the pharmaceutical college of our university. Fresh leaves were procured from the neem tree, washed and dried in a shaded area. The leaves were then powdered and extraction was carried out by maceration in rectified spirit.
Selection of cases
Following institutional research ethical approval and with informed patient consent, 36 single rooted anterior teeth requiring root canal treatment that did not respond to vitality tests (heat, cold and electric pulp testing) with a periapical radiolucency were selected for this study. None of the patients were on antibiotic treatment before or during sample collection.
These cases were randomly divided into six groups (allocation by lottery method) based upon the irrigant to be used, with six teeth in each group.
Group I — 2.5% SHC (prepared and obtained from the Hospital Pharmacy of the Medical College Hospital at our university)
Group II — 0.2% CHX (CMW, Bangalore Pharmaceuticals and Research Laboratory, Bangalore, India)
Group III — Use of the Neem Extract (ELE)
Group IV — Alternate use of 2.5% SHC and ELE
Group V — Alternate use of 0.2% CHX and ELE
Group VI — 0.9% saline (Parenteral Drugs India Pvt. Ltd., Mumbai, India) as control.
A volume of 3 mL of the irrigant was used in the root canal. If an irrigant combination was used, 1.5 mL of each ELE was followed by either 1.5 mL SHC or CHX.
The study was double-blinded with neither the patient nor the microbiologist being aware of the irrigant used.
Preparation of access cavity
The tooth surface was disinfected using Möller's technique.[15] After rubber dam isolation, the tooth, it's surroundings and the clamp were cleansed with 30% hydrogen peroxide for 1 min. An initial opening was made 1-2 mm into dentin with sterile tapering fissure diamond point at high speed with air-water coolant. The tooth was again swabbed with hydrogen peroxide for 10 s followed by application of 2% tincture of iodine for 1 min. The iodine was deactivated with sodium thiosulphate. After the penetration of the roof of the pulp chamber, a sterile surgical length no. 2 or no. 4 tungsten carbide round bur was used at slow speed, working the bur from the inside to expose the entire pulp chamber.
Method for collection of sample
Microbiological samples were obtained by a procedure outlined by Kuruvilla and Kamath, but with modifications.[16] Two sterile glass slides and two sterile cryo-vials (of 3 mL volume) containing Thioglycollate broth (TGB) were obtained from the Department of Microbiology (of the medical school at our university) for each case. The TGB (1.0 mL) was topped with a layer of sterile liquid paraffin (0.5 mL). The vial had a screw-on cap. A pre-operative long cone periapical radiograph was used to estimate the approximate length of the tooth. A sterile paper point (Dentsply Maillefer, CH 1338 Balluigues, Dentsply India Pvt. Ltd., India) pre-moistened with sterile distilled water (to provide a “pooling effect” of bacteria) was introduced into the root canal and the material smeared across a glass slide. Similarly, a sterile paper point and sterile barbed broach (Dentsply Maillefer, Dentsply India Pvt. Ltd, India) were inserted into the root canal to obtain the material and then placed in separate vials containing the TGB medium. This constituted the “pre-irrigant smear and culture”.
The canal was then irrigated with 3 mL of the irrigant or an irrigant combination using a sterile syringe and 27 gauge needle that did not bind to the canal walls over a 3 min period. The canal was dried with a sterile paper point and samples were taken for smear preparation and culture in a manner identical to pre-irrigant smear and culture methods. This constituted the “post-irrigant smear and culture”.
Laboratory processing for anaerobic culture
A semi-quantitative anaerobic technique was used to evaluate the microbial counts. Each sheep blood agar plate was divided into four quadrants. The samples were then streaked into these quadrants on separate plates (pre-irrigant and post-irrigant) using a calibration loop of 6 mm diameter that held 0.01 mL of TGB. The culture plates were incubated at 37°C in an anaerobic jar. After 72 h, the number of organisms were counted and expressed as the total colony forming units/mL (CFU/mL).
Criteria for data evaluation from anaerobic culture
The values obtained in this study were graded as follows:
Low count: <103 CFU/mL
Moderate count: 103-5 × 104 CFU/mL
Moderately high count: 5 × 104-105 CFU/mL
High Count: >105 CFU/mL.
Smear preparation with Gram's stain
The smears were Gram stained and observed with a light microscope under oil immersion field (OIF). From each sample, five insets with the highest colonies were seen and a mean score allotted for each sample and graded as per the following scale.
Criteria for evaluation of micro-organisms from Gram stained smears
Scanty: <5 micro-organisms/OIF +
Moderate: 6-30 micro-organisms/OIF ++
Moderately High: 31-100 micro-organisms/OIF +++
Abundant: >100 micro-organisms/OIF ++++.
