Abstract
Background & objectives:
Uterine leiomyomas (fibroids) are common cause of morbidity in women of reproductive age group. High intensity focused ultrasound with the imaging guidance of magnetic resonance imaging (MRI) known as magnetic resonance guided focused ultrasound sonication (MRgFUS) is now available. However, there are no available studies with this non invasive modality of treatment in Indian subjects. The objective of this study was to determine the safety and clinical efficacy of MRgFUS in the treatment of uterine fibroids.
Methods:
This prospective study included 32 consecutive women with clinically symptomatic uterine fibroids who were treated with MRgFUS from February 2011 to October 2011. Pre and post treatment symptom severity scores (SSS) were assessed at the time of enrolment and at one, three and six months follow up using a validated uterine fibroid symptom - quality of life questionnaire (UFS-QOL). Pre and post treatment fibroid volumes were compared immediately after treatment and at six months follow up using contrast enhanced MRI scan. Non perfused volume (NPV) ratios were calculated and correlated with fibroid volume reductions immediately after the treatment and at the end of six months follow up.
Results:
This procedure was well tolerated by the patients and procedure related adverse effects were non significant. Significant reductions in SSS were seen at one, three and six month intervals after the treatment (P<0.01). Significant reductions were noticed in fibroid volumes at six months follow up compared to pretreatment fibroid volumes (P<0.01). Significant positive correlations were observed between NPV ratios and reduction in fibroid volumes at six months follow-up (r=0.659, P<0.01).
Interpretation & conclusions:
MRgFUS is relatively a safe and effective non invasive treatment modality for treating uterine fibroids in selected patients. Its long term efficacy is yet to be tested and compared with other available minimally invasive treatment options.
Keywords: HIFU, MRgFUS, sonication, uterine fibroids
Uterine leiomyomas (fibroids) are common cause of morbidity in women of reproductive age group. About 25 per cent of women are affected during their lifetime1,2. Symptoms include heavy and prolonged menstrual flow, pelvic pain and pressure, pain in legs and back, pain during sexual intercourse, frequent micturition, constipation, abnormally enlarged abdomen and infertility. All available treatment modalities for uterine fibroids like hysterectomy and myomectomy are either invasive or minimally invasive. Uterine sparing treatments such as myomectomy and uterine artery embolization have become popular treatment options for uterine fibroids3. The advantages of these treatment modalities are lower morbidity and shorter recovery times when compared with hysterectomy. However, these procedures are associated with treatment specific limitations like fibroid size, location, and post embolization syndrome4,5. High intensity focused ultrasound (HIFU) is a new non invasive treatment modality for treating uterine fibroids. In HIFU ultrasound waves in the frequency range of 1-1.5 MHz are focused over a small volume of tissue by using a phased array transducer to generate thermal energy that causes coagulation necrosis of the tissue at that focal point2,6,7. During diagnostic ultrasound the ultrasound waves are distributed over a wide area and temperature elevation is negligible8. In HIFU ultrasound waves are focused over a small area to generate heat and cause coagulation necrosis of tissue at focal point. The therapeutic potential of ultrasound could not be utilized for a long time due to lack of proper image guidance9,10. With the advent of magnetic resonance imaging (MRI) it is now possible to apply this technology for the treatment of a wide range of clinical conditions. Excellent soft tissue resolution afforded by MRI enables accurate planning of target tissue. Images can be obtained in many planes and two and three dimensional images can be used for accurate treatment planning. MRI parameters have intrinsic sensitivity to temperature change and can be used for real time thermal mapping and tissue damage assessment following therapy11,12. The application of high intensity focused ultrasound with the imaging guidance of MRI is called magnetic resonance guided focused ultrasound (MRgFUS). So far, there are no available studies with this modality of treatment involving Indian subjects. The objective of this study was to determine the safety and clinical efficacy of this non invasive modality for the treatment of symptomatic uterine fibroids in the Indian patients.
