Abstract
Background & objectives:
Discrepancies exist in the reported prevalence of portal vein thrombosis (PVT), and its clinical characteristics and sites of occurrence need to be elucidated. The risk factors for PVT are also poorly understood. This single centre study was undertaken to determine the clinical characteristics, sites of occurrence, and risk factors associated with PVT in patients with liver cirrhosis.
Methods:
Hospitalized cirrhotic patients (N = 162) were segregated into the PVT and non-PVT groups. Indices possibly associated with PVT were measured and PVT was detected by both Doppler ultrasonography and computed tomography portal angiography. The portal vein diameter and flow velocity and splenic thickness were measured by ultrasonography.
Results:
PVT was found in 40 patients (24.7%); in 34 PVT patients (85%), the liver cirrhosis resulted from hepatitis B virus infections. Most (90%) patients were Child-Pugh classes B and C, with similar distribution between the groups. PVT was seen in 20 patients in the portal and superior mesenteric veins; ascites, abdominal pain, gastrointestinal bleeding, and jaundice were common findings in PVT patients. Haemoglobin levels and blood platelet counts (BPCs) were significantly lower and splenic thickness was greater in PVT than in non-PVT patients (P<0.01). There was a significant positive correlation between BPCs and platelet aggregation rates (R = 0.533, P<0.01).
Interpretation & conclusions:
The occurrence of PVT was 24.7 per cent, primarily in post-hepatitis B liver cirrhosis patients. PVT occurred mainly in the portal vein trunk and superior mesenteric vein. Different PVT sites may account for the differing clinical presentations. The lower levels of haemoglobin and BPCs as well as splenic thickening were associated with PVT. Splenic thickening may be a risk factor for PVT.
Keywords: Cirrhosis, hepatitis B virus, portal vein thrombosis, risk factors, splenic thickening
Portal vein thrombosis (PVT) is a complication of decompensated cirrhosis and is more likely to occur during late-stage liver cirrhosis1, with a prevalence of approximately 1 per cent in the general population2. Studies have shown that PVT develops in 5-20 per cent of cirrhotic patients3,4. There are discrepancies in the reported prevalence of PVT5,6. PVT may be classified into four categories according to its different sites of occurrence in the portal vein (PV) system7. However, additional research is required to determine if there is an anatomic site where PVT preferentially occurs. There is also a lack of clarity regarding whether the site of the PVT affects the clinical characteristics.
The aetiological factors of PVT have been defined and large case studies have improved the understanding of the condition8. Some of the factors that are considered to predispose for the development of PVT in patients with liver cirrhosis include inflammation, neoplasms, coagulation disorders, abdominal surgery, and trauma5. Further investigation of the common aetiological factors involved in liver cirrhosis, including hepatitis B and C viruses (HBV and HCV), alcohol, schistosomes, autoimmunity, and cryptogenic causes, is needed to determine their role in PVT development.
In cirrhotic patients, the primary pathogenic factors associated with PVT involve hepatic structural derangement secondary to slowing of the portal flow9; PVT is considered to be a multifactorial complication10,11. Both inherited and acquired thrombotic risk factors, as well as local anatomical and haemodynamic factors, appear to be involved in its occurrence12,13. The presence of PVT is also an indicator of the failure to control active variceal bleeding and prevent variceal rebleeding and is also significantly associated with increased mortality in patients with liver cirrhosis14. In addition, the presence of PVT influences the success and outcome of endovascular interventional treatments and liver transplantations performed due to liver cirrhosis and portal hypertension15.
The aim of the present study was to determine the clinical characteristics, and sites of PVT in cirrhotic patients attending a tertiary care hospital in PR China and to identify the risk factors associated with PVT.
Material & Methods
Patients: From March 2011 to March 2012, a total of 240 cirrhotic patients (174 men, 66 women) were enrolled in the study conducted at Tongji Hospital, a tertiary care hospital in Shanghai, PR China. Patients with hepatocellular carcinoma, other malignancies, known haemostatic disorders other than liver disease, bacterial infection, renal dysfunction, clinical history of peripheral venous thrombosis, Budd-Chiari syndrome, spleen resection, liver transplantation, endoscopic treatment, or anticoagulation therapy were excluded. Consequently, 162 patients were found to be eligible for inclusion in this study. HBV cirrhosis was defined by a positive diagnosis of HBV-related cirrhosis and the presence of the HBV surface antigen (HBsAg) in the absence of a history of alcohol consumption or other co-existing viral infections.
