Abstract
Background & objectives:
The association between α-adducin gene G614T polymorphism and essential hypertension (EH) is not clear. The present study was carried out to examine a possible association between α-adducin gene G614T mutation and essential hypertension in Chinese population.
Methods:
A total of 170 patients with essential hypertension (EH group) and 154 normotensive subjects (Control group) were genotyped for the cytoskeletal protein single nucleotide polymorphism G614T of the α-adducin gene by PCR-RFLP technique. Systolic blood pressure (SBP), diastolic blood pressure (DBP), body mass index (BMI), low density lipoprotein (LDL), high-density lipoprotein (HDL), high-sensitivity C-reactive protein (hs-CRP), left atrial diameter (LA DIA), left ventricular diameter (LV DIA) and other parameters were recorded in EH group.
Results:
There was significant association between EH and α-adducin genotypes (P<0.05). GT and TT genotypes in EH group had higher LDL levels as compared to GG carriers (P<0.05). The LDL concentration was significantly elevated in patients with GT and TT genotypes. The LDL levels also differed significantly in male patients with all the three genotypes.
Interpretation & conclusions:
A significant association was found between ADD1 gene G614T polymorphism and EH in Chinese patients. Further studies need to be done to confirm these findings in a large sample.
Keywords: α-adducin, essential hypertension, gene, LDL, polymorphisms
Essential hypertension (EH) is the most common cardiovascular disease worldwide. Epidemiological studies have shown that the incidence of hypertension is influenced by the genes, environmental factors and lifestyle of individuals. The occurrence of EH may be associated with a variety of gene mutations and variations including adducin gene (ADD)1,2,3. The α-adducin gene (ADD1) has been implicated in causing susceptibilty to hypertension, especially in relation to salt sensitivity4. ADD1 gene polymorphism G614T (rs4961) is found to result in an increased enzymatic activity of the outer medulla Na+-K+-ATPase prior to the development of hypertension in the Milan hypertensive strain of rats (MHS)5. The G614T polymorphism results in the amino acid substitution of glycine by tryptophan (Gly460Trp) which is reported to be associated with a salt sensitive form of hypertension patients6. There is no clear consensus on the α-adducin gene polymorphism (Gly460Trp) and risk of EH in Chinese population. Liu et al7,8 conducted a meta-analysis in an effort to systematically explore the possible association and suggested that the Gly460Trp polymorphism might increase the risk of hypertension in Chinese populations, especially in Han Chinese.
The potential involvement of α-adducin Gly460Trp gene mutation in the pathogenesis of EH has been demonstrated in a few studies9,10, but no definite conclusion could be drawn. The aim of the present study was, therefore, to analyze the possible association between G614T polymorphism of ADD1 gene and hypertesion phenotype in patients with EH.
Material & Methods
Subjects: Patients with EH (n=170) and normotensive controls (n=154) were consecutively selected from hypertension outpatient clinic and medical center, respectively, affiliated to the hospital of Zhejiang Medical College, Hangzhou, PR China from February to August 2010. The normotensive controls were the people who received common physical examination in the hospital. All subjects gave written informed consent for participation in the study and the study protocol was approved by the medical ethics committees of the Zhejiang Medical College. The EH group consisted of 97 male and 73 female patients with mean age of 57.4 ± 24 yr. Subjects with a history of diabetes mellitus and renal failure were excluded. Patients on antihypertensive drugs were excluded. The control group consisted of 81 males and 73 females with mean age of 56.9 ± 9.0 yr. These individuals came for routine physical examination in the hospital and had no family history of EH. The blood samples (3 ml) were collected after overnight fasting at morning without stasis in EDTA vacutainers. The patients were selected according to the Seventh Report of Joint National Committee on Detection, Evaluation and Treatment of High Blood Pressure (2003 JNC7) (i.e. systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mmHg were used as indication of hypertension). Patients with secondary hypertension, diabetes, abnormal liver and kidney function were excluded. All subjects were asked about smoking status. Body mass index (BMI) was calculated with the formula, weight (kg)/height (m2).
Quantitative parameters: Plasma high-sensitivity C-reactive protein (hsCRP) levels were measured within 2-3 h after collection of blood samples by high sensitivity enzyme immunoassay (Dade-Behring, Marburg Germany). Low-density lipoprotein (LDL) and high-density lipoprotein (HDL) were assessed using Flex Reagent Cartridges (Dade-Behring, Marburg Germany).
