Abstract
The use of antimalarial drugs in India has evolved since the introduction of quinine in the 17th century. Since the formal establishment of a malaria control programme in 1953, shortly after independence, treatments provided by the public sector ranged from chloroquine, the mainstay drug for many decades, to the newer, recently introduced artemisinin based combination therapy. The complexity of considerations in antimalarial treatment led to the formulation of a National Antimalarial Drug Policy to guide procurement as well as communicate best practices to both public and private healthcare providers. Challenges addressed in the policy include the use of presumptive treatment, the introduction of alternate treatments for drug-resistant malaria, the duration of primaquine therapy to prevent relapses of vivax malaria, the treatment of malaria in pregnancy, and the choice of drugs for chemoprophylaxis. While data on antimalarial drug resistance and both public and private sector treatment practices have been recently reviewed, the policy process of setting national standards has not. In this perspective on antimalarial drug policy, this review highlights its relevant history, analyzes the current policy, and examines future directions.
Keywords: Antimalarial, drug, India, malaria, treatment, policy
Introduction
Intensive eradication efforts against malaria led to its near elimination in the mid - 1960s. That was a golden period of motivated men and women, a powerful insecticide and susceptible vectors, and the wonder drug chloroquine whose safety and efficacy against both Plasmodium falciparum and P. vivax formed the mainstay of antimalarial treatment. The success could not last. Reported malaria cases in India peaked in 1976 and although the overall incidence decreased, the incidence of P. falciparum has remained stable1. The emergence and spread of antimalarial drug resistance in P. falciparum was a key contributor to this trend. The management of antimalarial drug resistance by control programmes consists of three primary activities: (i) reduce drug pressure, primarily through rational use, to prevent the emergence and subsequent spread of drug resistance, (ii) monitor the efficacy of current drug and future drugs under consideration, and (iii) create a robust pipeline, from research and development to regulatory registration, to ensure alternatives drugs in the future. Recently, Shah et al2 systematically reviewed data from the monitoring of antimalarial drug resistance in India during 1978-2007. However, the policy components related to all three activities are not well described. We discuss the evolution of antimalarial drug policy with the aim of evaluating the trends in policy changes.
Past: historical perspectives
There was no organized programme for malaria control in India in the pre-independence era; but there are records of epidemics and their control by the then Indian Medical Service. In 1912, a special malaria department was created in Mumbai (then Bombay). The department, apart from various surveillance and vector control activities, also distributed quinine and Cinchona febrifuge free of cost3. Large epidemics, and their classic investigations, were reported from Punjab, Bombay, and Bengal4. Quinine was the treatment of choice for malaria and distribution measures for prophylaxis and treatment existed in several areas5. In 1917, the Bengal Nagpur Railway and the East India Railways formed separate malaria control organizations for controlling malaria in and around stations. Similar programmes were undertaken in tea plantations of Assam and in Mysore by the Rockefeller Foundation6.
The first organized national programme in health - the National Malaria Control Programme was launched in 1953. In view of its initial successes, it was rechristened the National Malaria Eradication Programme (NMEP) in 1958 and developed organized surveillance for active case detection and treatment in 19611. A single dose of any 4-aminoquinoline was recommended as the presumptive treatment to all fever cases, while 8-aminoquinoline was added as the radical treatment to achieve gametocytocidal cure in falciparum and hypnozoiticidal cure in vivax malaria. By 1965, only 99,667 malaria cases were reported2, but the situation deteriorated in subsequent years in the face of administrative, political, and technical challenges (Fig. 1). Hence, the Modified Plan of Operations was introduced in 1977 which emphasized the reduction of disease burden in a cost-effective and integrated manner. Fever treatment depots (FTDs), which obtained blood smears prior to presumptive treating, and drug distribution centres (DDCs), which did not, were established at the village level to ensure the availability of antimalarials in remote and inaccessible areas1. Chloroquine resistant P. falciparum malaria was first reported in 1973 from the State of Assam in the northeast of the nation7. Under the modified plan, the emphasis on chemotherapy was also supported by measures to strengthen operational research by mapping areas with chloroquine resistant strains. In 1978, NMEP created six regional monitoring teams to routinely conduct therapeutic efficacy studies of antimalarials drugs which expanded to 13 teams by 19851.
Fig. 1.
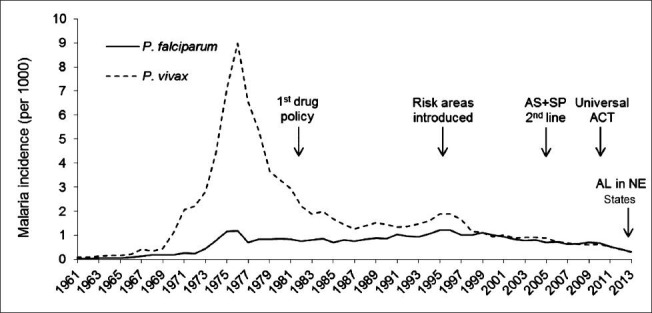
Reported malaria incidence and the evolution of the National Drug Policy for malaria in India, 1961-2013. AS, artesunate; SP, sulphadoxine-pyrimethamine; ACT, artemisinin based combination therapy; AL, artemether lumefantrine; NE, North-East.
