In this large cohort of human immunodeficiency virus–infected patients receiving first-line antiretroviral therapy in Zambia, individuals who started a tenofovir-containing regimen despite baseline renal dysfunction showed comparable mortality and renal function improvement to those not receiving tenofovir.
Keywords: renal function, tenofovir, Zambia
Abstract
Background. Although tenofovir disoproxil fumarate (TDF) use has increased as part of first-line antiretroviral therapy (ART) across sub-Saharan Africa, renal outcomes among patients receiving TDF remain poorly understood. We assessed changes in renal function and mortality in patients starting TDF- or non–TDF-containing ART in Lusaka, Zambia.
Methods. We included patients aged ≥16 years who started ART from 2007 onward, with documented baseline weight and serum creatinine. Renal dysfunction was categorized as mild (estimated glomerular filtration rate [eGFR], 60–89 mL/min), moderate (30–59 mL/min), or severe (<30 mL/min) according to the chronic kidney disease–epidemiology (CKD-EPI) formula. Differences in eGFR during ART were analyzed using linear mixed-effect models. The odds of developing moderate or severe eGFR decrease and mortality were assessed using logistic and competing risk regression, respectively.
Results. We included 62 230 adults, of which 38 716 (62.2%) initiated a TDF-based regimen. The proportion with moderate or severe renal dysfunction at baseline was lower in the TDF than in the non-TDF group (1.9% vs 4.0%). Among patients with no or mild renal dysfunction, those receiving TDF were more likely to develop moderate (adjusted odds ratio, 3.11; 95% confidence interval, 2.52–3.87) or severe ( 2.43; 1.80–3.28) eGFR decrease, although the incidence in such episodes was low. Among patients with moderate or severe renal dysfunction at baseline, renal function improved independently of ART regimen, and mortality rates were similar in both treatment groups.
Conclusions. TDF use did not attenuate renal function recovery or increase the mortality rate in patients with renal dysfunction. Further studies are needed to determine the role of routine renal function monitoring before and during ART use in Africa.
(See the Editorial Commentary by Estrella et al on pages 1481–3.)
In 2007, Zambia was one of the first countries in sub-Saharan Africa (SSA) to introduce tenofovir disoproxil fumarate (TDF) as a preferred nucleotide reverse-transcriptase inhibitor of first line antiretroviral therapy (ART) [1, 2]. The advantages of TDF include its high potency against human immunodeficiency virus (HIV) and hepatitis B infections, favorable resistance profile, good tolerability and safety, and availability as a coformulation with other antiretroviral agents in once-daily pills [3–5]. However, in industrialized countries, TDF has been associated with nephrotoxicity, including proximal tubulopathy and impaired glomerular filtration [6–11]. In SSA, a significant number of HIV-infected patients start ART with preexisting kidney dysfunction, due to either HIV-associated nephropathy or other causes such as hypertension or diabetes [12, 13]. Although the incidence of TDF-related kidney dysfunction seems to be low in most settings [8–10, 14], the effect of TDF on clinical outcomes in patients starting ART with moderate or severe renal dysfunction has not been studied previously, to our knowledge. In this report, we evaluated changes in renal function and mortality in patients starting TDF- or non–TDF-containing ART regimens in a large cohort of HIV infected adults in Lusaka, Zambia.
METHODS
Zambian National HIV Program
Care and treatment provided through the Zambian National HIV Program has been described elsewhere [15]. Along with a detailed medical history and physical examination, the baseline screening includes CD4 cell count and hemoglobin, serum creatinine, and aminotransferase levels. The choice of ART depends on laboratory test results, age and comorbid conditions. Before June 2007, stavudine (d4T) or zidovudine (AZT) with lamivudine (3TC) plus a nonnucleoside reverse-transcriptase inhibitor, either nevirapine (NVP) or efavirenz (EFV), were the recommended first-line agents. Thereafter, TDF replaced d4T and AZT as the preferred nucleotide reverse-transcriptase inhibitor alongside 3TC for patients with a creatinine clearance level ≥50 mL/min. However, because creatinine clearance was not calculated routinely, TDF was prescribed in patients with a serum creatinine level ≤120 µmol/L. For those with impaired renal function, abacavir (ABC) was prescribed instead of TDF.
Routine clinical follow-up visits were done every 3–6 months, and TDF was substituted with ABC in patients in whom severe renal dysfunction developed. Deaths are ascertained when reported by a family member, clinic staff member, or community health worker. Patient medical information is entered into an electronic database for clinical care, monitoring and evaluation, and reporting purposes [16]. These programmatic data have received local ethical approval for use in the International epidemiological Databases to Evaluate AIDS in Southern Africa (IeDEA-SA) network, a large regional collaboration of ART programs [17].