Material & Methods
This prospective study included 32 consecutive women with 51clinically symptomatic uterine fibroids (excessive and irregular menstrual bleeding, pelvic pain, pressure, urinary or bowel problems and anaemia) who attended GSL general hospital, Rajahmundry, Andhra Pradesh, India, from February 2011 to October 2011. They were first clinically examined by a gynecologist followed by ultrasound scan of the pelvis and based on the clinical symptoms and ultrasound findings they were subjected to screening MRI to assess the feasibility for MRgFUS treatment. The MR images of the pelvis were used to diagnose the presence of fibroids, their blood flow (perfusion volume) location, size and volume, characterize the tissue, and diagnose other uterine or pelvic conditions. These women were assessed for clinical symptoms and health related quality of life at the time of recruitment for the study and after treatment at one, three and six month follow up using a validated uterine fibroid symptom and quality of life questionnaire (UFS-QOL)5,13. The questionnaire uses eight questions assessed on a 5-point Likert scale to assess both bleeding and bulk-related symptoms due to uterine fibroids. Thus, the maximal raw score for the symptoms severity score is 40 points. However, the transformed score is typically reported on a single 100-point scale, with 100 points indicating maximal symptomatology. In validation studies normal women had an average transformed symptom score of approximately 20 points, and women with uterine fibroids had an average score of 40 points.
The women with a symptom severity score (SSS) of more than 41 points on UFS-QOL questionnaire qualified for the study. A 20 point reduction in SSS was considered as significant improvement. Immediately after the procedure and at six month follow up contrast enhanced MRI scan of the pelvis was performed to assess the nonperfused volume (NPV) or treated area and reduction in fibroid size. NPV ratio (NPV ratio = nonperfused volume/perfused volume expressed as percentage) was calculated and its relation to symptom relief as per UFS-QOL was assessed at 6 months follow up. The primary outcome of the study was symptom relief at one, three and six months and secondary outcome was reduction in the fibroid size at 6 months follow up and its relation to NPV ratio11,14,15.
The inclusion criteria were age above 18 yr, weight less than 110 kg, not more than four fibroids, fibroid size not more than 10 cm, location not more than 12 cm from anterior abdominal wall and at least 4 cm away from bone and nerve bundles. The exclusion criteria were pregnancy, massive scarring over lower abdomen, pelvic or systemic disease, contraindications for MRI like cardiac pacemaker, calcified fibroids and pedunculated fibroids. Written informed consent of the patients was obtained. This study protocol was approved by the institutional ethics review committee.
Selected patients reported to treatment centre in fasting state. An intravenous line and Foley's catheter were inserted. Conscious sedation was administered with fentanyl citrate or pentazocine. During MR guided focused ultrasound patients lied in the prone position on a modified MR gantry. The FUS machine integrates fully with a 1.5 T MR scanner (GE, Milwaukee, USA) and the transducer is situated within a bath of degassed water in the mid-section of the table. This allows a direct acoustic pathway from the transducer into the target fibroid, which was directly positioned above. To carry out MRgFUS safely an adequate acoustic window for the sound wave pathway was created which did not traverse abdominal wall scars or any loops of bowel. The goal of the therapy was to ablate maximum volume of the fibroid without damage to surrounding structures. The treatment parameters like fibroid volume, number of sonications, acoustic power and sonication frequency were initially determined by ultrasound ablation system (EXABLATE-2010, Insightec Haifa Israel). These were modified later on the basis of temperature mapping and feedback from patients regarding sonications related discomfort. On an average it took about 3-4 h for treating a 6 cm size fibroid.
Statistical analysis: Statistical analysis was performed using SPSS software trail version 16.0, USA. Symptom severity scores (SSS) were analyzed using ANOVA with Turkey's test. Student's paired t-test was used to compare the fibroid volumes before procedure and at six months follow up. Pearson coefficient of correlation was used to study the correlations between NPV ratio and reduction in fibroid size immediately after the procedure and at six months follow up. For all statistical analyses P<0.01 was considered significant.
