Abstract
Objectives
There is robust evidence from epidemiological studies that the offspring of older fathers have an increased risk of neurodevelopmental disorders such as schizophrenia and autism. Here we present a novel mechanism that may contribute to this association.
Methods
Narrative review.
Results
Because the male germ cell undergoes many more cell divisions across the reproductive age range, copy-errors taking place in the paternal germline are associated with de novo mutations in the offspring of older men. Recently it has been recognized that somatic mutations in male germ cells that modify proliferation via dysregulation of the RAS pathway can lead to within-testis expansion of mutant clonal lines. First identified in association with rare paternal age-effect disorders (e.g. Apert syndrome, achondroplasia), this process is known as ‘selfish spermatogonial selection’. This mechanism will (a) favor propagation of germ cells carrying pathogenic mutations, (b) increasingly skew the mutational profile of sperm as men age, and (c) result in an enrichment of de novo mutations in the offspring of older fathers that preferentially impact on specific cellular signaling pathways. This mechanism offers a parsimonious explanation not only for the association between advanced paternal age and various neurodevelopmental disorders, but also provides insights into the genetic architecture (role of de novo mutations), neurobiological correlates (altered cell cycle) and some epidemiological features of these disorders. We outline hypotheses to test this model.
Conclusions
In light of our current understanding of the genetic networks involved in neurocognitive disorders and the principles of selfish spermatogonial selection, we speculate that some pathogenic mutations associated with these disorders are the consequence of a selfish mechanism originating in the aging testis. Given the secular changes for delayed parenthood in most societies, this hypothesis has important public health implications.
Keywords: paternal age, selfish mutations, schizophrenia, autism, de novo mutation, spermatogonia, neurodevelopment
Introduction
Occasionally, seemingly distant fields of research intersect and can catalyze new discovery channels. In this review we outline how divergent clues from epidemiological and genetic studies in autism and schizophrenia research can be integrated with an innovative hypothesis originally proposed to explain the relatively high apparent mutational rate associated with congenital disorders such as Apert syndrome, achondroplasia and RASopathies. The ‘selfish spermatogonial selection’ hypothesis predicts that somatic mutations that promote clonal expansion within the male germline progenitors could skew the influence of paternally-derived de novo mutations. The proposed mechanism may provide a parsimonious explanation for diverse findings related to neurodevelopmental disorders such as schizophrenia and autism. A set of testable predictions are proposed to evaluate this hypothesis.
Key differences between male versus female gamete production
Germ cell development differs radically between human males and females - there are many more germline cell divisions in the life history of a sperm relative to that of an oocyte. In the female, germ cells undergo only 22 mitotic cell divisions in utero, which is followed by a long period of arrest until puberty. Oocyte maturation is completed when meiosis resumes a few minutes prior to ovulation. In the male by contrast, following 30 mitotic divisions during embryogenesis, spermatogenesis is re-initiated at puberty and adult stem cells (spermatogonial cells) undergo regular mitotic divisions once every 16 days to produce sperm. Assuming a simple turnover model, this means that in a 20-year-old male, the spermatogonial cells have undergone approximately 150 cell divisions. By age 50 years, this number is 840. Each time these cells divide, the entire genome is replicated and copy-errors (i.e. mutations) inevitably occur (1). These between-sex biological differences in gamete production are also associated with different profiles of de novo mutations. While chromosome non-disjunctions are meiotic in origin and tend to be associated with maternal effects (such as Down syndrome), point mutations, small insertion-deletion (indels), microsatellite repeats and non-recurrent copy number variations (CNVs) originate as a result of mitotic copy-errors () and are typically associated with a paternal origin (2-5). As whole-genome sequencing of single sperm has recently been achieved (6), such techniques applied to the sperm of men of different ages should reveal the nature of the de novo mutational load carried by individual male germ cells. Recently, whole-genome sequencing based on parents and their offspring has confirmed that ~80% of de novo mutations are paternal in origin and that the total number of mutations strongly correlates with paternal age - an increase of about two point mutations per year, corresponding to a doubling of paternally-derived mutations every 16.5 years was reported (7).
Advanced paternal age and mental disorders
It was noted over 30 years ago that schizophrenia occurred more frequently in the offspring of older fathers (8). However this observation had been largely forgotten until Malaspina and colleagues (9) suggested that this finding may be related to copy-error mutations in the male germline. Based on a large Israeli birth cohort, they found that paternal age was a significant predictor of schizophrenia risk. A recently published meta-analysis of this research (10) suggested that the relationship between paternal age and risk of schizophrenia was J-shaped: although it confirmed the increased risk in the offspring of older fathers, it also identified a smaller risk increase in the offspring of very young fathers, suggesting that other factors,in addition to a simple age-related increase in copy-error mutations, are likely to mediate this effect.
There is a growing body of epidemiological research linking advanced paternal age with other neuropsychiatric disorders and brain-related outcomes – these include autism and related spectrum disorders (11-13), bipolar disorder (14), epilepsy (15), sporadic Alzheimer’s disease (16), obsessive compulsive disorder (17) and impaired childhood cognitive ability (18). Thus, while the evidence-base is incomplete, the data suggests that advanced paternal age is associated with a wide range of brain-related adverse health outcomes (i.e. the exposure is nonspecific with respect to health outcomes). Within a community-based sample of healthy children, paternal age has been found to be significantly associated with cortical gray (but not white) matter volume (19). Finally, evidence from mouse models shows that the offspring of older sires have altered behavioural outcome (20, 21), altered brain structure (20) and increased de novo CNVs (22).
More recently, studies have suggested that grandpaternal age (specifically on the mother’s line) may also contribute to the risk of neurodevelopmental disorders (23, 24). This may be understood when considering that phenotypes associated with predisposing mutations tend to be more pronounced in males than in females,, therefore effectively leading to the skipping of generations. This grandpaternal age-effect suggests that mutations can accumulate across generations. Although mutations with larger phenotypic impacts are less likely to be transmitted to subsequent generations (e.g. schizophrenia and autism have markedly reduced fertility and fecundity), mutations associated with more subtle phenotypes or variable penetrance may be transmitted to offspring, and thus contribute to the ‘mutational burden’ in subsequent generations (see below). In light of secular trends to delay parenthood (25), Crow suggested that paternal-age related mutations could contribute to a transgenerational ‘mutational time-bomb’ (1).