Decompensated liver cirrhosis was diagnosed by clinical findings or morphological features and its severity was scored according to the Child-Pugh classification16. To explore the risk factors associated with PVT, the patients were classified into two groups: the PVT group (n = 40) and the non-PVT group (n = 122). All patients, including the 40 with PVT underwent computed tomography portal angiography (CTPA) and colour Doppler ultrasonography (CDUS) to rule out underlying hepatocellular carcinoma and to confirm PVT.
The study protocol was approved by the Tongji Hospital ethics committee. Written informed consent was obtained from each patient.
Sample collection and analyses: After fasting for at least 12 h, 20 ml of blood was collected from each patient and haemoglobin (Hb) levels and blood platelet counts (BPCs) were determined using a Sysmex XE- 2100 automated analyzer (Sysmex, Kobe, Japan). Prothrombin time (PT), activated partial prothrombin time (APTT), and fibrinogen levels were determined by routine coagulation methods, using a Sysmex CA6000 automated analyzer (Sysmex, Milton Keynes, UK). Total bilirubin (TBIL) and albumin levels were determined using a bromocresol green assay17 and spectrophotometric method18, respectively. The platelet aggregation rate (PAR) was determined by turbidometric platelet aggregometry (SC-2000; Beijing SUCCEEDER Technology, Beijing, China). Anticardiolipin antibodies (ACAs) were detected using an enzyme-linked immunosorbent assay (Byk Gulden, Milano, Italy), and D-dimers and high sensitivity C-reactive protein (hs-CRP) were detected using kits from Sun Biotech (Shanghai, China). Levels of intercellular adhesion molecule-1 (ICAM-1), alpha-interferon (IFN-α), and tissue necrosis factor alpha (TNF-α) were measured using enzyme-linked immunosorbent assays19; IgA, IgM, and IgG were detected by turbidometric methods20.
Imaging examination: CDUS examinations were performed using a colour Doppler ultrasound scanner with a 3.5-5 MHz convex probe (Mylab-90, Philips Electronics, Amsterdam, The Netherlands). The PV system was examined following current guidelines21. The PV diameter and portal flow velocity were calculated automatically by the instrument. Splenic thickness was measured perpendicular to the long axis of the spleen. All the patients were examined by CTPA to further confirm the presence of thrombosis, especially if thrombosis was not confirmed by CDUS.
Statistical analysis: The SPSS software package for Windows 2000 (ver. 11, IBM SPSS, Armonk, NY, USA) was used for all statistical analyses. All quantitative data were expressed as mean ± standard deviation (SD). Categorical variables were shown in terms of frequencies (percentages). The differences between the two groups were evaluated using the chi-square (χ2) or t test. The continuous variable (age) was examined by a normality test. The Pearson chi-square test was performed for categorical data and the Pearson bivariate correlation test was subsequently applied. Multivariate binary logistic regression was performed and the model was estimated using the stepwise backward method (Wald). The coefficients obtained from the logistic regression analyses were also expressed in terms of odds ratio with 95% confidence intervals.
Results
PVT was found in 40 patients [26 (65%) male patients (age range, 39-88 yr) and 14 (35%) female patients (age range, 61-82 yr)]; the overall presence was 24.7 per cent in this study. PVT occurred in 34 cirrhotic patients following HBV hepatitis, in two following HCV hepatitis, and in two patients each with either autoimmune or cryptogenic cirrhosis. However, PVT was not observed in either schistosomal or alcoholic cirrhosis patients. Viral hepatitis was, therefore, the aetiological factor in 90 per cent of the cirrhotic patients demonstrating PVT; the percentages of patients with these aetiological factors did not significantly differ between the two groups.
Univariate analysis showed that the ratios of both mean age and gender were similar between the two groups and that the percentages of patients who smoked, consumed alcohol, had hypertension, and had diabetes mellitus did not significantly differ between the two groups.