Results
A total of 32 women were treated with this procedure. The age varied between 21 to 48 yr with a mean age of 36.63 ± 6.23 yr (Fig. 1). Excessive bleeding and irregular cycles were the commonest complaints in more than 70 per cent of women. Abdominal and pelvic pain, pelvic pressure and urinary and bowel symptoms were present in 20 per cent of women. Five women presented with infertility and one with indwelling catheter for urinary retention. Fifty one fibroids were treated in these 32 women. The mean symptom severity score was 67.72 ± 6.56 at the time of enrolment for the study. After the treatment, the SSS was 35.09 ± 6.07 at one month follow up, 29.72 ± 5.27, at three months and 27.28 ± 5.46 at six months follow up. Significant reduction in SSS was seen at one, three and six months follow up compared to pre procedure SSS (P<0.01). Mean differences in SSS between pre and post procedure were 27.63, 33.0 and 35.43 at one, three and six months follow up (P<0.01). The mean perfused volume of the fibroids was 146.81 ± 64.98 cm3 before treatment. The mean non perfused volume of the fibroids immediately after the procedure on a gadolinium enhanced MRI scan was 102.88 ± 39.31 cm3 which represented 70 per cent of the pretreatment mean fibroid volume. The mean volume of the fibroids at the end of six months follow up was 87.31 ± 35.36 cm3 which represented 40 per cent reduction (P<0.01) in pretreatment fibroid volumes (Figs 2, 3). Significant positive correlations were seen (r=0.659, P<0.01) between NPV ratio and reduction in fibroid volume at six months follow up but not immediately after the procedure. No serious adverse effects were reported during the study period. Only one patient developed blisters over anterior abdominal wall which subsided in one week. Four patients reported leucorrhoea in the first week after procedure which subsided after two weeks. One patient who reported for treatment with indwelling catheter for retention of urine passed urine normally on the next day of the procedure.
Fig. 1.
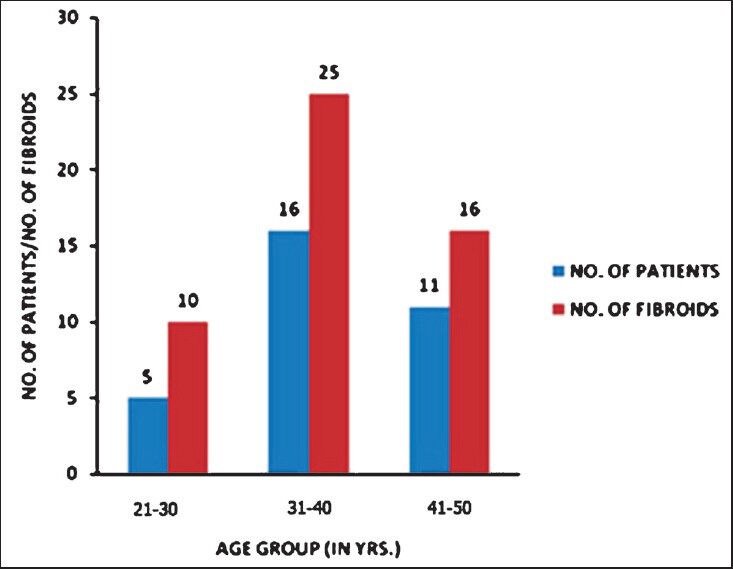
Distribution of number of fibroids in different age groups.
Fig. 2.
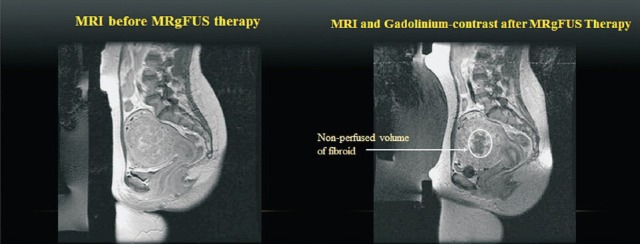
Pre and post treatment contrast enhanced T1W MRI images of the fibroid showing perfused and non perfused volumes.
Fig. 3.