Paternal age-effect mutations and selfish selection in the testis
Although the differences in male versus female gamete production provide a plausible explanation for the link between advanced paternal age and disease risk, recent evidence suggests that there could be more to this association than the simple accumulation of random copy-errors during spermatogenesis. We have previously defined paternal age-effect mutations as a small group of well-characterised genetic alterations that are associated with rare Mendelian dominant disorders and present with unusual characteristics (5, 26). The best known examples involve specific activating point mutations in the fibroblast growth factor receptor genes, FGFR2 (associated with Apert and other craniosynostosis syndromes) and FGFR3 (associated with achondroplasia and other short-limbed bone dysplasias) and in several members of the RTK/RAS and associated MAPK (Mitogen Activated Protein Kinase) signalling pathways, such as PTPN11/SHP2 and HRAS (associated with neuro-cardio-facio-cutaneous syndromes, such as Noonan and Costello syndromes, collectively termed RASopathies) (5). In the majority of cases, these point mutations are (a) acquired de novo, (b) exhibit a near-exclusive paternal origin, (c) have a high apparent germline mutation rate (up to 1,000-fold above background for Apert syndrome and achondroplasia, based on birth prevalence), and (d) are associated epidemiologically with a significant paternal age-effect (with unaffected fathers approximately 2 to 7 years older on average, than the population mean). Because of these features, paternal age-effect mutations provide an excellent model to study the effect of advanced paternal age at a molecular level.
Direct quantification of the levels of specific pathogenic mutations associated with paternal age-effect disorders in the sperm of healthy men of different ages (26-28) and in dissected whole testes (29-31) has been performed. The results gathered from these technically challenging experiments showed that paternal age-effect mutations are detected in the sperm (but not in the blood) of most men; that the measured levels account for the birth prevalence of the associated disorders; and positively correlate with donor’s age in line with the observed paternal age-effect. To explain these findings, Goriely et al (5, 26, 27) suggested that rather than simply accumulating through repeated copy-errors, the originating mutational hits occur infrequently (i.e. at rates similar to the background of genomic mutations). However, these are progressively enriched because they provide a selective advantage to the mutant spermatogonial cells in which they arose, leading to their clonal expansion. This clonal growth of mutant spermatogonial cells, which is likely to take place in the testes of all men and may occasionally be associated with testicular tumors (26), leads to the relative enrichment of mutant sperm over time. This process accounts for the exclusive paternal origin of these mutations, the epidemiological paternal age-effect and the high prevalence of the associated disorders. To distinguish this mechanism from the neutral ‘copy-error’ process involving the accumulation of random mutational hits over time, this phenomenon has been termed selfish spermatogonial selection (5). For the sake of brevity, we refer to this mechanism as ‘selfish selection’, for the remainder of this review, but it is most important to remember that this process is occurring in the spermatogonial progenitors, not in the mature sperm.
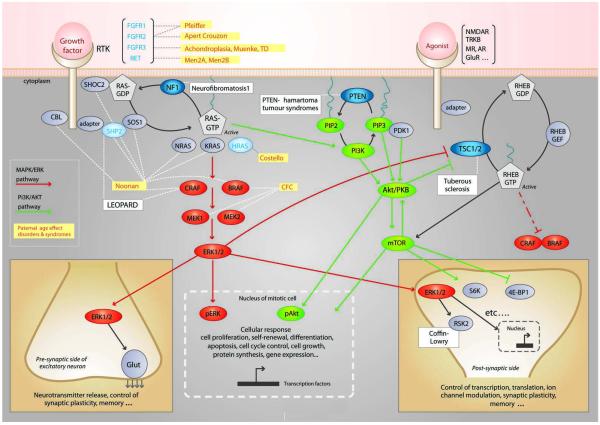
At a cellular level, selfish selection appears to operate through a mechanism that shares many of its features with oncogenesis. Indeed, all paternal age-effect genes are known oncogenes which are able to promote tumorigenesis in different cellular contexts (5). Strikingly, at a molecular level, there is a common denominator to selfish selection. Paternal age-effect genes cluster within a single pathway, the receptor tyrosine kinase (RTK)/RAS signaling cascade (Figure 1). Not surprisingly, this pathway is a key determinant of spermatogonial cell self-renewal and its role in controlling the balance of proliferation/differentiation during mammalian spermatogenesis has been well documented in murine models (5). This effect is mediated through control of several different transduction pathways downstream of RAS. The best characterised pathways include MAPK pathway (shown in red in Figure 1) for which ERK1/2 is a downstream effector, and the PI3K/AKT cascade for which mTOR is a downstream effector (in green in Figure 1). The RAS and RAS-related RHEB signaling pathways, that are commonly dysregulated in cancer, are widely connected with many essential cellular processes, and play a key role in neuronal function including synaptic plasticity (32, 33).
Figure 1.
Simplified overview of the receptor tyrosine kinase (RTK)-RAS and RAS-related RHEB signaling pathways. See (5) and 34 for more details on pathways. Germline disorders associated with mutations in specific genes along this pathway are indicated in boxes. The gene products that belong to the paternal age-effect class (as defined in text) are in blue and yellow boxes indicate related disorders. RASopathies include Noonan, Costello, LEOPARD and cardio-facio-cutaneous (CFC) syndromes and are caused by mutations in the RAS/MAPK/ERK pathway. Other proteins in the pathway and associated germline disorders for which evidence of direct involvement in the process of selfish spermatogonial selection/paternal age-effect is still lacking are indicated in black. Known tumor-suppressor genes in cancer are indicated by blue circles. The RAS pathway is involved in many cellular process and some of the consequences of pathway activation of are illustrated in the case of transduction occurring in a mitotically active cell (bottom, middle) or during neurotransmission and/or synaptic plasticity (bottom, left and right). Translocation of phosphorylated forms of ERK (pERK) or AKT (pAKT) into the nucleus of a mitotic cell triggers many different cellular responses such as cell growth, proliferation, differentiation, motility and apoptosis. Within excitatory neurons, a few examples of cellular responses triggered by the RAS orRHEB pathways are illustrated and involve molecules such as ribosomal S6 kinase (S6K and RSK2) and eukaryotic translation initiation factor 4E-binding protein 1 (4E-BP1).
Although this paradigm is based on a handful of paternal age-effect mutations of unusually large effect, it is likely that other selfish pathways operate in the context of the aging testis. For example, the signaling networks triggered by oncogenic activation of RAS are highly complex and involve crosstalk between multiple pathways (34). In particular we might expect other well-characterised oncogenic pathways such as PI3K/AKT and their effectors, which are key regulators of spermatogonial cell proliferation, to participate in this process. Thus, while this paper will focus on the canonical RAS-related pathways, we speculate that mutations in any gene that is expressed in spermatogonial cells and whose function involves regulation of homeostasis through control of proliferation/differentiation balance or cell cycle are potential targets of selfish selection (5). Similarly, selfish mutations are unlikely to be limited to point mutations with strong gain-of-function properties such as those described so far for paternal age-effect mutations; we predict that other genetic alterations such as CNVs, small indels, or regulatory mutations will also be targets of selfish selection.