It was also hypothesized that when greater amounts of organic material were present on the slide, the slide appeared darker in Gram's stain. Using this principle, tissue clearance was studied as an additional finding in this study.
Criteria for evaluation of tissue clearance from Gram stained smears
Four slides were standardized as follows:
Complete clearance
Lightly stained
Moderately stained
Darkly stained.
The “pre-irrigant” and “post-irrigant” stained smears were compared to the above standards and graded.
Statistical analysis of the data was perfomed using Statistical Package for Social Sciences (SPSS) version 12 (SPSS Inc, Chicago, USA). Evaluation of the percentage reduction in microbial counts within each group in either the anaerobic culture technique or the Gram stain method was analyzed with the Friedman test. Friedman test was also used to assess the percentage reduction in tissue load on Gram stained smears within each group. All inter group comparisons were subjected to the Mann Whitney U-test. Levels of significance were set at P < 0.05.
RESULTS
Our results indicate that the number of post-irrigant positive cultures (CFU/ml) were significantly lower than the pre-irrigant cultures in Groups 1-5 (P < 0.05). No significant reduction in colony counts was found between the pre-and post-irrigant cultures with saline. Figure 1 represents the percentage reduction in micro-organisms with the different irrigants and combinations. No significant differences were found between SHC, CHX and ELE. There were no statistically significant differences between SHC and SHC + ELE or SHC + CHX combinations. Even though CHX did not differ from the ELE + CHX combination (P = 0.473), we found it to differ significantly from a combination of ELE + SHC (P = 0.049). Similarly, ELE did not differ significantly with ELE + CHX combination (P = 0.265), but had a significant difference with the combination of ELE + SHC (P = 0.022). All irrigants and combinations differed significantly from saline.
Figure 1.
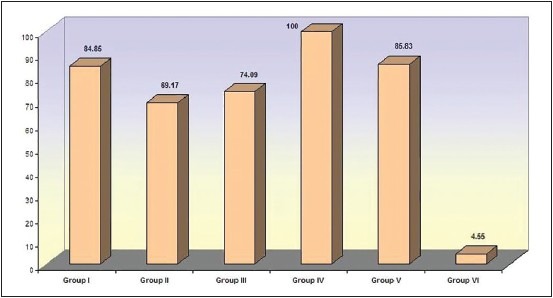
Comparison of percentage reduction of organisms in anaerobic culture (Group I - Sodium hypochlorite. Group II - Chlorhexidene gluconate. Group III - Ethanolic neem leaf extract. Group IV - Combination of sodium hypochlorite and ethanolic leaf extract. Group V - Combination of chlorhexidene and ethanolic leaf extract. Group VI - Saline)
The results from microbial counts on Gram-stained smears [Figure 2] indicate that the number of micro-organisms on the post-irrigant smears were significantly lower in all the groups. A statistically significant difference was found between saline and SHC (P = 0.009); a combination of SHC and ELE with saline (P = 0.017); and a combination of CHX and ELE with saline (P = 0.008). Comparisons between all other groups did not yield any difference.
Figure 2.
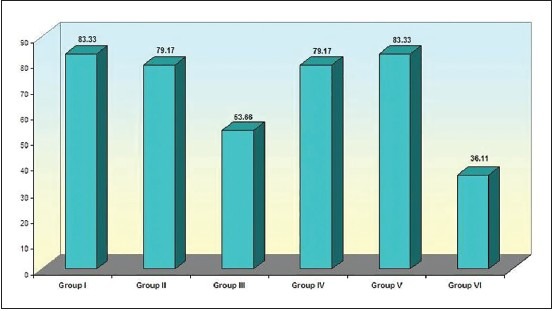
Comparison of percentage reduction of organisms in Gram-stained smears (Group I - Sodium hypochlorite. Group II - Chlorhexidene gluconate. Group III - Ethanolic Neem leaf extract. Group IV - Combination of sodium hypochlorite and ethanolic leaf extract. Group V - Combination of chlorhexidene and ethanolic leaf extract. Group VI - Saline)
The results from Gram-stained smears [Figure 3] indicate that tissue loads on the post-irrigant smears were significantly lower in all the groups. SHC + ELE differed significantly from saline (P = 0.026). Comparisons between other groups did not yield differences.
Figure 3.