Inclusion Criteria, Definitions, and Outcomes
We included patients aged ≥16 years who started ART in the capital city of Lusaka from June 2007, when the Zambian government instituted its national policy to incorporate TDF into first-line ART [1]. Those without documented serum creatinine and weight at baseline were excluded from the analysis cohort. Owing to a lag in rollout of national ART guidelines, not all the patients enrolled after June 2007 started a TDF-containing ART regimen. We categorized first-line regimens as either TDF–containing regimens (TDF + XTC [3TC or emtricitabine (FTC)] + NVP or EFV) or non–TDF-containing regimens (ABC or d4T or AZT + 3TC + NVP or EFV). Although boosted protease inhibitors (bPIs) may be incorporated into first-line regimens after nonnucleoside reverse-transcriptase inhibitor intolerance, they are typically reserved for second-line therapy and are almost never prescribed at ART initiation. In addition, because the coadministration of TDF with a bPI has been shown to increase the risk of nephrotoxicity and impair renal function [18–20], we excluded all patients who were receiving a bPI-based regimen. Because ABC was often prescribed to patients with renal dysfunction at baseline, including these patients could have introduced some degree of confounding by indication. To minimize this potential effect, we conducted secondary analyses that excluded patients receiving ABC.
Serum creatinine concentration was measured using the Cobas Integra 400 Plus automated chemistry analyzer (Roche Diagnostics) from 2007–2008, and the Olympus AU400 analyzer (Beckman Coulter Diagnostics) thereafter. Quality control of chemistry analyzers was performed according to manufacturer recommendations. We determined the estimated glomerular filtration rate (eGFR) using the chronic kidney disease–epidemiology (CKD-EPI) formula [21]. This method is considered more accurate than the Cockroft-Gault and modification of diet in renal disease (MDRD) equations for values >60 mL/min/1.73 m2 and has recently been shown to be accurate in HIV-infected populations as well [22]. In sensitivity analyses, we repeated the main analyses using the MDRD and the Cockroft-Gault formulas. In line with the Kidney Disease Outcomes Quality Initiative criteria [23, 24], we categorized renal function as follows: normal, eGFR ≥90 mL/min/1.73 m2; mild eGFR decrease, 60–89 mL/min/1.73 m2; moderate eGFR decrease, 30–59 mL/min/1.73 m2; and severe eGFR decrease, ≤29 mL/min/1.73 m2. Our outcomes of interest were (1) renal function after 6 and 12 months of ART, (2) the proportion of patients experience an episode of moderate or severe eGFR decrease while receiving ART, and (3) death.
Statistical Analyses
Baseline characteristics of patients starting TDF- and non–TDF-containing ART regimens were compared using χ2 and Mann-Whitney tests. We compared the median change in eGFR between patients receiving TDF and those not receiving TDF after 6 and 12 months of ART, according to baseline renal function category. To estimate the difference in renal function over time between the 2 study groups, we used a multivariable mixed-effects model (eGFR measurements within patients), using a random intercept with unstructured covariance correlation structure, stratified by baseline renal dysfunction category. In the TDF and non-TDF groups, we compared the proportions of patients with normal renal function or mild renal dysfunction at baseline who had an episode of moderate or severe eGFR decrease at 6 or 12 months . Adjusted estimates were obtained using a multivariable logistic regression model. We used a multivariable competing risk subdistribution model, described by Fine and Gray [25], to compare mortality rates between the 2 groups, measuring time from the initiation of first-line ART to the outcome of interest. Accounting for loss to follow-up, defined as not returning to the health care facility for >6 months, as a competing risk of death, limits the production of biased results. All multivariable regression analyses were adjusted for age, sex, calendar year, WHO stage, CD4 count, and hemoglobin. Anemia was defined as severe (hemoglobin <5.0 mmol/L [<8.0 g/dL]), moderate (5.0 to <6.2 mmol/L [8.0 to <10.0 g/dL] in women and 5.0 to <6.8 mmol/L [8.0 to <11.0 g/dL] in men), mild (6.2 to <7.4 mmol/L [10.0 to <12.0 g/dL] in women and 6.8 to <8.1 mmol/L [11.0 to <13.0 g/dL] in men), or none (≥7.4 mmol/L [≥12.0 g/dL] in women and ≥8.1 mmol/L [≥13.0 g/dL] in men). All statistical analyses were performed using Stata software (version 12.1; StataCorp).