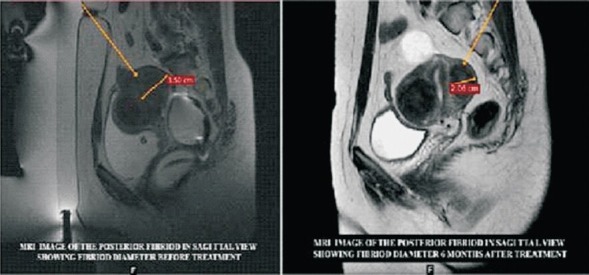
Pre and six months post treatment images of the same fibroid with marked size reduction.
Discussion
The outcome of the MRgFUS treatment in this study was on the basis of improvement in symptom severity scores and improvement in quality of life after the procedure. The results showed significant improvement in the SSS after the procedure at one, three and six months compared to symptom scores at the time of initial presentation. Although the relief of symptoms and improvement in the quality of life is promising about the effectiveness of this new non invasive ablative treatment in short term, its long term efficacy and sustainability is yet to be established. The reductions in SSS and fibroid volumes at the end of six months follow up after the MRgFUS treatment correlated positively with the NPV ratio. These observations were in accordance with the findings of other studies conducted outside India2,12,13,16. The common less serious adverse effects included leucorrhoea, fever, localized pain, erythema and swelling which disappeared in a few days. Serious adverse effects included, necrosis of non targeted tissue like bowel and bladder with perforation, nerve damage or haemorrhage in the treatment area, and skin burns resulting in ulceration and scar formation. various studies reported adverse effects in 1-3 per cent cases5,13.
Some investigators have correlated the therapeutic efficacy of MRgFUS with fibroid intensity in the pretreatment MRI images. Some other studies revealed that the treatment outcome was not influenced by the phase of menstrual cycle17,18. But in this study these aspects were not taken into consideration. Long term efficacy of this treatment is yet to be tested and compared with other available alternative minimally invasive treatment options. So far 24 months follow up studies are available which show lower recurrence rates (20%) when the treated volumes were around 60 per cent of the fibroid volume5,6.
The other treatment options available for uterine fibroids include hysterectomy, open myomectomy, laparoscopic and hysteroscopic myomectomy. Surgically guided thermoablative techniques like myolysis and cryoablation have shown variable response. Uterine artery embolization though widely used and less invasive can cause post embolization infection, fever, chronic vaginal discharge, fibroid extrusion and ovarian failure and requires expensive equipment and expertise1.
The advantages of MRgFUS over other therapies is that it is a day care procedure, can be conducted under conscious sedation, patient can communicate with the treating physician during treatment, can stop treatment herself and resume her daily activities from the next day of treatment. The other advantages with this treatment are uterus is conserved with chances of future pregnancy and it can be carried out even in anaemic patients and those who cannot withstand surgery. Spontaneous conception after treatment with MRgFUS for uterine fibroids has been reported19,20. Though this study included five subjects with uterine fibroids and infertility, so far there was no positive pregnancy outcome.
MRgFUS is also emerging as a treatment option for various other medical conditions like carcinoma breast, prostate, liver and palliation of bone metastasis21,22,23. The limitating factors for widespread application of this modality of treatment are (i) less than 50 per cent of subjects with uterine fibroids are eligible for treatment with this procedure, (ii) high cost of equipment, (iii) need for experienced radiologists and gynaecologists well versed with the procedure, and (iv) need for more comparative studies with other modalities of treatment to establish its long term efficacy.
To conclude, MRgFUS is a novel non invasive treatment option for treating uterine fibroids. It is relatively safe and effective in selected patients with minimal serious adverse effects and can be repeated if necessary5. The long term efficacy and durability of this treatment is yet to be tested and compared with other presently available minimally invasive treatment options. This study included a limited number of subjects and studies involving more number of subjects are required in future to establish its safety and efficacy.