Genotyping: Genomic DNA was extracted from peripheral blood using Blood Genome DNA Extraction Kit (TAKARA Biotechnology Co. Ltd., Dalian, Japan). The genotyping of G614T polymorphism of ADD1 was done by PCR-RFLP technique11. DNA fragments were amplified in a total volume of 50 μl PCR reaction mixture containing 10 × buffer 5 μl, 2.5 mM dNTP 4 μl, forward primer (5’-ctcctttgctagtgacggtgattc-3’) 0.5 μl, reverse primer (5’-gacttggcactgcttccattcgc-3’) 0.5 μl, double distilled water (DDW) 37.75 μl, Taq polymerase (TAKARA Biotechnology Co. Ltd., Japan) 0.25 μl and DNA 2 μl. Amplification was carried out under the following conditions: one cycle of 5 min at 95 °C, 35 cycles of 35 sec at 95 °C, 35 sec at 56 °C, and 35 sec at 72 °C, followed by 5 min at 72 °C. A mismatch, which introduces a Sau96I restriction site, was placed in the 3’ region of reverse primer to enable genotyping via restriction digest (the mismatched nucleotide is underlined and in heavier version. Amplified products were digested with Sau96I enzyme (NBE Inc., US) at 37 °C for 16 h. All products were loaded onto 3 per cent MS-6 agarose (TAKARA Biotechnology Co. Ltd., Dalian), and electrophoresed. Bands were visualized and typed after GelRed staining (Biotium Company, US). The length of PCR amplification product with G614T was 147 bp. The Sau 96I restriction enzymes were used to distinguish 614G/T, resulting in 122 bp and 25 bp fragments in the presence of the G allele. The polymorphism analysis was performed by two persons independently in a blind fashion. More than 10 per cent of the samples were randomly selected for confirmation, and the results were 100 per cent concordant (Figure).
Fig.
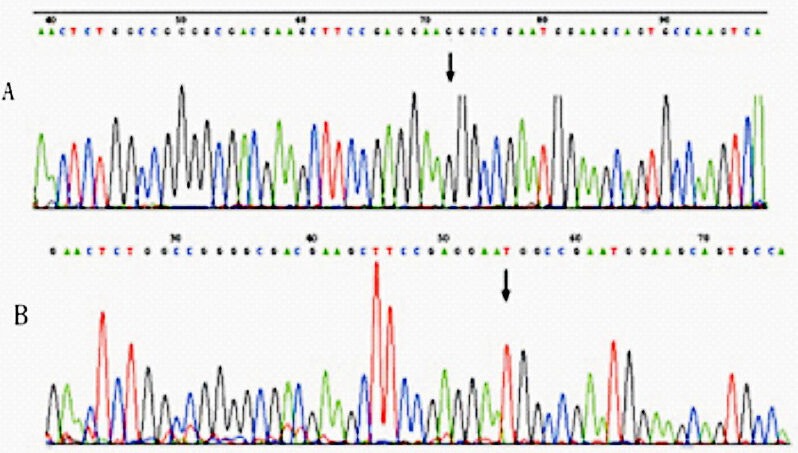
Chromatograms showing nucleotide sequence of ADD1 gene G614T polymorphism. (A) Normal allele with G, (B) mutant allele with T. Substitution of the nucleotide indicated by arrows.
Statistical analyses: The SPSS 16.0 software (SPPS Inc. USA) was used in this study. The expected frequencies of the ADD1 G614T genotypes were tested for the Hardy-Weinberg Equilibrium. Statistical differences for the distribution of genotypes G614T between EH and control groups were assessed by χ2 test. The relationships of the ADD1 genotypes with the clinicopathologic parameters of patients were tested by t-test. Logistic regression analysis was performed to assess the independent effect of each risk factor on the occurrence of EH. The odds ratio (OR) was calculated as estimators of relative risk, together with their 95% confidence intervals (95% CI). The difference in the clinicopathologic parameters according to ADD1 G614T genotype distributions was compared using ANOVA test.