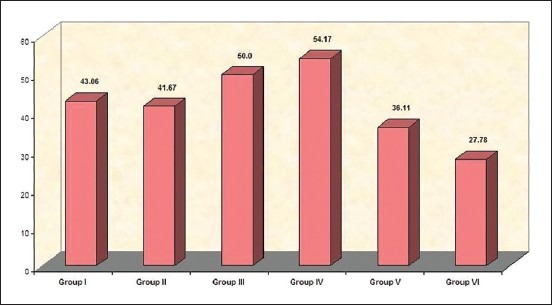
Comparison of percentage reduction of tissue load in Gram-stained smears (Group I — Sodium hypochlorite. Group II — Chlorhexidene gluconate. Group III — Ethanolic Neem leaf extract. Group IV — Combination of sodium hypochlorite and ethanolic leaf extract. Group V — Combination of chlorhexidene and ethanolic leaf extract. Group VI — Saline)
DISCUSSION
A. indica A. Juss is a useful traditional medicinal plant in India. Each part of the tree has some medicinal property. It is now considered a valuable source of unique natural products for development of medicines against various diseases.[17]
Past research has shown ELE of the neem tree to be effective against several microbes.[18] It's use within the root canal has been undocumented. The antimicrobial property of ELE against a mixed flora of anaerobic and facultative micro-organisms was tested in this study. ELE has low toxic potential and safety tolerance limits have been established at 176 mg/kg body weight.[19]3 mL of the ELE irrigant was used in this study, which was within the safety criteria.
The use of Gram staining to evaluate bacteria within root canals has been described.[20] The technique has been used to assess the antimicrobial effect of irrigants within root canals and tissue clearance based upon staining characteristics of the smear.[16] A similar approach has been used in the present study.
The leaf extract has several bioactive compounds that possess antibacterial capabilities. This may be attributed to the tetranortriterpenes that include, nimbin, nimbinin, nimbidinin, nimbolide and nimbidic acid.[5] The antibacterial action of the drug has been studied by Baswa et al. who found inhibition of cell membrane synthesis.[21] Besides the antimicrobial action, this group of compounds also demonstrates anti-inflammatory function (ability to prevent the production of prostaglandins, especially Prostaglandin E1 (PGEI) and 5-hydroxytryptamine) which is a desirable characteristic of the irrigant.[22] An immunomodulatory function has also been suggested for the neem drug that enables antigen presentation to immunocompetent cells.-[13] This study is the first to use a leaf extract within the root canal.
The irrigant combinations (ELE + SHC and ELE + CHX) had better antimicrobial properties than individual irrigants. This is indicative of synergistic action between the herbal and chemical irrigants, which potentiates their individual anti-microbial efficacy. It could be hypothesized that using the SHC + ELE combination as irrigants would prove beneficial.
Biofilms present within the root canal are hard to eliminate with current irrigants and techniques. They exhibit an increased resistance to all disinfectants.[23] A. indica has demonstrable antifungal action against Candida albicans biofilm on tooth substrate.[24] A neem-based irrigant has also shown prevention of Enterococcus faecalis adhesion to dentin.[25] The effect of an aqueous neem leaf extract on extracellular glucan produced by Streptococcus sanguis, which is responsible for co-aggregation of microorganisms to form colonies, has been demonstrated.[26] The gallotannin component of the extract had an inhibitory effect on insoluble glucan production and bacterial aggregation. Such results have also been reported by Wu-Yuan et al.[27] We can hypothesize similar action with ELE in our study. The extract may have disrupted the biofilm adhering to the root canal walls and aided in the permeation of SHC to produce it's antibacterial action. This may explain the synergism between the two irrigants.
ELE has anti-microbial properties attributable to the neem drug component. Ethanol is the solvent for the neem drug in ELE. Our in vitro study demonstrated that ethanol itself lacked antimicrobial properties.[14] It has been used to reduce the surface tension of SHC which helps increase spreading ability.[28] However, such mixtures are unstable and lack shelf-life due to the loss of available chlorine from SHC. In our study, ethanol was an integral part of the leaf extract and could have increased the spreading ability of the neem drug within the canal. When mixed with SHC within the root canal (SHC + ELE sequential irrigation), ethanol helped reduce the surface tension of SHC. This could further explain synergism between SHC and ELE.
The anti-microbial effect of the standard irrigants, SHC and CHX showed no difference even though the percentage reduction with SHC was higher (84.85%) than CHX (69.17%). Similar results have been previously reported,[16] though other studies have found differing results.[1]
Gram-stained smears were used for the detection of root canal flora. Significant differences were found between SHC and saline as well as combinations (SHC + ELE, CHX + ELE) and saline. The smears did not find a significant reduction in microbial counts between either ELE and saline or CHX and saline. This data does not co-relate with the anaerobic culture phase of the study. A possible explanation for this difference in results between the two methodologies could be the incomplete transfer of micro-organisms from the paper points onto the slides for gram smears. For anaerobic culture, the barbed broach and paper points were fully immersed within the TGB without attrition in the number of organisms.