Among the patients with PVT, four (10%) were in Child-Pugh class A, 20 (50%) in class B, and 16 (40%) in class C. Child-Pugh classes were not significantly different between the patients with and without PVT.
GI bleeding, abdominal pain, abdominal distention, ascites, jaundice, and hepatic encephalopathy were common clinical presentations among patients in the PVT group. Three of the 40 PVT patients died within 12 months of hospital admission; two died of gastrointestinal haemorrhaging and one died of surgical complications. Additionally, 17 of the 40 PVT patients were re-admitted due to gastrointestinal bleeding or hepatic encephalopathy within the 12 months following their initial admission; the remaining 20 PVT patients demonstrated stable conditions over the follow up period.
Careful examination of the clinical characteristics of the PVT patients indicated differences between the characteristics of patients with isolated PV trunk thrombosis and those with PV trunk and superior mesenteric vein or splenic vein thrombosis. For instance, ascites was most commonly observed in patients with isolated PV trunk thrombosis. However, gastrointestinal bleeding and abdominal pain were most common among PVT patients with superior mesenteric vein or splenic vein thrombosis. Jaundice was also commonly observed in patients with thrombosis in the branches of the PV (Table I).
Table I.
Frequency of different clinical characteristics in patients relative to the site of the portal vein thrombosis (PVT)
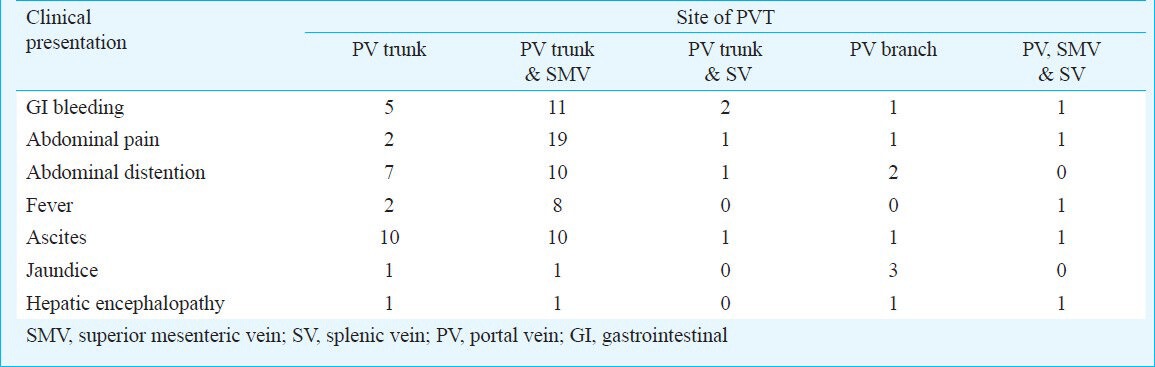
PVT occurred in the PV trunk in 12 (30%) patients, in the PV trunk and superior mesenteric vein in 20 (50%) patients, and in both the PV trunk and splenic vein in two (5%) patients. In five (12.5%) patients, thrombosis was found in a branch of the PV. PVT occurred in the PV trunk, splenic vein, and superior mesenteric vein at the same time in only one patient (2.5%; Table II). The thrombotic risk factors and the clinical and biochemical characteristics of patients with and without PVT are shown in Table III. The levels of fibrinogen, TBIL, PT, IgA, IgG, and IgM were lower in the PVT group than in the non-PVT group but this difference was not significant. The levels of albumin, APTT, ICAM-1, hs- CRP, and PAR were higher in the PVT patients than in the non-PVT patients, but the difference was not significant. The levels of IFN-α, TFN-α, and D-dimer were also not significantly different between the two groups. Univariate analysis showed that the PVT patients had significantly lower levels of Hb and PBC than the patients in the non-PVT group (P=0.003 and P<0.001, respectively). All the plasma samples were negative for ACA.
Table II.