Source: National Vector Borne Disease Control Programme, Delhi.
Under the eradication era: During the early days of the malaria programme in the 1950s-1970s the reduction of transmission occurred through vector control, primarily indoor residual spray operations. Case detection was geared towards identifying foci of transmission and not providing health care per se. The treatment aspect of eradication work sought to reduce morbidity among detected cases with little emphasis on radical cure until the latter maintenance phase of the programme as re-infection was though likely. No formal drug policies existed but the treatment en vogue was a 4-aminoquinoline (chloroquine or amodiaquine 10 mg/kg single-dose) for presumptive therapy with the addition of five days of primaquine (0.25 mg/kg for five days) regardless of the species present. For mass treatment in special situations, such as temporary labour camps, pyrimethamine (50 mg adult dose) was added for its sporontocidal action1.
First antimalarial drug policy: 1982: The first antimalarial drug policy was drafted in 1982 following the initial report of chloroquine resistance7 and the documentation of its presence in other States8,9,10,11. The policy recommended different regimens for different areas depending on the species prevalent and the chloroquine resistance status. Areas were designated as chloroquine-resistant based on the proportion of RIII cases (early treatment failure) found during sensitivity studies. In chloroquine sensitive areas, presumptive treatment was recommended in the form of single dose of chloroquine (10 mg/kg) for malaria cases detected by active case detection (ACD), DDCs, and FTDs. After confirmation of the diagnosis by microscopy, radical treatment in the form of single dose primaquine (0.75 mg/kg) was recommended for falciparum malaria with the use of sulphalene-pyrimethamine (SLP) (adult single dose 1000/50 mg) in cases where the patient did not respond to chloroquine. In chloroquine resistant areas, amodiaquine (10 mg/kg single dose) was recommended for presumptive treatment in patients detected through ACD, DDCs and FTDs while patients detected through passive case detection (PCD) were presumptively treated with SLP. In migrant labour, a single dose of primaquine would be added during presumptive treatment. Radical treatment for falciparum malaria was SLP plus single dose of primaquine12. In all areas the radical treatment for vivax malaria was chloroquine (10 mg/kg) and primaquine (0.25 mg/kg for five days). The five day regimen of primaquine was developed by the NMEP for its operational ease and reduced toxicity compared to the 14 days course and early reports of its comparable efficacy. The Table summarizes the revisions in the National Drug Policy for malaria in India.
Table.
Summary of different revisions of the National Drug Policy for malaria in India, 1950s-Present
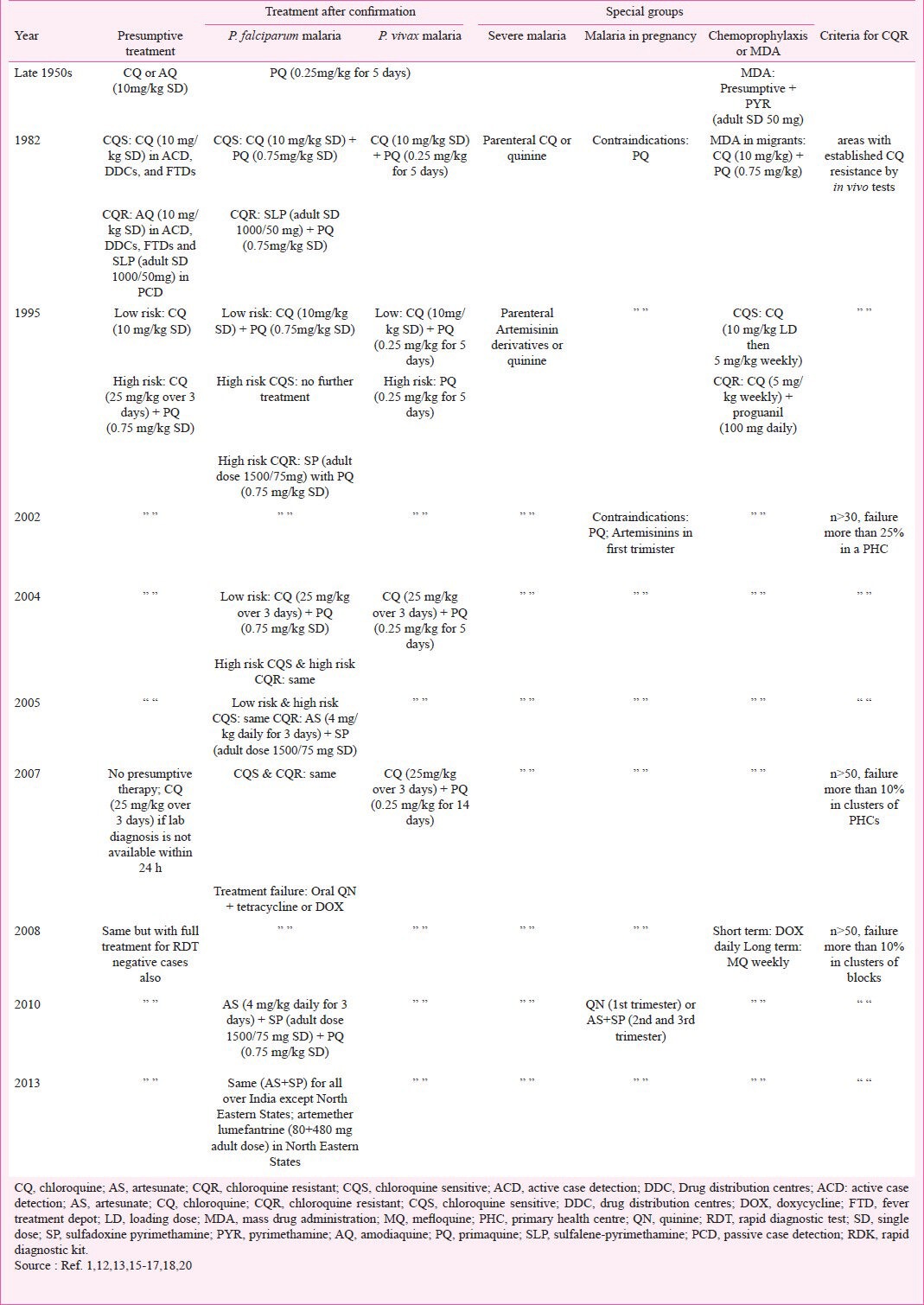
Modified presumptive treatment: 1995: The number of reported malaria cases dropped from 2.2 million in 1982 to 1.6 million in 1987 but again increased to 3 million by 19951. In light of several large epidemics of malaria with substantial mortality, the policy underwent a major revision in 19951. The NMEP stratified primary health centres (PHCs) into high and low risk areas based on the proportion of falciparum malaria cases, focus of chloroquine resistance in P. falciparum, slide positivity rate, and recorded malaria deaths. In low risk areas, presumptive and radical treatment and primaquine continued as recommended in the earlier policy1. In high risk areas, the full dose of chloroquine (25 mg/kg over three days) as opposed to the single dose of chloroquine (10 mg/kg), along with single dose of primaquine was recommended as radical treatment for all fever cases. Additional primaquine (0.25 mg/kg for five days) was provided for all confirmed vivax malaria