RESULTS
Baseline Characteristics
In this analysis, we included 62 230 HIV-infected adults who started ART between January 2007 and February 2011. Of these, 38 716 (62.2%) started a TDF-containing first-line regimen (Table 1). Individuals receiving TDF started ART with a more advanced stage of disease and were more likely to receive EFV than those not receiving TDF. Patients not receiving TDF-containing regimens were more likely to be female and to have started ART in earlier years. Age and hemoglobin were similar across treatment groups. Overall, patients receiving TDF were slightly more likely to have some degree of baseline renal dysfunction than those not receiving TDF-containing ART (16.7% vs 12.4%). However, the proportion of patients with moderate or severe renal dysfunction was 2 times higher in the non-TDF than in the TDF group (4.0% vs 1.9%). After ART initiation 70.4% of patients receiving TDF and 84.9% of those receiving non-TDF regimens had at least 1 repeated creatinine measurement available after either 6 or 12 months of ART.
Table 1.
Baseline Characteristics of Study Population by ART Regimen
| Characteristic | With TDF | Without TDF | P Value |
|---|---|---|---|
| (n = 38 716; 62.2%) | (n = 23 514; 37.8%) | ||
| Female, No. (%) | 21 891 (56.5) | 16 542 (70.4) | <.001 |
| Age, median (IQR), y | 34 (29–40) | 32 (27–38) | <.001 |
| WHO stage III/IV, No. (%) | 22 989 (59.4) | 11 218 (47.7) | <.001 |
| BMI, median (IQR), kg/m2 | 19.7 (17.8–22.1) | 20.8 (18.5–22.6) | <.001 |
| CD4 count, median (IQR), cells/μL | 151 (82–223) | 172 (98–251) | <.001 |
| Missing, No. (%) | 2333 (6.0) | 1858 (7.9) | |
| Anemia, No. (%) | <.001 | ||
| None | 10 050 (26.0) | 5650 (24.0) | |
| Mild | 13 291 (34.3) | 8486 (36.1) | |
| Moderate | 9709 (25.1) | 5429 (23.1) | |
| Severe | 3034 (7.8) | 1760 (7.5) | |
| Missing (%) | 2632 (6.8) | 2189 (9.3) | |
| eGFR decrease, No. (%) | <.001 | ||
| None | 32 247 (83.3) | 20 621 (87.7) | |
| Mild | 5741 (14.8) | 1967 (8.4) | |
| Moderate | 616 (1.6) | 676 (2.9) | |
| Severe | 110 (0.3) | 246 (1.1) | |
| Calendar year of ART start, No. (%) | <.001 | ||
| 2007 | 3204 (8.3) | 10 842 (46.1) | |
| 2008 | 10 155 (26.2) | 4589 (19.5) | |
| 2009 | 12 319 (31.8) | 4594 (19.5) | |
| 2010 | 12 110 (31.3) | 3297 (14.0) | |
| 2011 | 928 (2.4) | 192 (0.8) | |
| EFV-based ART, No. (%) | 23 367 (60.4) | 3913 (16.6) | <.001 |
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; EFV, efavirenz; eGFR, estimated glomerular filtration rate; IQR, interquartile range; TDF, tenofovir disoproxil fumarate; WHO, World Health Organization.
Changes in Renal Function in Patients With No or Mild eGFR Decrease at Baseline
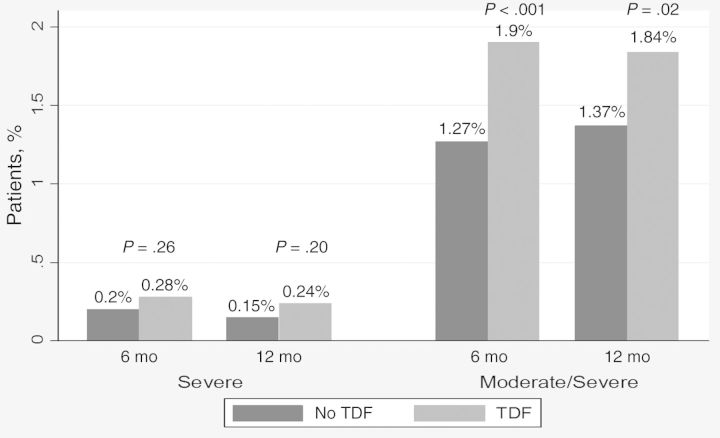
Among patients who started ART with no renal dysfunction, we observed a slight decline in renal function at both 6 and 12 months, irrespective of ART regimen (−15 mL/min in the TDF and −17 mL/min in the non-TDF group). There was no change in renal function over time in those with a mild eGFR decrease at baseline (Figure 1). In adjusted analyses, patients receiving TDF had slightly reduced renal function at 6 and 12 months, compared with those receiving other ART regimens, although differences were small (Table 2). The proportion of patients with no or mild baseline renal dysfunction with an incident episode of severe eGFR decrease at 6 or 12 months was higher in the TDF group, though this difference was not statistically significant (0.28% vs 0.20% [P = .26] at 6 months and 0.24% vs 0.15% [P = .20] at 12 months). For the outcome defined as incident episodes of moderate or severe eGFR decrease, the differences reached statistically significance; however, the numbers remained low (1.90% in the TDF vs 1.27% in the non-TDF group [P < .001] at 6 months and 1.84% vs 1.37% [P = .02] at 12 months; Figure 2). In adjusted analyses, patients receiving TDF were more likely to experience an episode of moderate or severe renal dysfunction than those receiving other regimens during the first year of ART (Table 2).