The tissue clearing ability of the irrigant was additionally evaluated by the staining characteristics of the Gram smears. A statistically significant difference was found only between SHC + ELE and saline. This method did not suggest of any difference in reduction in tissue load between SHC and saline. SHC has established tissue dissolving properties.[2] This suggests Gram smears are not reliable indicators of tissue clearance within the canal as it is dependent on the amount of tissue that adheres to the paper point.
CONCLUSIONS
Our study indicates that the ethanolic neem leaf extract can be used successfully within the root canal as an irrigant with demonstrable anti-microbial efficacy. SHC is important for good antimicrobial efficacy and tissue dissolution properties. A combination of SHC and ELE is synergistic antimicrobially. Further clinical research using this combination can aim at evaluating peri-radicular healing.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Siqueira JF, Jr, Batista MM, Fraga RC, de Uzeda M. Antibacterial effects of endodontic irrigants on black-pigmented gram-negative anaerobes and facultative bacteria. J Endod. 1998;24:414–6. doi: 10.1016/S0099-2399(98)80023-X. [DOI] [PubMed] [Google Scholar]
- 2.Naenni N, Thoma K, Zehnder M. Soft tissue dissolution capacity of currently used and potential endodontic irrigants. J Endod. 2004;30:785–7. doi: 10.1097/00004770-200411000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Tanomaru Filho M, Leonardo MR, Silva LA, Aníbal FF, Faccioli LH. Inflammatory response to different endodontic irrigating solutions. Int Endod J. 2002;35:735–9. doi: 10.1046/j.1365-2591.2002.00544.x. [DOI] [PubMed] [Google Scholar]
- 4.Ballal N, Kundabala M, Bhat K, Acharya S, Ballal M, Kumar R, et al. Susceptibility of Candida albicans and Enterococcus faecalis to Chitosan, Chlorhexidine gluconate and their combination in vitro. Aust Endod J. 2009;35:29–33. doi: 10.1111/j.1747-4477.2008.00126.x. [DOI] [PubMed] [Google Scholar]
- 5.Biswas K, Chattopadhyay I, Banerjee RK, Bandyopadhyay U. Biological activities and medicinal properties of neem (Azadirachta indica) Curr Sci. 2002;82:1337–45. [Google Scholar]
- 6.Jain A, Basal E. Inhibition of Propionibacterium acnes-induced mediators of inflammation by Indian herbs. Phytomedicine. 2003;10:34–8. doi: 10.1078/094471103321648638. [DOI] [PubMed] [Google Scholar]
- 7.Okpanyi SN, Ezeukwu GC. Anti-inflammatory and antipyretic activities of Azadirachta indica. Planta Med. 1981;41:34–9. doi: 10.1055/s-2007-971670. [DOI] [PubMed] [Google Scholar]
- 8.Pai MR, Acharya LD, Udupa N. Evaluation of antiplaque activity of Azadirachta indica leaf extract gel - A 6-week clinical study. J Ethnopharmacol. 2004;90:99–103. doi: 10.1016/j.jep.2003.09.035. [DOI] [PubMed] [Google Scholar]
- 9.Fabry W, Okemo PO, Ansorg R. Antibacterial activity of East African medicinal plants. J Ethnopharmacol. 1998;60:79–84. doi: 10.1016/s0378-8741(97)00128-1. [DOI] [PubMed] [Google Scholar]
- 10.Natarajan V, Pushkala S, Karuppiah VP, Prasad PV. Anti dermatophytic activity of Azardirachta indica (neem) by invitro study. Indian J Pathol Microbiol. 2002;45:311–3. [PubMed] [Google Scholar]
- 11.Fabry W, Okemo P, Ansorg R. Fungistatic and fungicidal activity of east African medicinal plants. Mycoses. 1996;39:67–70. doi: 10.1111/j.1439-0507.1996.tb00087.x. [DOI] [PubMed] [Google Scholar]
- 12.Nwosu MO, Okafor JI. Preliminary studies of the antifungal activities of some medicinal plants against Basidiobolus and some other pathogenic fungi. Mycoses. 1995;38:191–5. doi: 10.1111/j.1439-0507.1995.tb00048.x. [DOI] [PubMed] [Google Scholar]
- 13.Upadhyay SN, Dhawan S, Garg S, Talwar GP. Immunomodulatory effects of neem (Azadirachta indica) oil. Int J Immunopharmacol. 1992;14:1187–93. doi: 10.1016/0192-0561(92)90054-o. [DOI] [PubMed] [Google Scholar]