cases. In chloroquine-resistant areas, a single dose of sulphalene/sulphadoxine-pyrimethamine (SP) (adult single dose 1500/75 mg) was recommended for the treatment of falciparum malaria. The SP dose was increased from the two-tablet adult dose (1000/50 mg) recommended earlier to the three tablet adult dose (1500/75 mg) after studies suggesting higher efficacy of the latter. Amodiaquine was withdrawn from the drug policy since it possessed no advantage over chloroquine due to cross-resistance and was considered more toxic1. The World Health Organization (WHO) also recommended the withdrawal of amodiaquine at the time because of reported side effects14. The policy also approved the use of mefloquine in the country but only by a registered medical practitioner in cases of confirmed P. falciparum with ring stages and in chloroquine resistance areas. Finally, a review of the national drug policy was recommended every two years to keep up with the complex scenario and changing patterns in the country.
The stable millennium years: In 1998, the NMEP became the National Anti-Malaria Programme (NAMP) acknowledging the change of emphasis in the goals of control efforts. The 2001 review of the drug policy continued the recommendations of 1995 policy13. The criteria for the designation of chloroquine-resistant areas, more than 25 per cent treatment failure (RI-RIII) in at least 30 patients of one PHC, were stated in the policy. In 2003, NAMP acquired additional responsibilities and emerged as the National Vector Borne Disease Control Programme (NVBDCP). In 2003, the short follow up (7 day) drug resistance studies were also ended15.
Artemisinin combination therapy (ACT) and treatment after confirmation: 2005-2013: The WHO technical advisory group, while meeting in India in 2004, recommended the use of combination antimalarial therapy, particularly with artemisinin derivatives, in member countries for treating P. falciparum to delay the emergence of drug resistance. Artemisinin combination therapy (ACT) consists of an artemisinin derivative combined with a long acting partner antimalarial drug. In the 2005 drug policy, in light of SP monotherapy resistance and WHO recommendations, artesunate (AS) + SP replaced SP alone in the national drug policy for the treatment of confirmed falciparum malaria cases in chloroquine resistant areas in 200515. Injection artemisinin was to be restricted to severe malaria cases only but oral artemisinin could be used in cases which were resistant to chloroquine and SP. The use of artemisinin related compounds was not recommended in infants.
In 2007, several major changes occurred in the malaria drug policy. First, presumptive treatment, that is single dose chloroquine, was no longer recommended and the use of clinical diagnosis alone was rejected. The policy recommended investigating all suspected malaria cases by microscopy or with rapid diagnostic kits (RDK)16. In situations where diagnosis was not possible or the delay would be great, clinical treatment should use the full-dose, three days, of chloroquine until diagnosis was obtained. Second, the cut-off for designating an area as chloroquine-resistant was now only 10 per cent treatment failure given the recognition of the rapid spread of drug resistance as well as new cost-effectiveness analysis. Furthermore, clusters of PHCs, with a high (>30%) proportion of falciparum cases, around the resistant focus became the unit used for adopting second-line drug. Third, the anti-relapse treatment for P. vivax was extended to 14 days of therapy after definitive studies demonstrating the poor efficacy of the five day course. Other notable points were for cases in whom chloroquine and AS+SP failed, oral quinine plus tetracycline or doxycycline would be used. The policy also dictated the disuse of single dose of primaquine along with AS+SP given that artesunate itself reduces gametocyte carriage.