Figure 1.
Crude change in renal function during antiretroviral therapy, by baseline renal function and treatment group. Abbreviations: CKD-EPI, chronic kidney disease–epidemiology formula; eGFR, estimated glomerular filtration rate; GFR, glomerular filtration rate; TDF, tenofovir disoproxil fumarate.
Table 2.
Comparison of Renal Outcomes and Mortality Rates Between Patients Starting Antiretroviral Therapy With or Without TDFa
| Baseline eGFR Decrease | Difference in eGFR (95% CI),
mL/min |
Progression to Renal Dysfunction, OR (95%
CI) |
Mortality, sHR (95% CI) | ||
|---|---|---|---|---|---|
| At 6 mo | At 12 mo | Severe | Moderate/Severe | ||
| None | −8.64 (−9.40 to −7.88) | −9.93 (−10.7 to −9.14) | 3.09 (1.85–5.17) | 3.11 (2.52–3.87) | 1.22 (1.13–1.31) |
| Mild | −4.55 (−6.42 to −2.68) | −6.81 (−9.01 to −4.61) | 5.27 (1.19–23.2) | 2.43 (1.80–3.28) | 0.82 (.68–.98) |
| Moderate | −6.10 (−11.0 to −1.23) | −5.37 (−10.9 to .14) | 1.11 (.46–2.70) | NA | 0.79 (.58–1.07) |
| Severe | 19.3 (2.29–36.20) | 21.7 (4.33–39.10) | NA | NA | 0.89 (.52–1.52) |
Abbreviations: CI, confidence interval; eGFR, estimated glomerular filtration rate; NA, not applicable; OR, odds ratio; sHR, subhazard ration; TDF, tenofovir disoproxil fumarate.
a The comparison was done using the chronic kidney disease–epidemiology formula, with patients not receiving TDF serving as the reference group. All analyses are adjusted for age, sex, calendar year, baseline World Health Organization stage, CD4 cell count, and anemia status.
Figure 2.
Proportion of patients with no or mild renal dysfunction at baseline developing a moderate or severe eGFR decrease on ART. Abbreviations: ART, antiretroviral therapy; eGFR, estimated glomerular filtration rate; TDF, tenofovir disoproxil fumarate.
Changes in Renal Function in Patients With Moderate or Severe eGFR Decrease at Baseline
Independent of treatment regimen, individuals with moderate (n = 616) or severe (n = 110) renal dysfunction at baseline showed an improvement in renal function during ART. This included patents who received TDF despite severe renal failure (+30 mL/min after 12 months; Figure 1). In adjusted analyses, TDF was associated with marginally lower renal function at 1 year in individuals with baseline moderate renal dysfunction. On the contrary, among patients with available data, TDF use seemed to be associated with a higher eGFR during follow-up for those with severe renal dysfunction at ART initiation (adjusted difference, +21.7 mL/min; 95% confidence interval [CI], 4.33–39.10 at 1 year; Table 2). Finally, the odds of progression from moderate to severe dysfunction were not increased for patients starting a TDF-containing ART regimen (adjusted odds ratio, 1.11; 95% CI, .46–2.70; Table 2).