Genetic architecture of neurocognitive disorders and selfish selection
While not all de novo mutations associated with neuropsychiatric disorders are related to paternal age (e.g. 22q11.2 deletions), we note with interest that there is overlap between the molecular pathways that have been identified during exome/CNV screens for schizophrenia and autism and those implicated in selfish selection in the testis. In their large study of CNV in autism, Pinto et al described a noticeable enrichment of rare pathogenic CNVs - both inherited and de novo - disrupting genes involved in cellular proliferation, projection and motility, and RAS signaling (35). Studies analysing proband-parents trios in autism identified de novo mutations in genes clustering within molecular pathways implicated in tumorigenesis and control of early development such as RAS, p53 and β-catenin pathways (35-39). There is also evidence suggesting that individuals diagnosed with RASopathies have more difficulties in adaptive functioning (40) and show autistic traits (41). Thus, within the fields of autism and schizophrenia research, there has been an unexpected overlap between (a) candidate pathogenic variants in genes that are being discovered through genetic studies and (b) genes involved in tumorigenesis as well as selfish selection in the testis.
With respect to genetic risk factors, researchers have previously noted that some candidate genes linked to schizophrenia are also mutated in various cancers. These include protein kinase B (PKB, also known as AKT), a gene commonly mutated in cancer, and the gene neuregulin-which is associated with breast cancer, B cell leukemia/lymphoma and multiple myeloma (42, 43). Moreover, genome-wide association studies (GWAS) in schizophrenia have identified strong signals near the paternal age-effect genes FGFR1 (44) and FGFR2 (45), and there is convergent evidence linking disruption of AKT (42) and fibroblast growth factors signaling with schizophrenia (46) as well as a link between cancer-related pathways (e.g. PI3K/AKT, PTEN) and autism (47).
Weak selfish mutations and common diseases
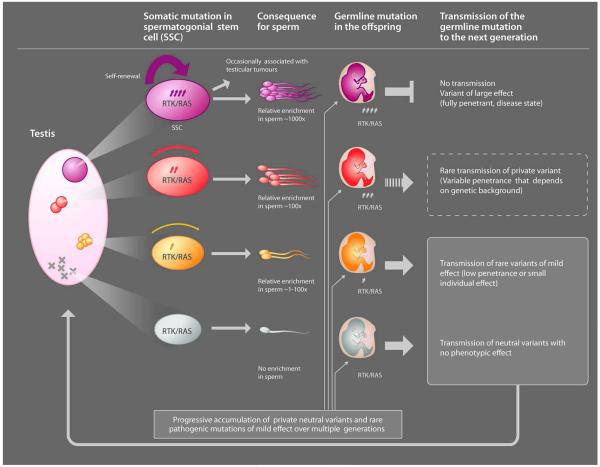
Although sperm studies of paternal age-effect mutations have highlighted that selfish selection relies on the activation of the RAS pathway, the role of RAS is clearly not limited to processes occurring in the testis. This pathway is a crucial mediator of intracellular signaling in many contexts and is recruited at different times in development to execute distinct functions including cell survival, proliferation, migration, differentiation and motility. Hence, dysregulation of the RAS pathway in the adult testis is predicted to have consequences that extend far beyond rare dominant disorders. To consider whether selfish selection could have a more pervasive role in complex disorders, we need to compare the effect of the strength of selfish selection occurring in the testis (that promotes clonal expansion and leads to relative enrichment in mutant sperm) and the impact that given mutations are anticipated to have on the fitness of the offspring who inherit the mutation upon germline transmission. Bearing in mind the strong pathogenic germline phenotypes they cause, paternal age-effect mutations - such as those commonly described in cancer and those associated with paternal age-effect disorders - will be associated with lethal/deleterious phenotypes that are unlikely to segregate in the population or have a long-term impact on disease burden (Figure 2 top, red mutations). However, selfish mutations providing a weaker selective advantage in the testes (that will be enriched to a lesser extent in sperm), are predicted to be associated with more subtle phenotypes (such as low/variable penetrance or susceptibility variants). Importantly, these mild selfish mutations are a potential source of heritable variations that could contribute to the genetic burden of common diseases (Figure 2; orange mutations) and to genetic heterogeneity (Figure 2; yellow mutations).
Figure 2.
Depiction of the process of selfish spermatogonial selection in the testis and its consequences for the offspring. The pink oval (left) represents the testis of an aging man in which mutations (represented by X or circles) have occurred randomly during the recurrent rounds of replication required for spermatogenesis. In gray (bottom of the diagram), functionally neutral mutations are not enriched in spermatogonial progenitors and are associated with a very low risk of transmission of individual genetic lesions in the offspring. In purple (top of the diagram), the mutant spermatogonial progenitor carries a ‘strong-effect’ mutation that is associated with overt dysregulation of the RTK/RAS pathway – such as a typical oncogenic mutation. These paternal age-effect mutations confer a strong selective advantage (represented by the thickness of the purple arrow) to the mutant spermatogonial stem cell, leading over time to the formation of large clones and relative enrichment in mutant sperm. Upon germline transmission to the offspring, strong paternal age-effect mutations can cause neonatal lethality or disease phenotypes and are eliminated by purifying selection as affected individuals are unlikely to reproduce. In red and orange (middle part of the diagram), are depicted intermediate scenarios for mutations with milder selective advantage (i.e. mutation of variable penetrance, weak gain-of-function) that are enriched over time in spermatogonial stem cells to a lesser extent (>1- to 100-fold). Unlike the strong effect mutations (purple), mildly pathogenic mutations are potentially transmissible over many generations, contributing to genetic heterogeneity and variable expressivity of disease phenotypes.
Does the selfish selection hypothesis have explanatory power?