Another revision in 2008 added the treatment of patients negative by RDK with full-dose chloroquine as the NVBDCP kits are monovalent and only detect P. falciparum17. The policy expanded the use of AS+SP to 117 districts across India which represented more than 90 per cent of the reported P. falciparum burden. The policy also recommended avoiding the use of mefloquine alone or in combination with artesunate in cerebral malaria. A flow diagram of the case management process was included for the first time to facilitate interpretation of the policy. Therapeutic efficacy studies continued to demonstrate a high prevalence of chloroquine resistance in falciparum malaria2,19. In 2010, the drug policy was further reviewed and revised with the use of AS+SP for treating falciparum malaria cases made universal all across the country18. For the first time the sulpha component of SP was specified as sulphadoxine instead of sulphalene/sulphadoxine. Single-dose primaquine was added to AS+SP, on day two, to reduce gametocyte carriage post-treatment since artesunate only acts against the immature forms.
In 2013, there was another policy change in the seven North Eastern States (Arunachal Pradesh, Assam, Manipur, Meghalaya, Mizoram, Nagaland and Tipura) in view of the resistance to partner drug SP. The combination was replaced by artemether lumefantrine in these States20.
Severe malaria, pregnancy, and prophylaxis: Initially, only parenteral chloroquine and quinine were recommended for the treatment of severe malaria cases. Parenteral artemisinin derivatives were introduced in the national drug policy in 1995 for treating severe and complicated malaria in addition to quinine, particularly in areas of chloroquine resistance or during quinine shortages1,13. Chloroquine was no longer recommended. Similarly, quinidine, under cardiac monitoring, was also recommended when quinine was not available. The 2002, the policy re-recommended injectable chloroquine for severe malaria, with precaution in children, in situations where injectable artesunate or quinine were unavailable. In 2005, the doses used for the artemisinin derivatives (artesunate, artemether, arteether, and artemisinin) were indicated, the minimum duration of treatment was seven days, followed by a full-course of ACT. In 2008, artemisinin was removed from the list of recommended derivatives17.
Till recently, quinine was the drug of choice for falciparum malaria in pregnancy though the emphasis of the national policy was on the drugs which were contraindicated rather than which were recommended. In 2001, the drug policy warned against the use of artemisinin derivatives in pregnant women. The present national drug policy recommends AS+SP in second and third trimesters though quinine is to be used in the first trimester until safety data for the artemisinin derivatives in the first trimester become available. For P. vivax malaria, chloroquine has been recommended18.
The national programme recommends chemoprophylaxis only for select groups from non-endemic areas (travelers, and military personnel) exposed to malaria in highly endemic areas. Among the population in endemic areas, chemoprophylaxis is only recommended in pregnant women. The 1995 drug policy recommended weekly chloroquine prophylaxis in chloroquine sensitive areas. In chloroquine resistant areas, besides weekly chloroquine, daily proguanil was recommended. Since 2008, the drug policy recommends daily doxycycline for short term prophylaxis (less than six weeks) and weekly mefloquine for long term prophylaxis18 with treatment beginning two days or two week before and ending after four weeks of return, respectively. Among migrant labourers, weekly case detection instead of chemoprophylaxis was recommended on operational grounds. The maximum duration for chloroquine treatment was limited to three years because of concerns of toxicity.
Present: SWOT analysis
Strengths: Artemisinin monotherapy was banned in India in 200918. The drug policy recommends antimalarial therapy only after parasitological confirmation of the diagnosis which will reduce drug pressure for resistance, prevent side-effects, decrease drug costs, and improve the management of other causes of febrile illness. The current first-line therapy for P. falciparum, AS+SP, showed 98.8 per cent treatment success across 25 sites in India during 2009 and 2010 over 28 days of follow up21. The programme has changed the ACT in North Eastern States to artemether lumefantrine in view of the resistance to partner drug SP20.
Chloroquine continues to be recommended for P. vivax malaria. Though there were reports of chloroquine resistance in P. vivax22, the therapeutic efficacy studies showed a 100 per cent efficacy. The joint NIMR-NVBDCP National Drug Resistance Monitoring System conducts both widespread and longitudinal measurement of the treatments used in both species through simultaneous in vivo and molecular methods. The policy process is now well-defined, consultative, and evidence-based in addition to expert opinion. Fig. 2 outlines the policy process for the formation of National Drug Policy for Malaria in India. The frequency of drug policy updates has also increased with three policy changes in the last five years. Finally, the policy has been translated into easy to follow case management guidelines for use by clinicians18.
Fig. 2.
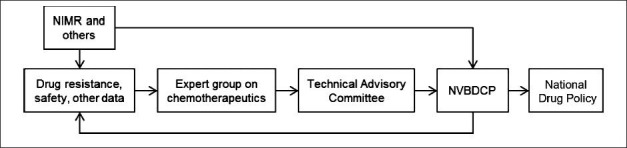
The policy process for the formation of National Drug Policy for malaria in India, 2001-onwards. NIMR, National Institute of Malaria Research (ICMR), New Delhi; NVBDCP, Natiional Vector Borne Disease Control Programme, Government of India, Delhi.