Mortality
During a total follow-up of 111 972 person-years, 2405 deaths (6.2%) were documented in the TDF group, and 1472 (6.3%) in the non-TDF group; 27.4% and 20.7% of patients, respectively, were lost to follow-up in each group. Overall, the degree of renal dysfunction at ART initiation predicted mortality risk. For instance, patients with severe renal dysfunction at baseline were twice as likely to die during follow-up as those with normal renal function (Table 3). In adjusted analyses stratified by baseline renal function, individuals who started a TDF-containing regimen with some degree of renal dysfunction were not more likely to die during follow-up than those receiving other treatment regimens (Table 2). Notably, there was no detectable difference in mortality rates between patients receiving TDF and those not receiving TDF and starting ART with a moderate (adjusted subhazard ratio, 0.79; 95% CI, .58–1.07) or severe (0.89; .52–1.52) eGFR decrease. Other important risk factors for death were male sex, advanced clinical stage of disease, low CD4 cell counts, and anemia (Table 3).
Table 3.
Risk Factors for Mortality
| Factor | Crude sHR (95% CI) | P Value | Adjusted sHR (95% CI) | P Value |
|---|---|---|---|---|
| TDF in regimen | <.001 | .001 | ||
| No | 1 | 1 | ||
| Yes | 1.13 (1.06–1.21) | 1.14 (1.05–1.23) | ||
| Baseline eGFR decrease | <.001 | <.001 | ||
| None | 1 | 1 | ||
| Mild | 1.19 (1.09–1.31) | 1.24 (1.12–1.37) | ||
| Moderate | 2.52 (2.16–2.94) | 1.80 (1.53–2.13) | ||
| Severe | 3.77 (2.96–4.80) | 2.00 (1.52–2.63) | ||
| Baseline CD4 count | <.001 | <.001 | ||
| <50 cells/μL | 1 | 1 | ||
| 50–199 cells/μL | 0.46 (.42–.51) | 0.60 (.56–.65) | ||
| >199 cells/μL | 0.23 (.20–.26) | 0.42 (.38–.47) | ||
| Anemia | <.001 | <.001 | ||
| None | 1 | 1 | ||
| Mild | 2.06(1.78–2.39) | 1.63 (1.45–1.83) | ||
| Moderate | 3.82 (3.32–4.40) | 2.70 (2.41–3.03) | ||
| Severe | 5.93 (1.78–2.39) | 3.88 (3.40–4.43) | ||
| Age (in years) | 1.01 (1.00–1.01) | <.001 | 1.01 (1.01–1.01) | <.001 |
| Sex | <.001 | <.001 | ||
| Male | 1 | 1 | ||
| Female | 0.81 (.75–.88) | 0.77 (.72–.83) | ||
| WHO stage | <.001 | <.001 | ||
| I or II | 1 | 1 | ||
| III | 2.34 (2.13–2.58) | 1.77 (1.62–1.93) | ||
| IV | 2.56 (2.21–2.97) | 2.12 (1.86–2.41) | ||
| Calendar year at ART start | <.001 | <.001 | ||
| 2007 | 1 | 1 | ||
| 2008 | 0.90 (.80–1.02) | 0.94 (.86–1.02) | ||
| 2009 | 0.76 (.67–.85) | 0.71 (.64–.78) | ||
| 2010 | 0.30 (.26–.34) | 0.49 (.43–.55) |
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; eGFR, estimated glomerular filtration rate; sHR, subhazard ratio; TDF, tenofovir disoproxil fumarate; WHO, World Health Organization.
Sensitivity Analyses
First, when we repeated the analyses using the MDRD and Cockcroft-Gault equations to measure eGFR, we found similar results for all the baseline renal eGFR categories (Supplementary Table 1A and 1B). Independently of the formula used, mortality and eGFR decrease during follow-up in patients receiving TDF despite moderate or severe baseline eGFR decrease were not higher than in patients receiving other regimens. Second, of the patients in the non-TDF group, 1787 (7.6%) started taking ABC. Excluding these patients from our analyses did not alter our results significantly (Supplementary Table 2).
DISCUSSION
In this large observational cohort of HIV-1–infected adults in Zambia, TDF did not have a significant effect on renal function changes or mortality rates in patients starting ART with moderate or severe renal dysfunction. Among individuals with no or mild eGFR decrease at baseline, those receiving TDF were more likely to experience an episode of moderate or severe renal dysfunction within the first 12 months of ART than were patients receiving other regimens; however, the overall occurrence of this outcome was rare.
Fifteen percent of the patients included in our study had some degree of renal dysfunction at ART initiation. This is lower than the proportions reported in other studies from SSA [12, 13]. These differences might have been driven by the degree of immunosuppression at ART start, which was less advanced in our study compared with the 2 other reports. Recent improvements in screening for coinfections associated with renal dysfunction, the avoidance of nephrotoxic drugs, and the change in CD4 threshold for ART initiation are additional factors that could explain this finding. The use of TDF is not recommended for patients with moderate or severe eGFR decrease according to most international guidelines. The Zambian national guidelines state that patients should start a TDF-containing regimen only if their creatinine clearance is >50 mL/min or their absolute serum creatinine level ≤120 µmol/L. Many clinicians use the absolute creatinine value to determine the ART regimen for reasons of convenience. As a result, TDF is sometimes prescribed to patients with an absolute creatinine level ≤120 µmol/L but undiagnosed moderate or severe renal eGFR decrease, as was the case for 726 patients (1.9%) receiving TDF in our analysis cohort.