Clinical characteristics of cirrhotic patients (n=162) with and without portal vein thrombosis (PVT)
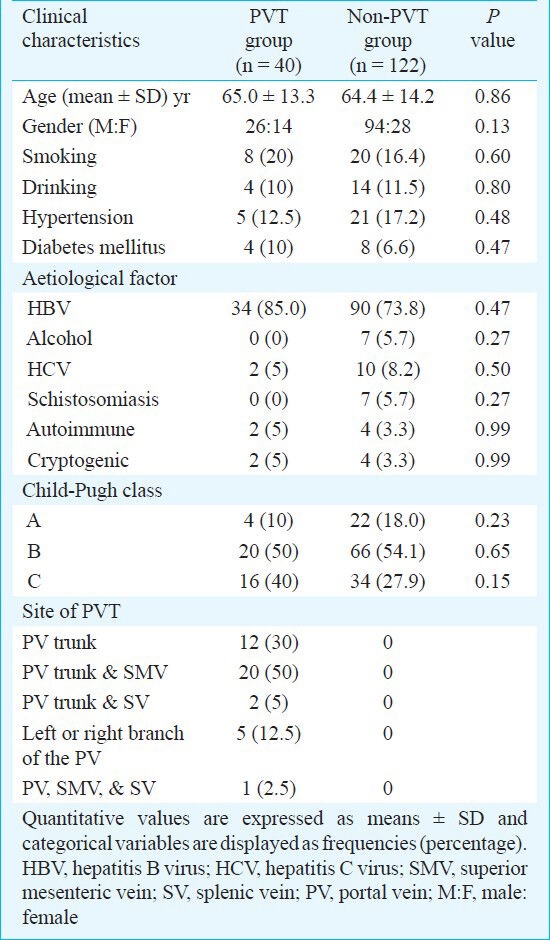
Table III.
Thrombotic risk factors and clinical and biochemical characteristics in patients with and without portal vein thrombosis (PVT)
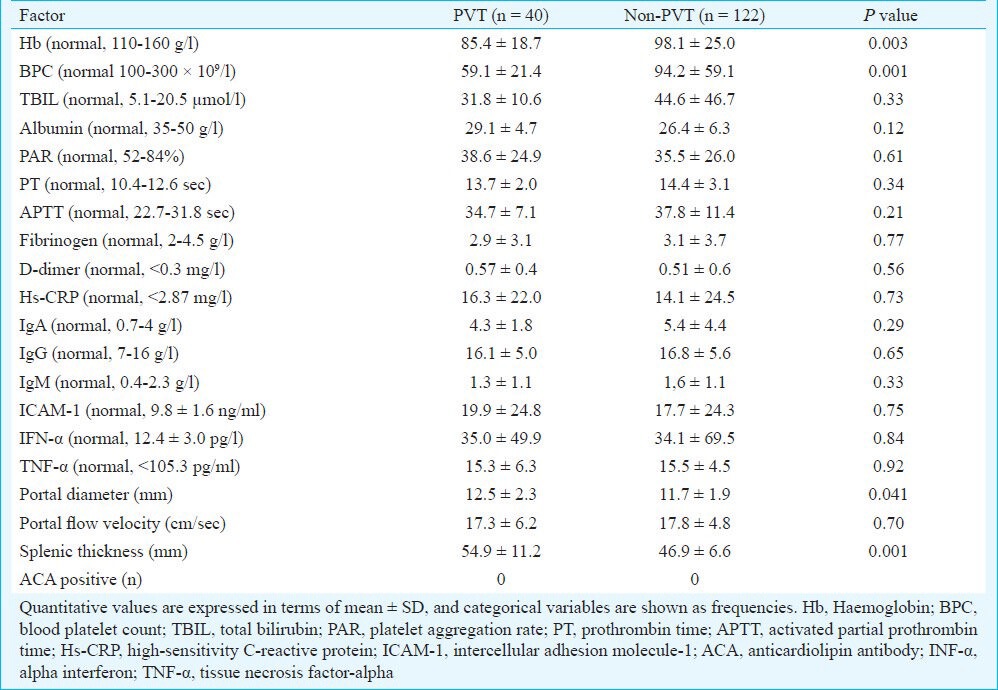
The diameters of the PVs and the splenic thicknesses showed significant differences between the two groups (P=0.041 and P=0.001, respectively). However, the portal flow velocities were not significantly different between the groups (Table III).