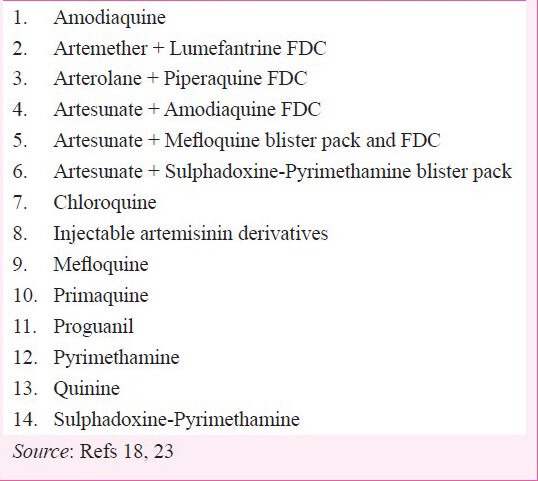
Weaknesses: The present ACT (AS+SP) being recommended all over India except North Eastern States is a blister pack. Compared to fixed-dose combinations (FDCs), blister packs where the individual drugs are co-packaged may have poorer adherence, the potential for monotherapy use, and even poorer bioavailability. Another challenge for the drug policy is access to the delivery systems used for malaria diagnosis and treatment in India. Citizens living in remote, inaccessible, or disturbed areas may have to undergo considerable hardship to reach publicly provided care and turn to self-treatment or the formal and, more often, informal private sector for care. Community-based care, while introduced in some places, is not available everywhere. On the provider side, there is lack of awareness of the National Drug Policy, and best practice in general, among the private sector. A host of available therapies (Box) shows a wide variation in treatment choice along with dose, duration, and co-administered drugs such as antibiotics. Physician and patient compliance to radical treatment (primaquine) is poor and may be contributing unnecessary burden in terms of additional transmission or relapses.
Box.
Currently registered antimalarial drugs in India
Opportunities: New ACTs have recently completed or are undergoing phase III studies23 and some are now registered. Phase III clinical trials have been completed for fixed dose ACTs including artesunate + mefloquine, dihydroartemisinin + piperaquine24, arterolane + piperaquine25, and pyronaridine + artesunate26. Arterolane is a synthetic analogue of artemisinin and has the potential to replace plant-derived artemisinin27. Trials are underway for combinations of current ACTs like artesunate + lumefantrine, artesunate+piperaquine, etc. Pharmacovigilance of antimalarial drugs is generating data on adverse events in patients which will help improve future policy. The case management of malaria has been extended to the village level in many areas through the use of community-based health workers. This should help promote more access and quicker treatment for suffering patients. The bivalent RDKs have recently been introduced and will improve the diagnosis.
Threats: Emerging resistance to antimalarial drugs poses the greatest threat to the National Drug Policy on malaria. While the results of in vitro sensitivity testing of antimalarial drugs in India have not shown any evidence of decreased sensitivity to artemisinin derivatives28, clinical resistance to artemisinin drugs has emerged along the nearby Thai-Myanmar and Thai-Cambodia borders29. The spread of resistance westwards, as happened with chloroquine, could jeopardize the most effective class of compounds we have for malaria treatment today. There is considerable evidence (clinical, in vitro, and molecular) of drug resistance to the partner drug used in the first-line ACT. Studies suggested the presence of double mutations in dhfr and single/double mutations in dhps30. Changes in these drug resistance markers are currently being monitored among patients enrolled in therapeutic efficacy studies in sentinel sites across the country31. The spread and increase in SP resistance, which is likely inevitable, may decrease the present high efficacy of AS+SP in India and necessitate the switch to a different combination therapy. Though data on the efficacy of AS+SP on mixed infections are sparse, we know that SP is not very effective against vivax malaria. Finally, the emergence of chloroquine resistance in P. vivax, as has happened elsewhere in the not too distant Western Pacific region32, would complicate the control of the species responsible for half of the national malaria burden.
Future: unresolved challenges
From antimalarial treatment to case management: A key transition from the malaria eradication era towards a modern malaria control programme is moving from drug distribution to case management. The former is concerned with an output, supplying drug, while the latter is an entire process from diagnosis to care to referrals and is concerned with quality. The change to the case management can be challenging where activities are influenced by many interconnected factors. While the process has been long initiated, and strengthened by policy changes such as the end of presumptive treatment, quality has room to improve. To begin with, indicators such as the time from fever to diagnosis and treatment need to be monitored. Another goal should be increasing the proportion of malaria cases from passive detection, which is better suited to quality care, than from active detection, which is needed when health systems are not available or accessible. Finally, at present there are no protocols for the management of malaria-negative fever patients who seek care.
Private sector treatment practices: The universe of malaria treatment practices in India is wide and diverse. The National Drug Policy for malaria seeks to be evidence-based best practice. However, the adherence of the private sector to correct treatment of malaria, according to species or severity, is generally poor though more extensive surveys are needed. In 2008, private sector treatment was the largest risk factor for receiving artemisinin monotherapy in a six State survey33. In 2009, the Drugs Controller General of India has banned the use, manufacture, sell and export of oral artemisinin monotherapy in the country. However, injectable artemisinin derivatives remain a preferred antimalarial treatment in rural areas33 for treating uncomplicated malaria. There is a need to rationalize the use of injectable artemisinin derivatives by limiting to severe malaria. While at present 80 per cent of medical care in India is privately provided, household survey data suggest that in rural areas of malaria endemic States only half of patients with fever seek private sector care34. This is still a substantial proportion. Strategies for communicating and promoting the quality of care, including print media, workshops, and even one-to-one interaction, in the private sector are needed.