Our study showed that kidney function in patients with severe baseline eGFR decrease improved during the first year of ART, a finding that was even more pronounced in those receiving TDF. Although the causes of renal dysfunction in patients starting ART in SSA have not been thoroughly investigated, it is assumed that HIV-associated nephropathy plays an important role in this setting [26]. As a consequence, even ART regimens containing potentially nephrotoxic agents can improve renal function in most of the patients with renal insufficiency. Similar to our findings, in a large cohort of American HIV-infected veterans, preexisting renal disease did not seem to worsen the effects of TDF [9]. Of the 3336 Development of AntiRetroviral Therapy in Africa trial patients included in the analysis of renal outcomes, only 237 (7.1%) had moderate and 7 (0.2%) had severe renal dysfunction at ART initiation [12, 14]; however, a detailed analysis of renal change by ART regimen was not performed for these patients [14]. In contrast, our patients who started a TDF-containing regimen with a moderate eGFR decrease were not more likely to experience severe renal dysfunction during ART than those receiving other regimens. As expected, mortality rates increased with worsening baseline eGFR decrease at baseline. However, TDF was not associated with higher mortality rates in patients with moderate or severe renal dysfunction at baseline. Taken together, these findings suggest that starting a TDF-containing regimen despite preexisting renal disease may be less harmful than previously thought.
In our study, patients who started ART with normal kidney function seemed to experience a slow decline in renal function during the first year, irrespective of ART regimen. It has been shown that even though ART seems to slow decline in creatinine clearance, a degree of renal function loss seems inevitable, even with durable viral suppression [27]. Therefore, ART may only improve kidney function if it is HIV or hepatitis B related. In line with recent publications from high-income countries that linked TDF with acute and chronic kidney dysfunction [8–10, 14], patients receiving TDF in our analysis experienced more pronounced reductions in renal function than those receiving non-TDF regimens. Furthermore, we observed a 2-fold increase in incident moderate or severe eGFR decreases in the TDF group. However, the numbers remained small, with <1% of patients experiencing an episode of severe renal dysfunction (eGFR < 30 mL/min) 6 or 12 months after ART initiation. Our findings show that the overall effect of TDF on renal outcomes in patients with normal baseline renal function is small. Considering the low magnitude or even the absence of this association in many previous reports [27, 28] and given the many advantages related to the use of TDF, poor access to renal function monitoring may not be a good justification for withholding TDF in first-line ART.
To our knowledge, this is the largest study assessing the association between TDF and renal dysfunction in SSA to date. We provide evidence on the safety of TDF-containing regimens in a nonselected population with highly prevalent kidney dysfunction. Whereas many studies have examined the renal safety of TDF in patients with normal baseline renal function, ours provides a detailed description of renal outcomes and mortality rates in patients who received TDF despite preexisting eGFR decrease.
Our main limitation was the significant proportion of patients who did not have a follow-up creatinine measurement (24.1%). Although we cannot exclude the nonrandom selection of patients in whom at least 1 measurement was performed, it is important to note that a significant proportion of individuals receiving TDF were also missing creatinine measurements, which reflects the situation of most ART programs in southern Africa. Although it is common practice to report renal outcomes as chronic kidney disease, defined as 2 eGFR measurements <60 mL/min ≥3 months apart, such diagnoses may be difficult in resource-constrained settings such as Zambia, where follow-up testing is not always routinely performed. For this reason, we were unable to further distinguish between acute and chronic renal conditions. Because single drug substitutions were not considered in our analyses, a fraction of the patients who developed renal dysfunction while receiving TDF might have been switched to a non–TDF-containing regimen, which might have had an effect on our mortality and severe eGFR decrease estimates. Some level of confounding by treatment allocation was also possible, because the patients included in this study were not randomized to receive either TDF or non-TDF regimens. Therefore, we adjusted our analyses for differences in baseline demographic and clinical characteristics. Finally, we could not evaluate long-term renal outcomes, and, because creatinine was the only routine measurement of renal function performed in this cohort, we could not assess the mechanisms of renal disease, such as proximal tubulopathy, which has been associated with the use of TDF [29].