Clues linking paternal age, cancer and neurodevelopmental disorders
There is some evidence (5) linking advanced paternal age with an increased risk of a range of cancer types (e.g. childhood brain tumors and leukemia, non-Hodgkin lymphoma, breast and testicular cancer). If advanced paternal age is associated with both risk of schizophrenia and cancer, then it might be predicted that cancer would be more common in schizophrenia patients. However, the epidemiological data on this issue are inconsistent (48-50), and the effects seem to vary depending on cancer types considered (51). On balance the studies find that the incidence of cancer in individuals with schizophrenia is lower than expected (52). Moreover, the decreased risk for several types of cancer has also been observed in unaffected family members of schizophrenic patients, providing further support for genetic protection against cancer in families with schizophrenia (48, 52, 53). Given the overlap between the molecular pathways involved in brain development and those associated with tumorigenesis, mutations that have been enriched in germ cells through mechanisms such as selfish selection are expected to modify the risk of both neurocognitive diseases and cancer when inherited by the offspring. As we learn more about the genetic basis of cancer and neuropsychiatric disorder, the precise relationship between these two broad categories of disorders may be clarified.
Clues related to minor physical anomalies, cell cycle and synaptic plasticity
The selfish selection hypothesis emerged from a group of congenital disorders that are often characterized by prominent craniofacial abnormalities (5). It has long been established that minor physical anomalies are more prevalent in schizophrenia (54) and autism (55).
Properties of cell lines such as those obtained from “neurosphere-derived” cells generated from olfactory mucosal biopsies from patients with schizophrenia and from healthy controls have been examined (56). The patient-derived cells showed significant dysregulation of genes involved in cell cycle control (e.g. in particular various cyclins and several CDK4 inhibitors controlling G1/S phase transition. Changes in expression of genes and/or proteins involved in cell cycle are of particular interest because increased cell proliferation has previously been demonstrated in olfactory biopsies from schizophrenia patients (57, 58). Induced pluripotent stem cells from individuals with LEOPARD syndrome (one of the RASopathies) have provided clues on the impact of these mutations on different derived cell types (59). It will be of interest to establish whether fibroblast-derived induced pluripotent stem (iPS) cells from individuals with neuropsychiatric disorders could be used to identify signaling pathways contributing to disease phenotype (60).
There is a growing body of evidence indicating that genes that were once thought to be involved only in cell cycle control also ‘moonlight’ in a range of critical functions in post-mitotic neurons (see review (61)). For example, cyclins have been implicated in axonal growth (62) and dendritic morphogenesis (63). Synaptic function is also being linked to genes better known for their role in cancer, cell cycle control and differentiation (64, 65) (Figure 1). These mechanisms may represent important clues for understanding the links between schizophrenia and cancer (66).
An integrative model and testable hypotheses
As mechanisms such as selfish selection operate in the aging human testis, it is anticipated that over the reproductive life of the male, mutations in germ cell progenitors that influence cell cycle/growth control pathways (in particular, within the RTK/RAS signaling cascade) will promote spermatogonial proliferation and result in relative enrichment of mutant sperm over time. Given that whole-genome sequencing data have confirmed that each newborn carries ~30-100 novel point mutations in their genome (7), this raises the possibility that a proportion of the paternally-derived mutations will preferentially carry alterations in genes that fall within these selfish pathways. These mutations would have variable consequences on phenotype and transmission to the next generation (Figure 2)
Conveniently, this hypothesis may provide insights into some more puzzling features of neurodevelopmental disorders, such as the nature of the ‘missing heritability’ associated with these genetic diseases (for more details see (67)). Selfish selection is predicted to promote a weak bias favouring fertilisation by sperm carrying mild pathogenic selfish alleles. Although this process occurs at each generation, the exact nature of the mutational hit involved is likely to be serendipitous and therefore is anticipated to be essentially unique (‘private’) and would only be identifiable during genome-wide unbiased sequencing screen (i.e. not during GWAS that only assess common variants). Thus this hypothesis aids formulation of a parsimonious framework to understand (a) the association between advanced paternal age and various neurocognitive disorders and some cancers, (b) the complex genetic architecture of neurodevelopmental disorders and the important role of de novo/private mutations clustering in key signaling pathways, (c) the high heritability, comorbidity and high prevalence of these deleterious conditions that are associated with low fertility, (d) the various clinical and epidemiological clues related to dysmorphogenesis, cell cycle properties and altered risk of comorbid cancer.
We readily acknowledge many limitations of this broad model. Not all neurodevelopmental disorders are caused by de novo mutations, nor do we propose that all genetic factors related to these disorders are restricted to RTK/RAS and related pathways. However, we hope that the selfish selection hypothesis will be useful as a catalyst for debate, data interpretation and new hypothesis-driven research. Below we list testable hypotheses based on this mechanism.
Whole-genome sequencing of family trios has demonstrated a significant excess of paternally-derived de novo mutations (7). Although, as a whole, the number of de novo mutations correlates with father’s age, selfish selection predicts that mutations dysregulating signaling pathways such RTK/RAS and their downstream effectors will be over-represented in the offspring of older fathers. The relationship between paternal age and de novo mutational load should therefore be examined independently for different categories of genetic alterations. Thus, the correlation between paternal age and de novo mutations in genes falling in selfish pathways will be greater than for neutral mutations.
Sequencing of multiple individual sperm from men of different ages will confirm the correlation between paternal age and excess of de novo mutations dysregulating selfish pathways such RTK/RAS.
Susceptibility variants for schizophrenia and autism risk will be significantly more likely to impact on pathways involved in cell cycle and RTK/RAS signaling cascade than would be predicted by chance.
Patient-derived cell lines (e.g. olfactory neurospheres, iPS cell lines) from patients with paternal-age related disorders such as schizophrenia or autism will be more likely to display phenotypic changes related to the RTK/RAS signaling pathway than would be predicted by chance.
Conclusions
So far, data have indicated that the RAS/MAPK pathway is a crucial mediator of selfish selection. As we learn more about the mechanisms controlling spermatogonial proliferation, the above hypotheses should expand beyond the canonical RTK/RAS cascade and include other signaling pathways that are potential target for selfish selection. Combining this knowledge with our understanding of the genetic networks involved in neurodevelopmental disorders should help define the extent of the overlap between the two processes. Ultimately, this will provide insights into a broader research question – are mutations associated with these complex disorders part of a ‘normal’ process that produces genetic variation with each generation and are therefore the unavoidable consequence of a selfish mechanism originating in the aging testis?
The selfish spermatogonial selection hypothesis provides an example of the creative intersection between diverse research fields – in this case, epidemiology, animal models, molecular and clinical genetics, genomics, oncology, cell biology and psychiatry. From a public health perspective, the impact of advanced paternal age on health outcomes may become more apparent over time, as parenthood is delayed in many societies as a result of educational and cultural factors (see review (25)). Because these mutations are proposed to accumulate over several generations, mechanisms related to selfish selection could have far-reaching consequences for the health of future generations.