Selecting future ACTs: The choice of optimal ACT for future use is not clear. Artesunate amodiaquine has the disadvantage of cross-resistance with chloroquine whose sensitivity is decreased nationwide in P. falciparum1,28. Artemether+lumefantrine is effective35 in India but has to be administered twice daily and can have erratic absorption. Arterolane+piperaquine is promising as a treatment for both species and has a long half-life but more data need to be generated25. AS+mefloquine was also effective36, India is largely mefloquine naïve from a resistance point of view, but has the disadvantage of neuropsychiatric complications and a higher cost than other ACTs. Evolutionary-epidemiological modeling suggests that the use of multiple first-line therapies may slow the spread of resistance although there is no empirical validation of the idea37. Switching to multiple ACTs, or region-wise ACTs, in the public sector may be beneficial, but there are several operational barriers for doing so from procurement and supply chain difficulties to training multiple levels, including community-based staff. One step regarding the regional policy has been taken by the programme by replacing AS+SP with artemether lumefantrine.
Gametocytocidal and antirelapse considerations: Current policy recommends a single dose of primaquine on the second day in falciparum and for 14 days in vivax malaria. For the former, the efficacy, optimal day of administration, dose, and safety are not well known though these are being evaluated in an on-going randomized controlled trial (CTRI/2012/12/003273). For the latter, the course is long and compliance, by both provider and patient, is not well-known though suspected to be poor. It is important to improve compliance to antirelapse therapy since upto 40 per cent P. vivax infections are known to relapse38. Strategies to improve anti-relapse primaquine treatment could include directly observed therapy or administering the same total dose over a short duration. Tafenoquine, a long half-life 8-aminoquinoline resulting in a quicker treatment course, could become an alternative choice of drug and is in clinical development39. Finally, there is a need to assess both the risks and benefits of primaquine therapy given its haemolytic potential. While glucose-6-phosphate dehydrogenase (G6PD) deficiency is rare in the general population, studies have documented its prevalence in up to 10-27 per cent of certain ethnic groups including tribal populations at higher risk for malaria40. However, primaquine is being used since several decades and no significant adverse events have been documented till date though these are not well monitored either. Tools for G6PD testing at the primary healthcare level could help address this challenge.
Preventing malaria during pregnancy: In the present National Drug Policy on malaria, personal protection measures are recommended for preventing malaria during pregnancy. There is a need to assess other methods of preventing malaria in this vulnerable group, particularly in regions where the burden may be high. Strategies for evaluation include intermittent screening and treatment, intermittent preventive treatment, and other protection measures during antenatal care. The first strategy is currently being evaluated (CTRI/2012/08/002921). Finally, more data on the safety and efficacy of different drugs are also needed. Trials are underway in India to compare two ACTs (AS+SP versus AS+mefloquine) for treating malaria during pregnancy23. Data from these efforts will be useful for future revisions.
Counterfeit antimalarials: Counterfeit and substandard antimalarials may pose a risk to patient health and antimalarial drug resistance in the country, with the North Eastern States near the China and Myanmar borders being particularly vulnerable41. In a limited study of chemist shops in two sites of India, 12 per cent of essential drugs, including antimalarial drugs, were of substandard quality42. Pre-procurement quality checks of antimalarial drugs are conducted by the procuring agency for public sector supply, but similar monitoring does not exist in the general retail market. Routine monitoring of the quality of drugs available on the market should be conducted, ideally by the drug regulatory agencies, in India. Even for public sector drugs, there is a need to check drug quality after dispatch and storage in field conditions where temperature, humidity, and physical placement may be adverse.
Conclusion
The National Drug Policy on malaria in India has evolved frequently and substantively since its inception in 1982. The current policy is up to date with the available evidence, both in India and from abroad. In addition to the policy document, a set of easy to use guidelines, in a frequently-asked-questions format is available in print and for download to be used by practitioners (http://mrcindia.org/TreatmentGuidelinesAddendum.pdf). Several unrealized opportunities and possible threats to the policy have been identified. Improving the National Drug Policy will require considerable participation and effort by, in addition to the national control programme, numerous other groups - academia, medical colleges, research institutes, regulatory agencies, the pharmaceutical industry, etc. - invested in malaria control for the country.
Acknowledgment
The authors thank the Indian Council of Medical Research, New Delhi, and National Institute of Malaria Research for providing facilities. Help from the National Vector Borne Disease Control Programme in providing various documents is also acknowledged.