In summary, in our analysis of a large observational cohort in Zambia, we show that patients receiving TDF-containing ART, despite preexisting renal disease, did not experience worse renal outcomes or increased mortality compared with those taking other regimens. Baseline screening of renal dysfunction should be encouraged, because dose adjustment of several antiretroviral agents is recommended in case of eGFR decrease. However, our data support the view that poor availability of renal function screening should not stop the prescription of TDF-containing ART, considering its numerous advantages over other combinations. Studies monitoring and evaluating the long-term effect of TDF in HIV-infected population in SSA are urgently needed.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank all study participants and staff of all participating sites
Author contributions. L. M., B. H. C., and G. W. designed the study. L. M., P. M., and G. W. performed the statistical analyses. L. M., P. M., and G. W. wrote the first draft of the manuscript. All authors contributed to the interpretation of the results and to the final version of the manuscript. L. M., P. M., and G. W. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. M. A. D. is coprincipal investigator of IeDEA-Southern Africa.
Financial support. This study was supported by the National Institutes of Health, through the IeDEA Southern Africa collaboration (grant 1U01AI069924) and through research training programs (grants R25TW009340 and D43TW001035). O. K. was supported by a PROSPER fellowship grant from the Swiss National Science Foundation (nr 32333B_150934). The funders had no role in study design, data collection and analysis, the decision to publish, or the preparation of the manuscript.
Potential conflicts of interests. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Ministry of Health, Zambia. 2007 Antiretroviral therapy protocols. Outprint Press, Lusaka; 2007. [Google Scholar]
- 2.Kilani B, Ammari L, Marrakchi C, et al. Seroepidemiology of HCV-HIV coinfection in Tunisia. Tunis Med. 2007;85:121–3. [PubMed] [Google Scholar]
- 3.Gallant JE, DeJesus E, Arribas JR, et al. Tenofovir DF, emtricitabine, and efavirenz vs. zidovudine, lamivudine, and efavirenz for HIV. N Engl J Med. 2006;354:251–60. doi: 10.1056/NEJMoa051871. [DOI] [PubMed] [Google Scholar]
- 4.Lacombe K, Gozlan J, Boelle PY, et al. Long-term hepatitis B virus dynamics in HIV-hepatitis B virus-co-infected patients treated with tenofovir disoproxil fumarate. AIDS. 2005;19:907–15. doi: 10.1097/01.aids.0000171404.07995.5c. [DOI] [PubMed] [Google Scholar]
- 5.Nelson MR, Katlama C, Montaner JS, et al. The safety of tenofovir disoproxil fumarate for the treatment of HIV infection in adults: the first 4 years. AIDS. 2007;21:1273–81. doi: 10.1097/QAD.0b013e3280b07b33. [DOI] [PubMed] [Google Scholar]
- 6.Cooper RD, Wiebe N, Smith N, Keiser P, Naicker S, Tonelli M. Systematic review and meta-analysis: renal safety of tenofovir disoproxil fumarate in HIV-infected patients. Clin Infect Dis. 2010;51:496–505. doi: 10.1086/655681. [DOI] [PubMed] [Google Scholar]
- 7.Gallant JE, Parish MA, Keruly JC, Moore RD. Changes in renal function associated with tenofovir disoproxil fumarate treatment, compared with nucleoside reverse-transcriptase inhibitor treatment. Clin Infect Dis. 2005;40:1194–8. doi: 10.1086/428840. [DOI] [PubMed] [Google Scholar]
- 8.Mocroft A, Kirk O, Reiss P, et al. Estimated glomerular filtration rate, chronic kidney disease and antiretroviral drug use in HIV-positive patients. AIDS. 2010;24:1667–78. doi: 10.1097/QAD.0b013e328339fe53. [DOI] [PubMed] [Google Scholar]
- 9.Scherzer R, Estrella M, Li Y, et al. Association of tenofovir exposure with kidney disease risk in HIV infection. AIDS. 2012;26:867–75. doi: 10.1097/QAD.0b013e328351f68f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laprise C, Baril JG, Dufresne S, Trottier H. Association between tenofovir exposure and reduced kidney function in a cohort of HIV-positive patients: results from 10 years of follow-up. Clin Infect Dis. 2013;56:567–75. doi: 10.1093/cid/cis937. [DOI] [PubMed] [Google Scholar]