Acknowledgements
The Wellcome Trust (091182) to AG and AOMW; the National Health and Medical Research Council, Australia (APP569528) to JJMcG; the US National Institute of Mental Health (RC1-MH088843; K24-5K24MH001699) to DM and the Swedish Research Council (2011-4659) to CH are acknowledged for financial support. We thank Dee McGrath for assistance with figures design.
Footnotes
Disclosures
AG, CMH, AOMW and DM report no conflicts of interest. JM has received research support from Eli Lilly and honorarium for educational talks from Astra Zeneca and Lundbeck.
References
- 1.Crow JF. The origins, patterns and implications of human spontaneous mutation. Nat Rev Genet. 2000;1(1):40–7. doi: 10.1038/35049558. [DOI] [PubMed] [Google Scholar]
- 2.Crow JF. A new study challenges the current belief of a high human male:female mutation ratio. Trends Genet. 2000;16(12):525–6. doi: 10.1016/s0168-9525(00)02136-3. [DOI] [PubMed] [Google Scholar]
- 3.Hehir-Kwa JY, Rodriguez-Santiago B, Vissers LE, de Leeuw N, Pfundt R, Buitelaar JK, Perez-Jurado LA, Veltman JA. De novo copy number variants associated with intellectual disability have a paternal origin and age bias. J Med Genet. 2011;48(11):776–8. doi: 10.1136/jmedgenet-2011-100147. [DOI] [PubMed] [Google Scholar]
- 4.Sun JX, Helgason A, Masson G, Ebenesersdottir SS, Li H, Mallick S, Gnerre S, Patterson N, Kong A, Reich D, Stefansson K. A direct characterization of human mutation based on microsatellites. Nat Genet. 2012;44(10):1161–5. doi: 10.1038/ng.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goriely A, Wilkie AOM. Paternal age effect mutations and selfish spermatogonial selection: causes and consequences for human disease. Am J Hum Genet. 2012;90(2):175–200. doi: 10.1016/j.ajhg.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang J, Fan HC, Behr B, Quake SR. Genome-wide Single-Cell Analysis of Recombination Activity and De Novo Mutation Rates in Human Sperm. Cell. 2012;150(2):402–12. doi: 10.1016/j.cell.2012.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kong A, Frigge ML, Masson G, Besenbacher S, Sulem P, Magnusson G, Gudjonsson SA, Sigurdsson A, Jonasdottir A, Wong WS, Sigurdsson G, Walters GB, Steinberg S, Helgason H, Thorleifsson G, Gudbjartsson DF, Helgason A, Magnusson OT, Thorsteinsdottir U, Stefansson K. Rate of de novo mutations and the importance of father’s age to disease risk. Nature. 2012;488(7412):471–5. doi: 10.1038/nature11396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hare EH, Moran PA. Raised parental age in psychiatric patients: evidence for the constitutional hypothesis. Br J Psychiatry. 1979;134:169–77. doi: 10.1192/bjp.134.2.169. [DOI] [PubMed] [Google Scholar]
- 9.Malaspina D, Harlap S, Fennig S, Heiman D, Nahon D, Feldman D, Susser ES. Advancing paternal age and the risk of schizophrenia. Arch Gen Psychiatry. 2001;58(4):361–7. doi: 10.1001/archpsyc.58.4.361. [DOI] [PubMed] [Google Scholar]
- 10.Miller B, Messias E, Miettunen J, Alaraisanen A, Jarvelin MR, Koponen H, Rasanen P, Isohanni M, Kirkpatrick B. Meta-analysis of Paternal Age and Schizophrenia Risk in Male Versus Female Offspring. Schizophr Bull. 2011;37(5):1039–47. doi: 10.1093/schbul/sbq011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reichenberg A, Gross R, Weiser M, Bresnahan M, Silverman J, Harlap S, Rabinowitz J, Shulman C, Malaspina D, Lubin G, Knobler HY, Davidson M, Susser E. Advancing paternal age and autism. Arch Gen Psychiatry. 2006;63(9):1026–32. doi: 10.1001/archpsyc.63.9.1026. [DOI] [PubMed] [Google Scholar]
- 12.Shelton JF, Tancredi DJ, Hertz-Picciotto I. Independent and dependent contributions of advanced maternal and paternal ages to autism risk. Autism Res. 2010;3(1):30–9. doi: 10.1002/aur.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hultman CM, Sandin S, Levine SZ, Lichtenstein P, Reichenberg A. Advancing paternal age and risk of autism: new evidence from a population-based study and a meta-analysis of epidemiological studies. Mol Psychiatry. 2011;16(12):1203–12. doi: 10.1038/mp.2010.121. [DOI] [PubMed] [Google Scholar]
- 14.Frans EM, Sandin S, Reichenberg A, Lichtenstein P, Langstrom N, Hultman CM. Advancing paternal age and bipolar disorder. Arch Gen Psychiatry. 2008;65(9):1034–40. doi: 10.1001/archpsyc.65.9.1034. [DOI] [PubMed] [Google Scholar]
- 15.Vestergaard M, Mork A, Madsen KM, Olsen J. Paternal age and epilepsy in the offspring. Eur J Epidemiol. 2005;20(12):1003–5. doi: 10.1007/s10654-005-4250-2. [DOI] [PubMed] [Google Scholar]
- 16.Bertram L, Busch R, Spiegl M, Lautenschlager NT, Muller U, Kurz A. Paternal age is a risk factor for Alzheimer disease in the absence of a major gene. Neurogenetics. 1998;1(4):277–80. doi: 10.1007/s100480050041. [DOI] [PubMed] [Google Scholar]
- 17.Wu Y, Liu X, Luo H, Deng W, Zhao G, Wang Q, Zhang L, Ma X, Murray RA, Collier DA, Li T. Advanced paternal age increases the risk of schizophrenia and obsessive-compulsive disorder in a Chinese Han population. Psychiatry Res. 2012;198(3):353–9. doi: 10.1016/j.psychres.2012.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saha S, Barnett A, Foldi C, Burns T, Eyles D, Buka S, McGrath J. Advanced Paternal Age Is Associated With Impaired Neurocognitive Outcomes during Infancy and Childhood. PLoS Med. 2009;6(3):e10000040. doi: 10.1371/journal.pmed.1000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaw P, Gilliam M, Malek M, Rodriguez N, Greenstein D, Clasen L, Evans A, Rapoport J, Giedd J. Parental age effects on cortical morphology in offspring. Cereb Cortex. 2012;22(6):1256–62. doi: 10.1093/cercor/bhr194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foldi CJ, Eyles DW, McGrath JJ, Burne TH. Advanced paternal age is associated with alterations in discrete behavioural domains and cortical neuroanatomy of C57BL/6J mice. Eur J Neurosci. 2010;31(3):556–64. doi: 10.1111/j.1460-9568.2010.07074.x. [DOI] [PubMed] [Google Scholar]