References
- 1.Sharma RS, Sharma GK, Dhillon GPS. New Delhi: Ministry of Health and Family Welfare, National Malaria Eradication Programme, Directorate General of Health Services, Government of India; 1996. Epidemiology and control of malaria in India 1996. [Google Scholar]
- 2.Shah NK, Dhillon GP, Dash AP, Arora U, Meshnick SR, Valecha N. Antimalarial drug resistance of Plasmodium falciparum in India: changes over time and space. Lancet Infect Dis. 2011;11:57–64. doi: 10.1016/S1473-3099(10)70214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Covell G. Bombay: Government Central Press; 1928. Malaria in Bombay, 1928. [Google Scholar]
- 4.Watts S. British development policies and malaria in India: 1897-c.1929. Past Present. 1999;165:141–82. doi: 10.1093/past/165.1.141. [DOI] [PubMed] [Google Scholar]
- 5.Muraleedharan VR. Quinine (Cinchona) and the incurable malaria: Indiac. 1900-1930s. Parasitologia. 2000;42:91–100. [PubMed] [Google Scholar]
- 6.Stapleton DH. Lessons of history? Anti-malaria strategies of the International Health Board and the Rockefeller Foundation from the 1920s to the era of DDT. Public Health Rep. 2004;119:206–15. doi: 10.1177/003335490411900214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sehgal PN, Sharma MID, Sharma SL, Gogai S. Resistance to chloroquine in falciparum malaria in Assam state, India. J Commun Dis. 1973;5:175–80. [Google Scholar]
- 8.Guha AK, Roy JR, Das S, Roy RG, Pattanayak S. Results of chloroquine sensitivity tests in Plasmodium falciparum in Orissa State. Indian J Med Res. 1979;70(Suppl):40–7. [PubMed] [Google Scholar]
- 9.Chakravarty SC, Dwivedi SR, Das S, Phukan D, Roy RG, Pattanayak S. Response of Plasmodium falciparum to chloroquine in the Meghalaya State. Indian J Med Res. 1979;70(Suppl):34–9. [PubMed] [Google Scholar]
- 10.Dwivedi SR, Sahu H, Yadava RL, Roy RG, Pattanayak S. In vivo chloroquine sensitivity tests of Plasmodium falciparum in some parts of Uttar Pradesh and Haryana States. Indian J Med Res. 1979;70(Suppl):20–2. [PubMed] [Google Scholar]
- 11.Choudhury B, Dutt SC, Roy RG, Pattanayak S. Chloroquine resistant P. falciparum in Chandrapur district of Maharastra state. J Commun Dis. 1981;13:142–4. [PubMed] [Google Scholar]
- 12.New Delhi: National Malaria Eradication Programme, Directorate General of Health Services, Ministry of Health and Family Welfare, Government of India; 1986. Directorate of the National Malaria Eradication Programme. Malaria and its control in India. [Google Scholar]
- 13.Delhi: National Anti Malaria Programme, Directorate of Health Services, Government of India, Delhi; 2001. National Anti Malaria Programme. National drug policy on malaria. [Google Scholar]
- 14.Geneva: WHO; 1990. Practical chemotherapy of malaria: Report of a WHO Scientific Group, WHO Tech Rep Ser; 805; pp. 1–141. [PubMed] [Google Scholar]
- 15.Delhi: National Anti Malaria Programme, Directorate of Health Services, Government of India; 2005. National Anti Malaria Programme. National Drug Policy on malaria. [Google Scholar]
- 16.National Drug Policy on malaria. Directorate of Health Services, Government of India, Delhi: NVBDCP; 2007. National Vector Borne Disease Control Programme (NVBDCP) [Google Scholar]
- 17.National Drug Policy on malaria. (Delhi: NBVDCP), Directorate of Health Services, Government of India, Delhi; 2008. National Vector Borne Disease Control Programme (NVBDCP) [Google Scholar]
- 18.2nd ed. New Delhi: NIMR; 2011. National Institute of Malaria Research (NIMR). Guidelines for diagnosis and treatment of malaria in India. [Google Scholar]
- 19.Valecha N, Joshi H, Mallick PK, Sharma SK, Kumar A, Tyagi PK, et al. Low efficacy of chloroquine: time to switch over to artemisinin-based combination therapy for falciparum malaria in India. Acta Trop. 2009;111:21–8. doi: 10.1016/j.actatropica.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 20.National Drug Policy on malaria. Directorate of National Vector Borne Disease Control Programme, Directorate General of Health Services, Ministry of Health and Family Welfare, Government of India; 2013. [accessed on December 21, 2013]. National Vector Borne Disease Control Programme. Available from: http://nvbdcp.gov.in/Doc/National-Drug-Policy-2013.pdf . [Google Scholar]
- 21.Mishra N, Singh JP, Srivastava B, Arora U, Shah NK, Ghosh SK, et al. Monitoring antimalarial drug resistance in India via sentinel sites: outcomes and risk factors for treatment failure, 2009-2010. Bull World Health Organ. 2012;90:895–904. doi: 10.2471/BLT.12.109124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dua VK, Kar PK, Sharma VP. Chloroquine resistant Plasmodium vivax malaria in India. Trop Med Int Health. 1996;1:816–9. doi: 10.1111/j.1365-3156.1996.tb00116.x. [DOI] [PubMed] [Google Scholar]
- 23.Clinical Trials Registry of India. [accessed on November 22, 2010]. Available from: http://ctri.nic.in/Clinicaltrials/do/login1 .