- 11.Fux CA, Simcock M, Wolbers M, et al. Tenofovir use is associated with a reduction in calculated glomerular filtration rates in the Swiss HIV Cohort Study. Antivir Ther. 2007;12:1165–73. [PubMed] [Google Scholar]
- 12.Reid A, Stohr W, Walker AS, et al. Severe renal dysfunction and risk factors associated with renal impairment in HIV-infected adults in Africa initiating antiretroviral therapy. Clin Infect Dis. 2008;46:1271–81. doi: 10.1086/533468. [DOI] [PubMed] [Google Scholar]
- 13.Mulenga LB, Kruse G, Lakhi S, et al. Baseline renal insufficiency and risk of death among HIV-infected adults on antiretroviral therapy in Lusaka, Zambia. AIDS. 2008;22:1821–7. doi: 10.1097/QAD.0b013e328307a051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stohr W, Reid A, Walker AS, et al. Glomerular dysfunction and associated risk factors over 4–5 years following antiretroviral therapy initiation in Africa. Antivir Ther. 2011;16:1011–20. doi: 10.3851/IMP1832. [DOI] [PubMed] [Google Scholar]
- 15.Stringer JS, Zulu I, Levy J, et al. Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: feasibility and early outcomes. JAMA. 2006;296:782–93. doi: 10.1001/jama.296.7.782. [DOI] [PubMed] [Google Scholar]
- 16.Fusco H, Hubschman T, Mweeta V, Chi B, Levy J, Sinkala M, Stringer J. Electronic patient tracking supports rapid expansion of HIV care and treatment in resource-constrained settings. Third IAS Conference on HIV Pathogenesis and Treatment Rio de Janeiro; 24–27 July 2005; Brazil. Abstract MoPe112C37. [Google Scholar]
- 17.Egger M, Ekouevi DK, Williams C, et al. Cohort Profile: the international epidemiological databases to evaluate AIDS (IeDEA) in sub-Saharan Africa. Int J Epidemiol. 2012;41:1256–64. doi: 10.1093/ije/dyr080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Young J, Schafer J, Fux CA, et al. Renal function in patients with HIV starting therapy with tenofovir and either efavirenz, lopinavir or atazanavir. AIDS. 2012;26:567–75. doi: 10.1097/QAD.0b013e32834f337c. [DOI] [PubMed] [Google Scholar]
- 19.Ryom L, Mocroft A, Kirk O, et al. Association between antiretroviral exposure and renal impairment among HIV-positive persons with normal baseline renal function: the D:A:D study. J Infect Dis. 2013;207:1359–69. doi: 10.1093/infdis/jit043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kearney BP, Mathias A, Mittan A, Sayre J, Ebrahimi R, Cheng AK. Pharmacokinetics and safety of tenofovir disoproxil fumarate on coadministration with lopinavir/ritonavir. J Acquir Immune Defic Syndr. 2006;43:278–83. doi: 10.1097/01.qai.0000243103.03265.2b. [DOI] [PubMed] [Google Scholar]
- 21.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inker LA, Wyatt C, Creamer R, et al. Performance of creatinine and cystatin C GFR estimating equations in an HIV-positive population on antiretrovirals. J Acquir Immune Defic Syndr. 2012;61:302–9. doi: 10.1097/QAI.0b013e31826a6c4f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–47. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 24.Levey AS, de Jong PE, Coresh J, et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011;80:17–28. doi: 10.1038/ki.2010.483. [DOI] [PubMed] [Google Scholar]
- 25.Fine JP, Gray R. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 26.Gerntholtz TE, Goetsch SJ, Katz I. HIV-related nephropathy: a South African perspective. Kidney Int. 2006;69:1885–91. doi: 10.1038/sj.ki.5000351. [DOI] [PubMed] [Google Scholar]
- 27.Leport C, Bouteloup V, Rossert J, et al. Long-term evolution and determinants of renal function in HIV-infected patients who began receiving combination antiretroviral therapy in 1997–1999, ANRS CO8 APROCO-COPILOTE. Clin Infect Dis. 2009;49:1950–4. doi: 10.1086/648445. [DOI] [PubMed] [Google Scholar]
- 28.Jones R, Stebbing J, Nelson M, et al. Renal dysfunction with tenofovir disoproxil fumarate-containing highly active antiretroviral therapy regimens is not observed more frequently: a cohort and case-control study. J Acquir Immune Defic Syndr. 2004;37:1489–95. doi: 10.1097/01.qai.0000138983.45235.02. [DOI] [PubMed] [Google Scholar]
- 29.Zimmermann AE, Pizzoferrato T, Bedford J, Morris A, Hoffman R, Braden G. Tenofovir-associated acute and chronic kidney disease: a case of multiple drug interactions. Clin Infect Dis. 2006;42:283–90. doi: 10.1086/499048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.