- 14.Dutta A, Kundabala M. Antimicrobial efficacy of endodontic irrigants from Azadirachta indica: An in vitro study. Acta Odontol Scand. 2013;71:1594–8. doi: 10.3109/00016357.2013.780290. [DOI] [PubMed] [Google Scholar]
- 15.Möller AJ. Microbiological examination of root canals and periapical tissues of human teeth. Methodological studies. Odontol Tidskr. 1966;74(Suppl):1–380. [PubMed] [Google Scholar]
- 16.Kuruvilla JR, Kamath MP. Antimicrobial activity of 2.5% sodium hypochlorite and 0.2% chlorhexidine gluconate separately and combined, as endodontic irrigants. J Endod. 1998;24:472–6. doi: 10.1016/S0099-2399(98)80049-6. [DOI] [PubMed] [Google Scholar]
- 17.van der Nat JM, van der Sluis WG, de Silva KT, Labadie RP. Ethnopharmacognostical survey of Azadirachta indica A. Juss (Meliaceae) J Ethnopharmacol. 1991;35:1–24. doi: 10.1016/0378-8741(91)90131-v. [DOI] [PubMed] [Google Scholar]
- 18.Vanka A, Tandon S, Rao SR, Udupa N, Ramkumar P. The effect of indigenous neem Azadirachta indica [correction of (Adirachta indica)] mouth wash on Streptococcus mutans and lactobacilli growth. Indian J Dent Res. 2001;12:133–44. [PubMed] [Google Scholar]
- 19.Tandan S, Chandra S, Gupta S, Tripathi H, Lal J. Pharmacological effects of Azadirachta indica leaves. Fitoterapia. 1990;LXI:75–8. [Google Scholar]
- 20.Pajari U, Ahola R, Bäckman T, Hietala EL, Tjäderhane L, Larmas M. Evaluation of Gram's method of staining for the prognosis of root canal treatment in nonvital dental pulps. Oral Surg Oral Med Oral Pathol. 1993;76:91–6. doi: 10.1016/0030-4220(93)90301-j. [DOI] [PubMed] [Google Scholar]
- 21.Baswa M, Rath CC, Dash SK, Mishra RK. Antibacterial activity of Karanj (Pongamia pinnata) and Neem (Azadirachta indica) seed oil: A preliminary report. Microbios. 2001;105:183–9. [PubMed] [Google Scholar]
- 22.Chattopadhyay R, Chattopadhyay R, Mitra S. Possible mechanism of anti-inflammatory activity of a indica leaf extract. Indian J Pharmacol. 1993;25:99–100. [Google Scholar]
- 23.Luddin N, Ahmed HM. The antibacterial activity of sodium hypochlorite and chlorhexidine against Enterococcus faecalis: A review on agar diffusion and direct contact methods. J Conserv Dent. 2013;16:9–16. doi: 10.4103/0972-0707.105291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tyagi SP, Sinha DJ, Garg P, Singh UP, Mishra CC, Nagpal R. Comparison of antimicrobial efficacy of propolis, Morinda citrifolia, Azadirachta indica (Neem) and 5% sodium hypochlorite on Candida albicans biofilm formed on tooth substrate: An in-vitro study. J Conserv Dent. 2013;16:532–5. doi: 10.4103/0972-0707.120973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosaline H, Kandaswamy D, Gogulnath D, Rubin M. Influence of various herbal irrigants as a final rinse on the adherence of Enterococcus faecalis by fluorescence confocal laser scanning microscope. J Conserv Dent. 2013;16:352–5. doi: 10.4103/0972-0707.114365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolinsky LE, Mania S, Nachnani S, Ling S. The inhibiting effect of aqueous Azadirachta indica (Neem) extract upon bacterial properties influencing in vitro plaque formation. J Dent Res. 1996;75:816–22. doi: 10.1177/00220345960750021301. [DOI] [PubMed] [Google Scholar]
- 27.Wu-Yuan CD, Chen CY, Wu RT. Gallotannins inhibit growth, water-insoluble glucan synthesis, and aggregation of mutans streptococci. J Dent Res. 1988;67:51–5. doi: 10.1177/00220345880670011001. [DOI] [PubMed] [Google Scholar]
- 28.Cunningham WT, Cole JS, 3rd, Balekjian AY. Effect of alcohol on the spreading ability of sodium hypochlorite endodontic irrigant. Oral Surg Oral Med Oral Pathol. 1982;54:333–5. doi: 10.1016/0030-4220(82)90105-0. [DOI] [PubMed] [Google Scholar]