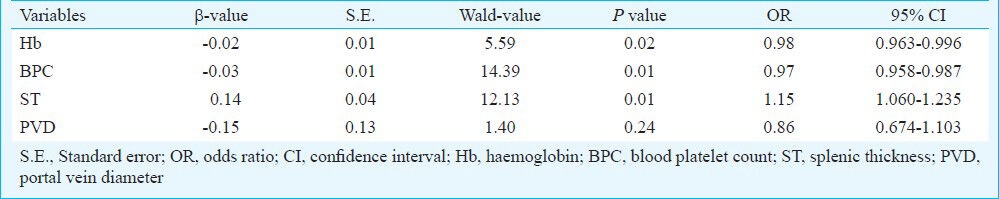
A logistic regression analysis model was applied to the variables associated with the presence of PVT. This analysis showed that decreased levels of BPC and Hb and the presence of splenic thickening were the variables independently associated with PVT (Table IV).
Table IV.
Logistic regression analysis examining the relationship between the presence of portal thrombosis as the dependent variable and other independent variables (Hb, BPC, portal vein diameter, splenic thickness). For the selection of relevant variables, a backward stepwise model (Wald) was applied
A significant positive correlation was observed between PAR and BPC, as shown in the Figure. The value of R was 0.533 (P<0.01).
Fig.
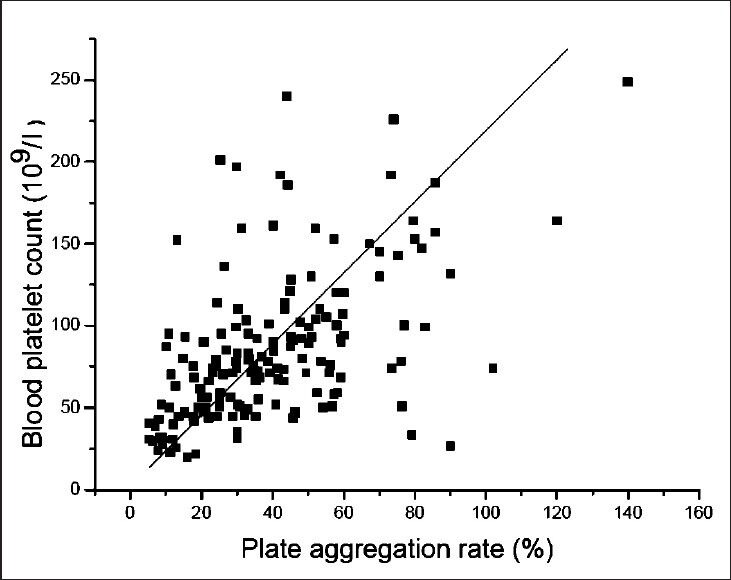
X axis expresses the plate aggregation (PAR), and Y axis expresses the blood platelet count (BPC). A positive correlationship was seen between PAR and BPC (P<0.01).
Discussion
PVT was found in 24.7 per cent patients in this study, confirming the increased occurrence of PVT among cirrhotic patients compared with the general population22. Among the patients in both groups, HBV was observed to be the single major cause of cirrhosis. These results support those of a previous report indicating that HBV is the major risk factor for PVT in Southeast Asian populations23.
PVT was primarily observed to occur in the PV trunk, with the superior mesenteric vein being the second most common site. This suggests that extension of the thrombosis beyond the PV occurred preferentially towards the mesenteric vein. In the PVT patients, the most common clinical presentations were gastrointestinal bleeding, abdominal pain, abdominal distention, fever, jaundice, and hepatic encephalopathy. However, the signs were not identical for the patients with PVT in different locations. Further studies need to be done to show whether the site of PVT affects the clinical characteristics of the disease in a patient.
Some of the previously hypothesized risk factors of PVT, such as age, gender, smoking status, alcohol consumption history, hypertension, and diabetes mellitus, were not associated with PVT in this study. Furthermore, various laboratory markers (fibrinogen, TBIL, PT, IgA, IgG, IgM, albumin, APTT, ICAM-1, D-dimer hs-CRP, IFN-α, and TNF-α), portal flow velocity, and the presence of ACAs did not appear to affect the risk of developing PVT; Child-Pugh classes were also similar between the groups with and without PVTs. The absence of ACA in the plasma of the patients in this study was particularly remarkable, given that the presence of these antibodies has been previously associated with thrombotic events24.