- 24.Valecha N, Phyo AP, Mayxay M, Newton PN, Krudsood S, Keomany S, et al. An open-label, randomised study of dihydroartemisinin-piperaquine versus artesunate-mefloquine for falciparum malaria in Asia. PLoS One. 2010;5:e11880. doi: 10.1371/journal.pone.0011880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valecha N, Krudsood S, Tangpukdee N, Mohanty S, Sharma SK, Tyagi PK, et al. Arterolane maleate plus piperaquine phosphate for treatment of uncomplicated Plasmodium falciparum malaria: a comparative, multicenter, randomized clinical trial. Clin Infect Dis. 2012;55:663–71. doi: 10.1093/cid/cis475. [DOI] [PubMed] [Google Scholar]
- 26.Poravuth Y, Socheat D, Rueangweerayut R, Uthaisin C, Phyo A, Valecha N, et al. Pyronaridine-artesunate versus chloroquine in patients with acute Plasmodium vivax malaria: a randomized, double-blind, non-inferiority trial. PLoS One. 2011;6:e14501. doi: 10.1371/journal.pone.0014501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valecha N, Looareesuwan S, Martensson A, Abdulla SM, Krudsood S, Tangpukdee N, et al. Arterolane, a new synthetic trioxolane for treatment of uncomplicated Plasmodium falciparum malaria: a phase II, multicenter, randomized, dose-finding clinical trial. Clin Infect Dis. 2010;51:684–91. doi: 10.1086/655831. [DOI] [PubMed] [Google Scholar]
- 28.Anvikar AR, Sharma B, Sharma SK, Ghosh SK, Bhatt RM, Kumar A, et al. In vitro assessment of drug resistance in Plasmodium falciparum in five States of India. Indian J Med Res. 2012;135:494–9. [PMC free article] [PubMed] [Google Scholar]
- 29.Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, et al. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361:455–67. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahmed A, Bararia D, Vinayak S, Yameen M, Biswas S, Dev V, et al. Plasmodium falciparum isolates in India exhibit a progressive increase in mutations associated with sulfadoxine - pyrimethamine resistance. Antimicrob Agents Chemother. 2004;48:879–89. doi: 10.1128/AAC.48.3.879-889.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.New Delhi: National Institute of Malaria Research (Indian Council of Medical Research); 2011. [accessed on May 6, 2012]. National Institute of Malaria Research. Annual Report 2010-11. Available from: http://www.mrcindia.org/annual-rep/2010-11.pdf . [Google Scholar]
- 32.Baird JK. Chloroquine resistance in Plasmodium vivax. Antimicrob Agents Chemother. 2004;48:4075–83. doi: 10.1128/AAC.48.11.4075-4083.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mishra N, Anvikar AR, Shah NK, Kamal VK, Sharma SK, Srivastava HC, et al. Prescription practices and availability of artemisinin monotherapy in India: where do we stand? Malar J. 2011;10:360. doi: 10.1186/1475-2875-10-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.National Institute of Malaria Research. Household Survey and Health Facility Survey for In-depth Review of NVBDCP (Malaria) 2006. [accessed on December 17, 2013]. Available from: http://nvbdcp.gov.in/Round-9/Annexure-10%20%20IDR_Mal.pdf .
- 35.Valecha N, Srivastava P, Mohanty SS, Mitra P, Sharma SK, Tyagi PK, et al. Therapeutic efficacy of artemether-lumefantrine in uncomplicated falciparum malaria in India. Malar J. 2009;8:107. doi: 10.1186/1475-2875-8-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valecha N, Srivastava B, Dubhashi NG, Krishnamoorthy Rao BHK, Kumar A, Ghosh SK, et al. Safety and efficacy and population pharmacokinetics of fixed-dose combination of artesunate-mefloquine in the treatment of acute uncomplicated Plasmodium falciparum malaria in India. J Vector Borne Dis. 2013;50:258–64. [PubMed] [Google Scholar]
- 37.Boni MF, Smith DL, Laxminarayan R. Benefits of using multiple first - line therapies against malaria. Proc Natl Acad Sci USA. 2008;105:14216–21. doi: 10.1073/pnas.0804628105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adak T, Valecha N, Sharma VP. Plasmodium vivax polymorphism in a clinical drug trial. Clin Diagn Lab Immunol. 2001;8:891–4. doi: 10.1128/CDLI.8.5.891-894.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Llanos-Cuentas A, Lacerda MV, Rueangweerayut R, Krudsood S, Gupta SK, Kochar SK, et al. Tafenoquine plus chloroquine for the treatment and relapse prevention of Plasmodium vivax malaria (DETECTIVE): multicentre, double-blind, randomised, phase 2b dose-selection study. Lancet. 2013 doi: 10.1016/S0140-6736(13)62568-4. piiS0140-6736(13)62568-4. [DOI] [PubMed] [Google Scholar]
- 40.Mohanty D, Mukherjee MB, Colah RB. Glucose-6-phosphate dehydrogenase deficiency in India. Indian J Pediatr. 2004;71:525–9. doi: 10.1007/BF02724295. [DOI] [PubMed] [Google Scholar]
- 41.Dash AP, Valecha N, Anvikar AR, Kumar A. Malaria in India: challenges and opportunities. J Biosci. 2008;33:583–92. doi: 10.1007/s12038-008-0076-x. [DOI] [PubMed] [Google Scholar]
- 42.Bate R, Tren R, Mooney L, Hess K, Mitra B, Debroy B, et al. Pilot study of essential drug quality in two major cities in India. PLoS One. 2009;4:e6003. doi: 10.1371/journal.pone.0006003. [DOI] [PMC free article] [PubMed] [Google Scholar]