- 21.Smith RG, Kember RL, Mill J, Fernandes C, Schalkwyk LC, Buxbaum JD, Reichenberg A. Advancing paternal age is associated with deficits in social and exploratory behaviors in the offspring: a mouse model. PLoS One. 2009;4(12):e8456. doi: 10.1371/journal.pone.0008456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flatscher-Bader T, Foldi CJ, Chong S, Whitelaw E, Moser RJ, Burne THJ, Eyles DW, McGrath JJ. Increased de novo copy number variants in the offspring of older males. Translational Psychiatry. 2011;1(e34) doi: 10.1038/tp.2011.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frans EM, McGrath JJ, Sandin S, Lichtenstein P, Reichenberg A, Langstrom N, Hultman CM. Advanced paternal and grandpaternal age and schizophrenia: a three-generation perspective. Schizophr Res. 2011;133(1-3):120–4. doi: 10.1016/j.schres.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frans E, Sandin S, Reichenberg A, Langstrom N, Lictenstein P, McGrath J, Hultman C. Autism Risk Develops Across Generations: A Population Based Study of Advancing Grandpaternal and Paternal Age. JAMA Psychiatry. 2013 doi: 10.1001/jamapsychiatry.2013.1180. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bray I, Gunnell D, Davey Smith G. Advanced paternal age: how old is too old? J Epidemiol Community Health. 2006;60(10):851–3. doi: 10.1136/jech.2005.045179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goriely A, Hansen RM, Taylor IB, Olesen IA, Jacobsen GK, McGowan SJ, Pfeifer SP, McVean GA, Rajpert-De Meyts E, Wilkie AOM. Activating mutations in FGFR3 and HRAS reveal a shared genetic origin for congenital disorders and testicular tumors. Nat Genet. 2009;41(11):1247–52. doi: 10.1038/ng.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goriely A, McVean GA, Rojmyr M, Ingemarsson B, Wilkie AOM. Evidence for selective advantage of pathogenic FGFR2 mutations in the male germ line. Science. 2003;301(5633):643–6. doi: 10.1126/science.1085710. [DOI] [PubMed] [Google Scholar]
- 28.Yoon SR, Qin J, Glaser RL, Jabs EW, Wexler NS, Sokol R, Arnheim N, Calabrese P. The ups and downs of mutation frequencies during aging can account for the Apert syndrome paternal age effect. PLoS Genet. 2009;5(7):e1000558. doi: 10.1371/journal.pgen.1000558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qin J, Calabrese P, Tiemann-Boege I, Shinde DN, Yoon SR, Gelfand D, Bauer K, Arnheim N. The molecular anatomy of spontaneous germline mutations in human testes. PLoS Biol. 2007;5(9):e224. doi: 10.1371/journal.pbio.0050224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi SK, Yoon SR, Calabrese P, Arnheim N. A germ-line-selective advantage rather than an increased mutation rate can explain some unexpectedly common human disease mutations. Proc Natl Acad Sci U S A. 2008;105(29):10143–8. doi: 10.1073/pnas.0801267105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi SK, Yoon SR, Calabrese P, Arnheim N. Positive selection for new disease mutations in the human germline: evidence from the heritable cancer syndrome multiple endocrine neoplasia type 2B. PLoS Genet. 2012;8(2):e1002420. doi: 10.1371/journal.pgen.1002420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Samuels IS, Saitta SC, Landreth GE. MAP’ing CNS development and cognition: an ERKsome process. Neuron. 2009;61(2):160–7. doi: 10.1016/j.neuron.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wiegert JS, Bading H. Activity-dependent calcium signaling and ERK-MAP kinases in neurons: a link to structural plasticity of the nucleus and gene transcription regulation. Cell Calcium. 2011;49(5):296–305. doi: 10.1016/j.ceca.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 34.Schubbert S, Shannon K, Bollag G. Hyperactive Ras in developmental disorders and cancer. Nat Rev Cancer. 2007;7(4):295–308. doi: 10.1038/nrc2109. [DOI] [PubMed] [Google Scholar]
- 35.Pinto D, Pagnamenta AT, Klei L, Anney R, Merico D, Regan R, Conroy J, Magalhaes TR, Correia C, Abrahams BS, Almeida J, Bacchelli E, Bader GD, Bailey AJ, Baird G, Battaglia A, Berney T, Bolshakova N, Bolte S, Bolton PF, Bourgeron T, Brennan S, Brian J, Bryson SE, Carson AR, Casallo G, Casey J, Chung BH, Cochrane L, Corsello C, Crawford EL, Crossett A, Cytrynbaum C, Dawson G, de Jonge M, Delorme R, Drmic I, Duketis E, Duque F, Estes A, Farrar P, Fernandez BA, Folstein SE, Fombonne E, Freitag CM, Gilbert J, Gillberg C, Glessner JT, Goldberg J, Green A, Green J, Guter SJ, Hakonarson H, Heron EA, Hill M, Holt R, Howe JL, Hughes G, Hus V, Igliozzi R, Kim C, Klauck SM, Kolevzon A, Korvatska O, Kustanovich V, Lajonchere CM, Lamb JA, Laskawiec M, Leboyer M, Le Couteur A, Leventhal BL, Lionel AC, Liu XQ, Lord C, Lotspeich L, Lund SC, Maestrini E, Mahoney W, Mantoulan C, Marshall CR, McConachie H, McDougle CJ, McGrath J, McMahon WM, Merikangas A, Migita O, Minshew NJ, Mirza GK, Munson J, Nelson SF, Noakes C, Noor A, Nygren G, Oliveira G, Papanikolaou K, Parr JR, Parrini B, Paton T, Pickles A, Pilorge M, Piven J, Ponting CP, Posey DJ, Poustka A, Poustka F, Prasad A, Ragoussis J, Renshaw K, Rickaby