Theoretically, Hb levels are expected to be an independent risk factor for PVT in this study. In fact, because splenomegaly decreases the Hb level and hypersplenism is more severe in PVT patients, the lower Hb level may be more appropriately attributed to splenomegaly. Thus, although Hb was associated with PVT, it was not an independent risk factor of PVT. Similarly, inconsistent conclusions have been drawn from different studies regarding the association of BPC with PVT25,26. Lower BPCs can also be attributed to splenomegaly since splenomegaly is more evident in PVT patients than in non-PVT patients. In addition, the positive correlation between BPC and PAR suggests that the defect associated with the platelets may be the result of their decreased numbers. Extrahepatic portal venous obstruction has previously been shown to result in a decreased PAR in 83 per cent of the patients in one study27. Therefore, the qualitative changes observed in BPCs were speculated to contribute to the formation of PVT, and these were considered more important than the quantitative changes in BPCs during the development of PVTs, although BPCs might not be a true independent risk factor for PVT formation.
Portal flow velocities of <15 cm/sec have been previously identified as an independent risk factor of PVT formation28. However, the present findings were dissimilar from previous reports in that the portal flow velocities for the patients in the two groups were greater than 15 cm/sec and no significant difference was observed in the velocities for the groups. Thus, PVT may not occur if the only alteration that has occurred is a change in portal flow velocity.
Another study showed that preoperative splenic vein diameter was a risk factor for postsplenectomy portal or splenic vein thrombosis29, which is supported by the present results. A widened portal diameter might be involved in the development of PVT. However, multivariate analyses failed to detect a correlation between portal diameter and PVT. The present results indicated that splenic thickness was associated with PVT occurrence. Splenomegaly has been associated with portal hypertension, with greater splenic enlargement resulting in more aggravated portal hypertension30. Further, the resultant risk increases during PVT development due to the presence of aggravated portal hypertension3. Thus, the occurrence of splenic thickening may contribute to the formation of PVT.
The current study had some limitations. First, all the data for this study were obtained from a single center, involving a relatively small number of subjects with or without PVT. A larger sample needs to be investigated to understand the features of PVT. Second, small numbers of patients with aetiologies other than hepatitis B was also a limitation of this study, and more cirrhotic patients with aetiologies other than hepatitis B should be enrolled into the study in future clinical practice.
In conclusion, this single centre study showed PVT in 24.7 per cent cirrhotic Chinese patients, with PVT mainly occurring in patients with post-hepatitis B liver cirrhosis. The lower levels of haemoglobin and BPCs as well as splenic thickening were no underline associated with PVT. Splenic thickening is likely to increase the risk of PVT. Further studies are required to evaluate the relevance of these parameters in a larger population and to identify additional risk factors.
Acknowledgment
This work was supported by the Natural Science Foundation of China (81070343) and the Shanghai Excellent Academic Pacesetters Program (08×D14045).