Results
No significant differences were observed with respect to age, sex, smoking status, serum HDL, CRP levels between hypertensive subjects and the controls. However, the EH group had a significantly (P<.05) higher blood pressure (Table I), BMI and the serum LDL concentration compared to the control group. Table II shows the genotype distributions and the allele frequencies of ADD1 G614T polymorphism in the two groups. A significant difference in genotype distributions between hypertensives and controls was noted with the observed power of 0.899. The TT genotype was significantly (P<.05) higher in hypertensives as compared with controls (TT: 31.18 vs. 16.88%). From the “Genetic Power Calculator”, the genotypic risk for TT was 12.27 per cent. For G and T allele frequencies, there was a significant (P<.05) difference between hypertensives and controls as well (G: 41.76 vs. 57.47%, T: 58.24 vs. 42.53%). The frequencies of the genotypes were significantly different between the patient and control groups (P<0.05). Both genotypes and alleles frequencies in control and EH groups were in Hardy-Weinberg equilibrium. By univariate analysis, it was found that EH was influenced by BMI, LDL and ADD1 gene 614T allele. By multivariate logistic regression, T allele (OR=2.217, 95% CI: 1.243-3.953, P=0.007<0.05/3=0.017) was the independent risk factor of essential hypertension (Table III).
Table I.
Clinical characteristics of essential hypertensive (EH) and normotensive groups
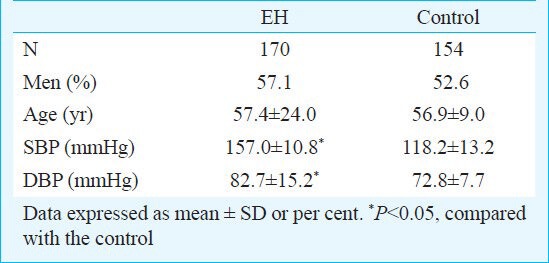
Table II.
Allele frequencies of α-adducin gene (ADD1) G614T polymorphism in normotensive and hypertensive groups
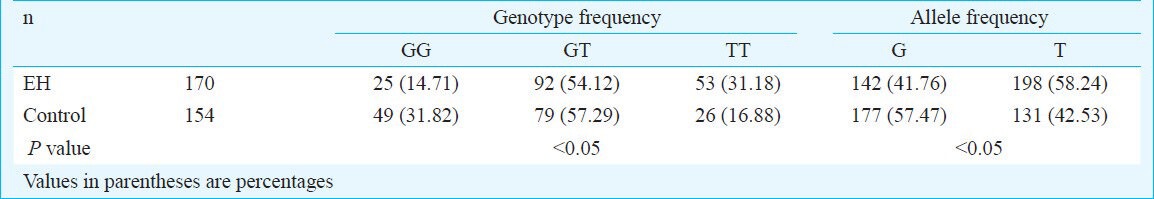
Table III.
Clinical characteristics of each genotype in essential hypertensive groups
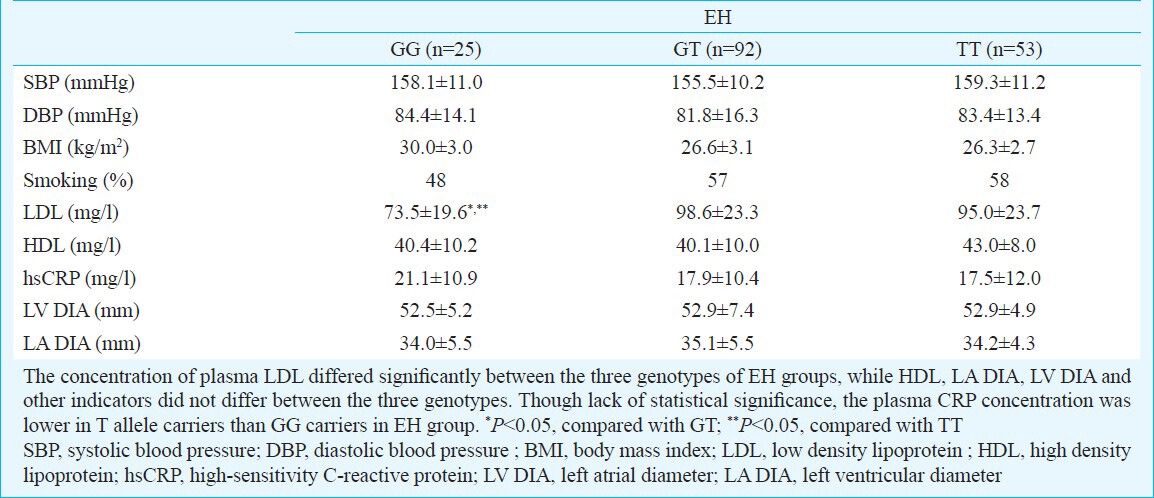
The demographic and clinical characteristics in EH group were studied according to the ADD1 G614T genotype distributions. It was found that the concentration of plasma LDL differed significantly between the three genotypes in EH group (Table III). But the level of LDL was not associated with ADD1 gene G614T polymorphism in the control group (data not shown). When subdivided according to gender, no significant association was observed with respect to clinical characteristics in female patients, while LDL was significantly elevated in the male patients (P<0.05). Though there was lack of statistical significance, the plasma CRP concentration was lower in patients who were T allele carriers than in those with GG genotype.