Acknowledgment
The authors thank Dr Ganni Bhaskararao, Chairman GSL group of institutions who introduced this technology for the first time in south India in a teaching hospital, Prof (Dr) T. Hanumantharao Medical Superintendent GSL general hospital, Prof (Dr) Y.V. Sharma principal and the teaching and technical staff of Radiology and Gynaecology departments for their constant support during the study. The authors also thank Shri Paul and Shri More Technical staff of Insightec Israel who trained them in MRgFUS applications and Shri N. Lakshmanrao, Assistant Professor of statistics for statistical analysis.
References
- 1.Stewart EA, Gedroyc WM, Tempany CM, Quade BJ, Inbar Y, Ehrenstein T, et al. Focused ultrasound treatment of uterine fibroid tumors: safety and feasibility of a noninvasive thermoablative technique. Am J Obstet Gynecol. 2003;189:48–54. doi: 10.1067/mob.2003.345. [DOI] [PubMed] [Google Scholar]
- 2.Fennessy FM, Tempany CM. MRI guided focused ultrasound surgery of uterine leiomyomas. Acad Radiol. 2005;12:1158–66. doi: 10.1016/j.acra.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 3.Manyonda IT, Gorti M. Costing magnetic resonance-guided focused ultrasound surgery, a new treatment for symptomatic fibroids. BJOG. 2008;115:551–3. doi: 10.1111/j.1471-0528.2007.01656.x. [DOI] [PubMed] [Google Scholar]
- 4.Fennessy FM, Tempany CM. A review of magnetic resonance imaging-guided focused ultrasound surgery of uterine fibroids. Top Magn Reson Imaging. 2006;17:173–9. doi: 10.1097/RMR.0b013e3180337e1f. [DOI] [PubMed] [Google Scholar]
- 5.Hudson SB, Stewart EA. Magnetic resonance-guided focused ultrasound surgery. Clin Obstet Gynecol. 2008;51:159–66. doi: 10.1097/GRF.0b013e318161e91f. [DOI] [PubMed] [Google Scholar]
- 6.Tempany CM. From the RSNA refresher courses: Image-guided thermal therapy of uterine fibroids1. Radiographics. 2007;27:1819–26. doi: 10.1148/rg.276075096. [DOI] [PubMed] [Google Scholar]
- 7.Hesley GK, Felmlee JP, Gebhart JB, Dunagan KT, Gorny KR, Kesler JB, et al. Noninvasive treatment of uterine fibroids: early mayo clinic experience with magnetic resonance imaging-guided focused ultrasound. Mayo Clin Proc. 2006;81:936–42. doi: 10.4065/81.7.936. [DOI] [PubMed] [Google Scholar]
- 8.Rabinovici J, Inbar YA, Ravel A, Zalel Y, Gomori JM, Itzchak Y, et al. Clinical improvement and shrinkage of uterine fibroids after thermal ablation by magnetic resonance-guided focused ultrasound surgery. Ultrasound Obstet Gynecol. 2007;30:771–7. doi: 10.1002/uog.4099. [DOI] [PubMed] [Google Scholar]
- 9.Chapman A, Gail ter Haar. Thermal ablation of uterine fibroids using MR-guided focused ultrasound-a truly non-invasive treatment modality. Eur Radiol. 2007;17:2505–11. doi: 10.1007/s00330-007-0644-8. [DOI] [PubMed] [Google Scholar]
- 10.Hokland SL, Pedersen M, Salomir R, Quesson B, Stodkilde-Jørgensen H, Moonen CT. MRI-guided focused ultrasound: methodology and applications. IEEE Trans Med Imaging. 2006;25:723–31. doi: 10.1109/tmi.2006.873296. [DOI] [PubMed] [Google Scholar]
- 11.Fennessy FM, Tempany CM, McDanold NJ, So MJ, Hesley G, Gostout B, et al. Uterine leiomyomas: MRImaging-guided focused ultrasound surgery - results of different treatment protocols. Radiology. 2007;243:885–93. doi: 10.1148/radiol.2433060267. [DOI] [PubMed] [Google Scholar]