References
- 1.Mangia A, Villani MR, Cappucci G, Santoro R, Ricciardi R, Facciorusso D, et al. Causes of portal venous thrombosis in cirrhotic patients: the role of genetic and acquired factors. Eur J Gastroenterol Hepatol. 2005;17:745–51. doi: 10.1097/00042737-200507000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Ogren M, Bergqvist D, Bjorck M, Acosta S, Eriksson H, Sternby NH. Portal vein thrombosis: prevalence, patient characteristics and lifetime risk: a population study based on 23796 consecutive autopsies. World J Gastroenterol. 2006;12:2115–9. doi: 10.3748/wjg.v12.i13.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fimognari FL, Violi F. Portal vein thrombosis in liver cirrhosis. Intern Emerg Med. 2008;3:213–8. doi: 10.1007/s11739-008-0128-0. [DOI] [PubMed] [Google Scholar]
- 4.Gaiani S, Bolondi L, Li Bassi S, Zironi G, Siringo S, Barbara L. Prevalence of spontaneous hepatofugal portal flow in liver cirrhosis. Clinical and endoscopic correlation in 228 patients. Gastroenterology. 1991;100:160–7. doi: 10.1016/0016-5085(91)90596-d. [DOI] [PubMed] [Google Scholar]
- 5.Zocco MA, Di Stasio E, De Cristofaro R, Novi M, Ainora ME, Ponziani F, et al. Thrombotic risk factors in patients with liver cirrhosis: correlation with MELD scoring system and portal vein thrombosis development. J Hepatol. 2009;51:682–9. doi: 10.1016/j.jhep.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 6.Francoz C, Belghiti J, Vilgrain V, Sommacale D, Paradis V, Condat B, et al. Splanchnic vein thrombosis in candidates for liver transplantation: usefulness of screening and anticoagulation. Gut. 2005;54:691–7. doi: 10.1136/gut.2004.042796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Webster GJ, Burroughs AK, Riordan SM. Portal vein thrombosis - new insights into aetiology and management. Aliment Pharmacol Ther. 2005;21:1–9. doi: 10.1111/j.1365-2036.2004.02301.x. [DOI] [PubMed] [Google Scholar]
- 8.Harmanci O, Bayraktar Y. Portal hypertension due to portal venous thrombosis: etiology, clinical outcomes. World J Gastroenterol. 2007;13:2535–40. doi: 10.3748/wjg.v13.i18.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weber A, Krebs S, Lenhardt C, Wagenpfeil S, Schmid RM, Schulte-Frohlinde E. Correlation of routinely used coagulation parameters and presence of portal vein thrombosis in patients with liver cirrhosis. Hepatol Res. 2009;39:882–7. doi: 10.1111/j.1872-034X.2009.00531.x. [DOI] [PubMed] [Google Scholar]
- 10.Leebeek FW, Smalberg JH, Janssen HL. Prothrombotic disorders in abdominal vein thrombosis. Neth J Med. 2012;70:400–5. [PubMed] [Google Scholar]
- 11.Shetty S, Ghosh K. Thrombophilic dimension of Budd chiari syndrome and portal venous thrombosis - a concise review. Thromb Res. 2011;127:505–12. doi: 10.1016/j.thromres.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 12.Amitrano L, Brancaccio V, Guardascione MA, Margaglione M, Iannaccone L, D’Andrea G, et al. Inherited coagulation disorders in cirrhotic patients with portal vein thrombosis. Hepatology. 2000;31:345–8. doi: 10.1002/hep.510310213. [DOI] [PubMed] [Google Scholar]
- 13.Denninger MH, Chait Y, Casadevall N, Hillaire S, Guillin MC, Bezeaud A, et al. Cause of portal or hepatic venous thrombosis in adults: the role of multiple concurrent factors. Hepatology. 2000;31:587–91. doi: 10.1002/hep.510310307. [DOI] [PubMed] [Google Scholar]
- 14.Englesbe MJ, Kubus J, Muhammad W, Sonnenday CJ, Welling T, Punch JD, et al. Portal vein thrombosis and survival in patients with cirrhosis. Liver Transplantation. 2010;16:83–90. doi: 10.1002/lt.21941. [DOI] [PubMed] [Google Scholar]