Discussion
ADD1 G614T polymorphism plays a potential biological role in the development of high blood pressure12. Several previous studies revealed that this variant was a likely candidate for studying association with hypertension status13,14,15,16. In this study, there was a significant difference in α-adducin genotype between EH and control groups. The levels of LDL were significantly increased in persons with GT and TT genotypes in EH group as compared with GG genotypes.
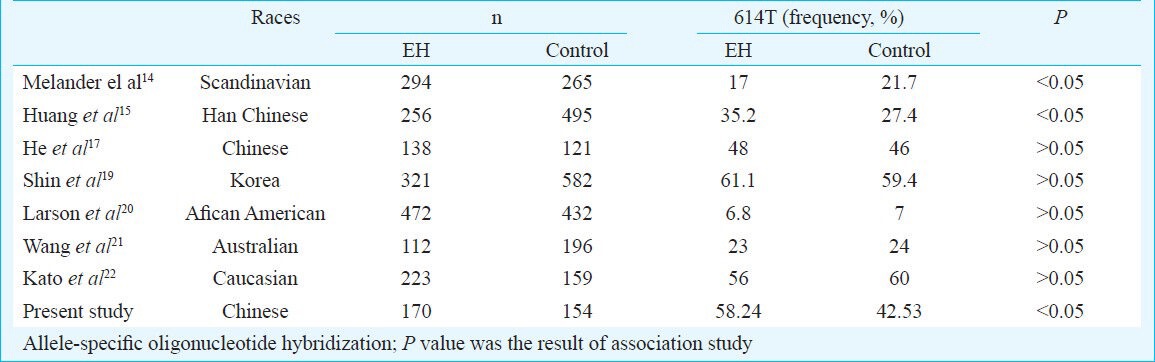
The frequency of α-adducin gene G614T varies in different populations. In our study, the frequency of α-adducin gene 614T in south China was 42.53 per cent in normotensives and 58.24 per cent in hypertensives. The frequency of α-adducin gene 614T allele in hypertensives and controls were 46 and 48 per cent in Chinese17, 54 and 60 per cent in Japanese17, 59 and 61 per cent in Koreans18 and 53 and 33 per cent in Americans14.
Several studies have demonstrated the potential involvement of α-adducin in the pathogenesis of EH, but no definite conclusion could be drawn (Table IV). Factors like ethnic diversity, sample sizes are expected to be the cause for these inconclusive results found. A sample of 904 African Americans (from Jackson, Mississippi) was examined for α- adducin gene 614T association with hypertension20. The results showed that African Americans not only have a higher prevalence of hypertension, but also the condition strikes at an early age, with greater severity, often ending in death when compared with whites in the United States. No association was found between hypertension status and the ADD1 G614T polymorphism in American Blacks23, white population of USA, Australia21,22,23,24. A case-control study conducted in a large population from Sassari, Italy, did not find any association of the α-adducin T allele with hypertensives while the study in a large population from Milan, Italy confirmed a positive association25. No significant association was found in a well characterized Japanese population22 and Korean population that was also Asian descent19. G614T polymorphism in Chinese Han population of Shang Hai also reported absence of association with hypertension26. Adjusted for the conventional risk factors of hypertension, alpha-adducin polymorphism has been shown to play an independent role on systolic blood pressure in Indians living in Car Nicobar Island16. Our study showed that there was significant association between G614T substitution of ADD1 gene and hypertesion phenotype of EH patients in South China.
Table IV.