- 12.Hindley J, Gedroyc WM, Regan L, Stewart E, Tympany C, Hynyen K, et al. MRI guidance of focused ultrasound therapy of uterine fibroids: early results. Am J Roentgenol. 2004;183:1713–9. doi: 10.2214/ajr.183.6.01831713. [DOI] [PubMed] [Google Scholar]
- 13.Steward EA, Gostout B, Rabinovici J, Kim HS, Regan L, Tympany CM. Sustained relief of leiomyoma symptoms by using focused utrasound surgery. Obstet Gynecol. 2007;110:279–87. doi: 10.1097/01.AOG.0000275283.39475.f6. [DOI] [PubMed] [Google Scholar]
- 14.Smart OC, Hindley JT, Regan L, Gedroyc WM. Magnetic resonance guided focused ultrasound surgery of uterine fibroids - the tissue effects of GnRH agonist pre-treatment. Eur J Radiol. 2006;59:163–7. doi: 10.1016/j.ejrad.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 15.McDanold N, Tempany CM, Fennessy FM, So MJ, Rybicki FJ, Stewart EA, et al. Uterine leiomyomas: MR imaging-based thermometry and thermal dosimetry during focused ultrasound thermal ablation. Radiology. 2006;240:263–72. doi: 10.1148/radiol.2401050717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stewart EA, Rabinovici J, Tempany CM, Inbar Y, Regan L, Gostout B, et al. Clinical outcomes of focused ultrasound surgery for the treatment of uterine fibroids. Fertil Steril. 2006;85:22–9. doi: 10.1016/j.fertnstert.2005.04.072. [DOI] [PubMed] [Google Scholar]
- 17.So MJ, Fennessy FM, Zou KH, McDannold N, Hynynen K, Jolesz FA, et al. Does the phase of menstrual cycle affect MR-guided focused ultrasound surgery of uterine leiomyomas? Eur J Radiol. 2006;59:203–7. doi: 10.1016/j.ejrad.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Funaki K, Fukunishi H, Funaki T, Sawada K, Kahi Y, Maruo T. Magnetic resonance-guided focused ultrasound surgery for uterine fibroids: relationship between the therapeutic effects and signal intensity of preexisting T2-weighted magnetic resonance images. Am J Obstet Gynecol. 2007;196:184.e1–6. doi: 10.1016/j.ajog.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 19.Hanstede MM, Tympany CM, Stewart EA. Focused ultrasound surgery of intramural leiomyomas may facilitate fertility: a case report. Fertil Steril. 2007;88:497.e5–7. doi: 10.1016/j.fertnstert.2006.11.103. [DOI] [PubMed] [Google Scholar]
- 20.Rabinovici J, David M, Fukunishi H, Morita Y, Gostout BS, Stewart EA MRgFUS Study Group. Pregnancy outcome after magnetic resonance-guided focused ultrasound surgery (MRgFUS) for conservative treatment of uterine fibroids. Fertil Steril. 2010;93:199–209. doi: 10.1016/j.fertnstert.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Machtinger R, Inbar Y, Ben-Baruch G, Korach J, Rabinovici J. MRgFUS for pain relief as palliative treatment in recurrent cervical carcinoma: a case report. Gynecol Oncol. 2008;108:241–3. doi: 10.1016/j.ygyno.2007.08.079. [DOI] [PubMed] [Google Scholar]
- 22.Catane R, Beck A, Inbar Y, Rabin T, Shabshin N, Hengst S, et al. MR-guided focused ultrasound surgery (MRgFUS) for the palliation of pain in patients with bone metastases - preliminary clinical experience. Ann Oncol. 2007;18:163–7. doi: 10.1093/annonc/mdl335. [DOI] [PubMed] [Google Scholar]
- 23.Okada A, Murakami T, Mikami K, Onishi H, Tanigawa N, Marukawa T, et al. A case of hepatocellular carcinoma treated by MR-guided focused ultrasound ablation with respiratory gating. Magn Reson Med Sci. 2006;5:167–71. doi: 10.2463/mrms.5.167. [DOI] [PubMed] [Google Scholar]