- 15.Qi X, Bai M, Yang Z, Yuan S, Zhang C, Han G, et al. Occlusive portal vein thrombosis as a new marker of decompensated cirrhosis. Med Hypotheses. 2011;76:522–6. doi: 10.1016/j.mehy.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 16.Amitrano L, Anna Guardascione M, Brancaccio V, Margaglione M, Manguso F, Iannaccone L, et al. Risk factors and clinical presentation of portal vein thrombosis in patients with liver cirrhosis. J Hepatol. 2004;40:736–41. doi: 10.1016/j.jhep.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Imbert-Bismut F, Ratziu V, Pieroni L, Charlotte F, Benhamou Y, Poynard T. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet. 2001;357:1069–75. doi: 10.1016/S0140-6736(00)04258-6. [DOI] [PubMed] [Google Scholar]
- 18.Kazmierczak SC, Robertson AF, Catrou PG, Briley KP, Kreamer BL, Gourley GR. Direct spectrophotometric method for measurement of bilirubin in newborns: comparison with HPLC and an automated diazo method. Clin Chem. 2002;48:1096–7. [PubMed] [Google Scholar]
- 19.Tripathy D, Mohanty P, Dhindsa S, Syed T, Ghanim H, Aljada A, et al. Elevation of free fatty acids induces inflammation and impairs vascular reactivity in healthy subjects. Diabetes. 2003;52:2882–7. doi: 10.2337/diabetes.52.12.2882. [DOI] [PubMed] [Google Scholar]
- 20.Potapnev MP, Belevtsev MV, Bortkevich LG, Grinev VV, Martsev SP, Kravchuk ZI, et al. Significance of serum immunoglobulin G for leukocytosis and prognosis in childhood B-lineage acute lymphoblastic leukemia. Pediatric Blood & Cancer. 2004;42:421–6. doi: 10.1002/pbc.20014. [DOI] [PubMed] [Google Scholar]
- 21.Sabba C, Merkel C, Zoli M, Ferraioli G, Gaiani S, Sacerdoti D, et al. Interobserver and interquipment variability of echo-Doppler examination of the portal vein: effect of a cooperative training program. Hepatology. 1995;21:428–33. doi: 10.1002/hep.1840210225. [DOI] [PubMed] [Google Scholar]
- 22.Ogren M, Bergqvist D, Bjorck M, Acosta S, Eriksson H, Sternby NH. Portal vein thrombosis: prevalence, patient characteristics and lifetime risk: a population study based on 23,796 consecutive autopsies. World J Gastroenterol. 2006;12:2115–9. doi: 10.3748/wjg.v12.i13.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korn L, Chirayu A. High incidence of hepatitis B infection-associated cirrhosis and hepatocellular carcinoma in the Southeast Asian patients with portal vein thrombosis. BMC Gastroenterol. 2011;11:66. doi: 10.1186/1471-230X-11-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirohata Y, Murata A, Abe S, Otsuki M. Portal vein thrombosis associated with antiphospholipid syndrome. J Gastroenterol. 2001;36:574–8. doi: 10.1007/s005350170063. [DOI] [PubMed] [Google Scholar]
- 25.Ushitora Y, Tashiro H, Takahashi S, Amano H, Oshita A, Kobayashi T, et al. Splenectomy in chronic hepatic disorders: portal vein thrombosis and improvement of liver function. Dig Surg. 2011;28:9–14. doi: 10.1159/000321886. [DOI] [PubMed] [Google Scholar]
- 26.Zhang D, Hao J, Yang N. Protein C and D-dimer are related to portal vein thrombosis in patients with liver cirrhosis. J Gastroenterol Hepatol. 2010;25:116–21. doi: 10.1111/j.1440-1746.2009.05921.x. [DOI] [PubMed] [Google Scholar]
- 27.Bajaj JS, Bhattacharjee J, Sarin SK. Coagulation profile and platelet function in patients with extrahepatic portal vein obstruction and non-cirrhotic portal fibrosis. J Gastroenterol Hepatol. 2001;16:641–6. doi: 10.1046/j.1440-1746.2001.02392.x. [DOI] [PubMed] [Google Scholar]
- 28.Zoli M, Iervese T, Merkel C, Bianchi G, Magalotti D, Marchesini G, et al. Prognostic significance of portal hemodynamics in patients with compensated cirrhosis. J Hepatol. 1993;17:56–61. doi: 10.1016/s0168-8278(05)80521-5. [DOI] [PubMed] [Google Scholar]
- 29.Danno K, Ikeda M, Sekimoto M, Sugimoto T, Takemasa I, Yamamoto H, et al. Diameter of splenic vein is a risk factor for portal or splenic vein thrombosis after laparoscopic splenectomy. Surgery. 2009;145:457–64. doi: 10.1016/j.surg.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 30.Bolognesi M, Merkel C, Sacerdoti D, Nava V, Gatta A. Role of spleen enlargement in cirrhosis with portal hypertension. Dig Liver Dis. 2002;34:144–50. doi: 10.1016/s1590-8658(02)80246-8. [DOI] [PubMed] [Google Scholar]