J, Roberts W, Roeder K, Roge B, Rutter ML, Bierut LJ, Rice JP, Salt J, Sansom K, Sato D, Segurado R, Sequeira AF, Senman L, Shah N, Sheffield VC, Soorya L, Sousa I, Stein O, Sykes N, Stoppioni V, Strawbridge C, Tancredi R, Tansey K, Thiruvahindrapduram B, Thompson AP, Thomson S, Tryfon A, Tsiantis J, Van Engeland H, Vincent JB, Volkmar F, Wallace S, Wang K, Wang Z, Wassink TH, Webber C, Weksberg R, Wing K, Wittemeyer K, Wood S, Wu J, Yaspan BL, Zurawiecki D, Zwaigenbaum L, Buxbaum JD, Cantor RM, Cook EH, Coon H, Cuccaro ML, Devlin B, Ennis S, Gallagher L, Geschwind DH, Gill M, Haines JL, Hallmayer J, Miller J, Monaco AP, Nurnberger JI, Jr., Paterson AD, Pericak-Vance MA, Schellenberg GD, Szatmari P, Vicente AM, Vieland VJ, Wijsman EM, Scherer SW, Sutcliffe JS, Betancur C. Functional impact of global rare copy number variation in autism spectrum disorders. Nature. 2010;466(7304):368–72. doi: 10.1038/nature09146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Dushlaine C, Kenny E, Heron E, Donohoe G, Gill M, Morris D, Corvin A. Molecular pathways involved in neuronal cell adhesion and membrane scaffolding contribute to schizophrenia and bipolar disorder susceptibility. Mol Psychiatry. 2011;16(3):286–92. doi: 10.1038/mp.2010.7. [DOI] [PubMed] [Google Scholar]
- 37.O’Roak BJ, Vives L, Girirajan S, Karakoc E, Krumm N, Coe BP, Levy R, Ko A, Lee C, Smith JD, Turner EH, Stanaway IB, Vernot B, Malig M, Baker C, Reilly B, Akey JM, Borenstein E, Rieder MJ, Nickerson DA, Bernier R, Shendure J, Eichler EE. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature. 2012;485(7397):246–50. doi: 10.1038/nature10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kirov G, Pocklington AJ, Holmans P, Ivanov D, Ikeda M, Ruderfer D, Moran J, Chambert K, Toncheva D, Georgieva L, Grozeva D, Fjodorova M, Wollerton R, Rees E, Nikolov I, Lagemaat LN, Bayes A, Fernandez E, Olason PI, Bottcher Y, Komiyama NH, Collins MO, Choudhary J, Stefansson K, Stefansson H, Grant SG, Purcell S, Sklar P, O’Donovan MC, Owen MJ. De novo CNV analysis implicates specific abnormalities of postsynaptic signalling complexes in the pathogenesis of schizophrenia. Mol Psychiatry. 2012;17(2):142–53. doi: 10.1038/mp.2011.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levy D, Ronemus M, Yamrom B, Lee YH, Leotta A, Kendall J, Marks S, Lakshmi B, Pai D, Ye K, Buja A, Krieger A, Yoon S, Troge J, Rodgers L, Iossifov I, Wigler M. Rare de novo and transmitted copy-number variation in autistic spectrum disorders. Neuron. 2011;70(5):886–97. doi: 10.1016/j.neuron.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 40.Pierpont EI, Pierpont ME, Mendelsohn NJ, Roberts AE, Tworog-Dube E, Rauen KA, Seidenberg MS. Effects of germline mutations in the Ras/MAPK signaling pathway on adaptive behavior: cardiofaciocutaneous syndrome and Noonan syndrome. Am J Med Genet A. 2010;152A(3):591–600. doi: 10.1002/ajmg.a.33268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Corbin I, Desachy G, Rauen K, Weiss L. Autism Traits in the RASopathies (Program Number 99); 62nd Annual Meeting of The American Society of Human Genetics; San Francisco, California. 2012. [Google Scholar]
- 42.Emamian ES. AKT/GSK3 signaling pathway and schizophrenia. Front Mol Neurosci. 2012;5:33. doi: 10.3389/fnmol.2012.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Britsch S. The neuregulin-I/ErbB signaling system in development and disease. Adv Anat Embryol Cell Biol. 2007;190:1–65. [PubMed] [Google Scholar]
- 44.Shi Y, Li Z, Xu Q, Wang T, Li T, Shen J, Zhang F, Chen J, Zhou G, Ji W, Li B, Xu Y, Liu D, Wang P, Yang P, Liu B, Sun W, Wan C, Qin S, He G, Steinberg S, Cichon S, Werge T, Sigurdsson E, Tosato S, Palotie A, Nothen MM, Rietschel M, Ophoff RA, Collier DA, Rujescu D, Clair DS, Stefansson H, Stefansson K, Ji J, Wang Q, Li W, Zheng L, Zhang H, Feng G, He L. Common variants on 8p12 and 1q24.2 confer risk of schizophrenia. Nat Genet. 2011;43(12):1224–7. doi: 10.1038/ng.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O’Donovan MC, Norton N, Williams H, Peirce T, Moskvina V, Nikolov I, Hamshere M, Carroll L, Georgieva L, Dwyer S, Holmans P, Marchini JL, Spencer CC, Howie B, Leung HT, Giegling I, Hartmann AM, Moller HJ, Morris DW, Shi Y, Feng G, Hoffmann P, Propping P, Vasilescu C, Maier W, Rietschel M, Zammit S, Schumacher J, Quinn EM, Schulze TG, Iwata N, Ikeda M, Darvasi A, Shifman S, He L, Duan J, Sanders AR, Levinson DF, Adolfsson R, Osby U, Terenius L, Jonsson EG, Cichon S, Nothen MM, Gill M, Corvin AP, Rujescu D, Gejman PV, Kirov G, Craddock N, Williams NM, Owen MJ. Analysis of 10 independent samples provides evidence for association between schizophrenia and a SNP flanking fibroblast growth factor receptor 2. Mol Psychiatry. 2009;14(1):30–6. doi: 10.1038/mp.2008.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Terwisscha van Scheltinga AF, Bakker SC, Kahn RS. Fibroblast growth factors in schizophrenia. Schizophr Bull. 2010;36(6):1157–66. doi: 10.1093/schbul/sbp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crespi B. Autism and cancer risk. Autism Res. 2011;4(4):302–10. doi: 10.1002/aur.208. [DOI] [PubMed] [Google Scholar]
- 48.Lichtermann D, Ekelund J, Pukkala E, Tanskanen A, Lonnqvist J. Incidence of cancer among persons with schizophrenia and their relatives. Arch Gen Psychiatry. 2001;58(6):573–8. doi: 10.1001/archpsyc.58.6.573. [DOI] [PubMed] [Google Scholar]