Frequency of α-adducin gene (ADD1) G614T varies in different populations
In our study, the concentration of plasma LDL was higher in hypertensives carrying at least one 614T allele than in GG homozygotes. Furthermore, no association of LDL concentration and the mutation was observed in female hypertensives. Males with 614T allele had higher LDL, suggesting risk for cardiovascular diseases. Castejon et al27 reported that the concentration of plasma LDL was significantly different in the GG and GT healthy groups. However, because of the small sample size (n=90), TT homozygotes were not detected. In our study, TT homozygotes were detected and EH patients with T allel had higher plasma LDL level. The mechanism by which ADD polymorphism influences LDL levels is not clear and further study is needed to investigate underlying factors in detail.
In conclusion, our results indicate towards genetic association between α-adducin gene G460T polymorphism and hypertension. Further studies need to be done on the association of this polymorphism with hypertension in different ethnic groups with larger samples.
References
- 1.Clark CJ, Davies E, Anderson NH, Farmer R, Friel EC, Fraser R, et al. Alpha-adducin and angiotensin I-converting enzyme polymorphisms in essential hypertension. Hypertension. 2000;36:990–4. doi: 10.1161/01.hyp.36.6.990. [DOI] [PubMed] [Google Scholar]
- 2.Nicod J, Frey BM, Frey FJ, Ferrari P. Role of the á-adducin genotype on renal disease progression. Kidney Int. 2002;61:1270–5. doi: 10.1046/j.1523-1755.2002.00275.x. [DOI] [PubMed] [Google Scholar]
- 3.Rajan S, Ramu P, Umamaheswaran G, Adithan C. Association of aldosterone synthase (CYP11B2 C-344T) gene polymorphism & susceptibility to essential hypertension in a south Indian Tamil population. Indian J Med Res. 2010;132:379–85. [PubMed] [Google Scholar]
- 4.Chen S, Wang H, Lu X, Liu DP, Chen J, Jaquish CE, et al. Polymorphisms in the GNB3 and ADD1 genes and blood pressure in a Chinese population. Hum Genet. 2010;128:137–43. doi: 10.1007/s00439-010-0834-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tripodi G, Florio M, Ferrandi M, Modica R, Zimdahl H, Hubner N, et al. Effect of Addl gene transfer on blood pressure in reciprocal eongenie strains of Milan rats. Biochem Biophys Res Commun. 2004;324:562–8. doi: 10.1016/j.bbrc.2004.09.079. [DOI] [PubMed] [Google Scholar]
- 6.Suonsyrja T, Hannila-Handelberg T, Fodstad H, Donner K, Kontula K, Hiltunen TP. Renin angiotensin system and alpha-adducin gene polymorphisms and their relation to responses to antihypertensive drugs: results from the GENRES study. Am J Hypertens. 2009;22:169–75. doi: 10.1038/ajh.2008.343. [DOI] [PubMed] [Google Scholar]
- 7.Liu K, Liu Y, Liu J, Wang Z, Lou Y, Huang Y, et al. α-Adducin Gly460Trp polymorphism and essential hypertension risk in Chinese: a meta-analysis. Hypertens Res. 2011;34:389–99. doi: 10.1038/hr.2010.252. [DOI] [PubMed] [Google Scholar]
- 8.Liu K, Liu J, Huang Y, Liu Y, Lou Y, Wang Z, et al. Alpha-adducin Gly460Trp polymorphism and hypertension risk: a meta-analysis of 22 studies including 14303 cases and 15961 controls. PLoS One. 2010;5:e13057. doi: 10.1371/journal.pone.0013057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li YY. α-Adducin Gly460Trp gene mutation and essential hypertension in a Chinese population: a meta-analysis including 10,960 subjects. PLoS One. 2012;7:e30214. doi: 10.1371/journal.pone.0030214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrandi M, Molinari I, Torielli L, Padoani G, Salardi S, Rastaldi MP, et al. Adducin- and ouabain-related gene variants predict the antihypertensive activity of rostafuroxin, part 1: experimental studies. Sci Transl Med. 2010;2:59ra86. doi: 10.1126/scitranslmed.3001815. [DOI] [PubMed] [Google Scholar]