- 49.Bushe CJ, Bradley AJ, Wildgust HJ, Hodgson RE. Schizophrenia and breast cancer incidence: a systematic review of clinical studies. Schizophr Res. 2009;114(1-3):6–16. doi: 10.1016/j.schres.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 50.Guan NC, Termorshuizen F, Laan W, Smeets HM, Zainal NZ, Kahn RS, De Wit NJ, Boks MP. Cancer mortality in patients with psychiatric diagnoses: a higher hazard of cancer death does not lead to a higher cumulative risk of dying from cancer. Soc Psychiatry Psychiatr Epidemiol. 2012 doi: 10.1007/s00127-012-0612-8. [DOI] [PubMed] [Google Scholar]
- 51.Tabares-Seisdedos R, Dumont N, Baudot A, Valderas JM, Climent J, Valencia A, Crespo-Facorro B, Vieta E, Gomez-Beneyto M, Martinez S, Rubenstein JL. No paradox, no progress: inverse cancer comorbidity in people with other complex diseases. Lancet Oncol. 2011;12(6):604–8. doi: 10.1016/S1470-2045(11)70041-9. [DOI] [PubMed] [Google Scholar]
- 52.Catts VS, Catts SV, O’Toole BI, Frost AD. Cancer incidence in patients with schizophrenia and their first-degree relatives - a meta-analysis. Acta Psychiatr Scand. 2008;117(5):323–36. doi: 10.1111/j.1600-0447.2008.01163.x. [DOI] [PubMed] [Google Scholar]
- 53.Ji J, Sundquist K, Ning Y, Kendler KS, Sundquist J, Chen X. Incidence of Cancer in Patients With Schizophrenia and Their First-Degree Relatives: A Population-Based Study in Sweden. Schizophr Bull. 2012 doi: 10.1093/schbul/sbs065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Compton MT, Walker EF. Physical manifestations of neurodevelopmental disruption: are minor physical anomalies part of the syndrome of schizophrenia? Schizophr Bull. 2009;35(2):425–36. doi: 10.1093/schbul/sbn151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Angkustsiri K, Krakowiak P, Moghaddam B, Wardinsky T, Gardner J, Kalamkarian N. Hertz-Picciotto I, Hansen RL. Minor physical anomalies in children with autism spectrum disorders. Autism. 2011;15(6):746–60. doi: 10.1177/1362361310397620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matigian N, Abrahamsen G, Sutharsan R, Cook AL, Vitale AM, Nouwens A, Bellette B, An J, Anderson M, Beckhouse AG, Bennebroek M, Cecil R, Chalk AM, Cochrane J, Fan Y, Feron F, McCurdy R, McGrath JJ, Murrell W, Perry C, Raju J, Ravishankar S, Silburn PA, Sutherland GT, Mahler S, Mellick GD, Wood SA, Sue CM, Wells CA, Mackay-Sim A. Disease-specific, neurosphere-derived cells as models for brain disorders. Dis Model Mech. 2010;3(11-12):785–98. doi: 10.1242/dmm.005447. [DOI] [PubMed] [Google Scholar]
- 57.Feron F, Perry C, Hirning MH, McGrath J, Mackay-Sim A. Altered adhesion, proliferation and death in neural cultures from adults with schizophrenia. Schizophr Res. 1999;40(3):211–8. doi: 10.1016/s0920-9964(99)00055-9. [DOI] [PubMed] [Google Scholar]
- 58.McCurdy RD, Feron F, Perry C, Chant DC, McLean D, Matigian N, Hayward NK, McGrath JJ, Mackay-Sim A. Cell cycle alterations in biopsied olfactory neuroepithelium in schizophrenia and bipolar I disorder using cell culture and gene expression analyses. Schizophr Res. 2006;82(2-3):163–73. doi: 10.1016/j.schres.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 59.Carvajal-Vergara X, Sevilla A, D’Souza SL, Ang YS, Schaniel C, Lee DF, Yang L, Kaplan AD, Adler ED, Rozov R, Ge Y, Cohen N, Edelmann LJ, Chang B, Waghray A, Su J, Pardo S, Lichtenbelt KD, Tartaglia M, Gelb BD, Lemischka IR. Patient-specific induced pluripotent stem-cell-derived models of LEOPARD syndrome. Nature. 2010;465(7299):808–12. doi: 10.1038/nature09005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brennand KJ, Gage FH. Modeling psychiatric disorders through reprogramming. Dis Model Mech. 2012;5(1):26–32. doi: 10.1242/dmm.008268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Frank CL, Tsai LH. Alternative functions of core cell cycle regulators in neuronal migration, neuronal maturation, and synaptic plasticity. Neuron. 2009;62(3):312–26. doi: 10.1016/j.neuron.2009.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lasorella A, Stegmuller J, Guardavaccaro D, Liu G, Carro MS, Rothschild G, de la Torre-Ubieta L, Pagano M, Bonni A, Iavarone A. Degradation of Id2 by the anaphase-promoting complex couples cell cycle exit and axonal growth. Nature. 2006;442(7101):471–4. doi: 10.1038/nature04895. [DOI] [PubMed] [Google Scholar]
- 63.Kim AH, Puram SV, Bilimoria PM, Ikeuchi Y, Keough S, Wong M, Rowitch D, Bonni A. A centrosomal Cdc20-APC pathway controls dendrite morphogenesis in postmitotic neurons. Cell. 2009;136(2):322–36. doi: 10.1016/j.cell.2008.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Knafo S, Esteban JA. Common pathways for growth and for plasticity. Curr Opin Neurobiol. 2012;22(3):405–11. doi: 10.1016/j.conb.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 65.Huang YS, Jung MY, Sarkissian M, Richter JD. N-methyl-D-aspartate receptor signaling results in Aurora kinase-catalyzed CPEB phosphorylation and alpha CaMKII mRNA polyadenylation at synapses. EMBO J. 2002;21(9):2139–48. doi: 10.1093/emboj/21.9.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Y, He G, He L, McGrath J. Do shared mechanisms underlying cell cycle regulation and synaptic plasticity underlie the reduced incidence of cancer in schizophrenia? Schizophr Res. 2011;130(1-3):282–4. doi: 10.1016/j.schres.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 67.Goriely A, Wilkie AOM. Missing heritability: paternal age effect mutations and selfish spermatogonia. Nat Rev Genet. 2010;11(8):589. doi: 10.1038/nrg2809-c1. [DOI] [PubMed] [Google Scholar]