- 11.Morrison AC, Doris PA, Folsom AR, Nieto FJ, Boerwinkle E. Atherosclerosis Risk in Communities Study. G-protein beta3 subunit and alpha-adducin polymorphisms and risk of subclinical and clinical stroke. Stroke. 2001;32:822–9. doi: 10.1161/01.str.32.4.822. [DOI] [PubMed] [Google Scholar]
- 12.Ramu P, Umamaheswaran G, Shewade DG, Swaminathan RP, Balachander J, Adithan C. Gly460Trp polymorphism of the ADD1 gene and essential hypertension in an Indian population: A meta-analysis on hypertension risk. Indian J Hum Genet. 2010;16:8–15. doi: 10.4103/0971-6866.64938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beeks E, Janssen RG, Kroon AA, Keulen ET, Geurts JM, de Leeuw PW, et al. Association betwecn the alpha-adducin Gly460Trp polymorphism and systofie blood pressure in familial combined hyperlipidemia. Am J Hypertens. 2001;l4:1185–90. doi: 10.1016/s0895-7061(01)02216-6. [DOI] [PubMed] [Google Scholar]
- 14.Melander O, Bengtsson K, Orho-Melander M, Lindblad U, Forsblom C, Rastam L, et al. Role of the Gly460Trp polymorphism of the alpha- adducin gene in primary hypertension in Scandinavians. J Hum Hypertens. 2000;14:43–6. doi: 10.1038/sj.jhh.1000942. [DOI] [PubMed] [Google Scholar]
- 15.Huang XH, Sun K, Song Y, Zhang HY, Yang Y, Hui RT. Association of alpha adducin gene and G-protein beta3-subunit gene with essential hypertension in Chinese. Zhonghua Yi Xue Za Zhi. 2007;87:1682–4. [PubMed] [Google Scholar]
- 16.Manimunda SP, Sugunan AP, Benegal V, Balakrishna N, Rao MV, Pesala KS. Association of hypertension with risk factors & hypertension related behaviour among the aboriginal Nicobarese tribe living in Car Nicobar Island, India. Indian J Med Res. 2011;133:287–93. [PMC free article] [PubMed] [Google Scholar]
- 17.He X, Zhu DL, Chu SL, Jin L, Xiong MM, Wang GL, et al. Alpha-Adducin gene and essential hypertensionin in China. Clin Exp Hypertens. 2001;23:579–89. doi: 10.1081/ceh-100106828. [DOI] [PubMed] [Google Scholar]
- 18.Katsuya T, Ishikawa K, Sugimoto K, Rakugi H, Ogihara T. Salt sensitivity of Japanese from the viewpoint of gene polymorphism. Hypertens Res. 2003;26:521–5. doi: 10.1291/hypres.26.521. [DOI] [PubMed] [Google Scholar]
- 19.Shin MH, Chung EK, Kim HN, Park KS, Nam HS, Kweon SS, et al. Alpha-adduein Gly460Trp polymorphism and essential hypertension in Korea. J Korean Med Sci. 2004;19:812–4. doi: 10.3346/jkms.2004.19.6.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Larson N, Hutchinson R, Boerwinkle E. Lack of association of 3 functional gene variants with hypertension in African Americans. Hypertension. 2000;35:1297–300. doi: 10.1161/01.hyp.35.6.1297. [DOI] [PubMed] [Google Scholar]
- 21.Wang WY, Adams DJ, Glenn CL, Morris BJ. The Gly460Trp variant of alpha adducin is not associated with hypertension in white Anglo-Australians. Am J Hypertens. 1999;12:632–6. doi: 10.1016/s0895-7061(99)00004-7. [DOI] [PubMed] [Google Scholar]
- 22.Kato N, Sugiyama T, Nabika T, Morita H, Kurihara H, Yazaki Y, et al. Lack of association between the alpha-adduein locus and essential hypertension in the Japanese population. Hypertension. 1998;31:730–3. doi: 10.1161/01.hyp.31.3.730. [DOI] [PubMed] [Google Scholar]
- 23.Martinez Cantarin MP, Ertel A, Deloach S, Fortina P, Scott K, Burns TL, et al. Variants in genes involved in functional pathways associated with hypertension in African Americans. Clin Transl Sci. 2010;3:279–86. doi: 10.1111/j.1752-8062.2010.00242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang JG, Staessen JA, Barlassina C, Fagard R, Kuznetsova T, Struijker-Boudier HA, et al. Association between hypertension and variation in the aipha-and beta-adducin genes in a white population. Kidney Int. 2002;62:215–9. doi: 10.1046/j.1523-1755.2002.00691.x. [DOI] [PubMed] [Google Scholar]
- 25.Glorioso N, Manunta P, Filigheddu F, Troffa C, Stella P, Barlassina C, et al. The role of alpha-adducin polymorphism in blood pressure and sodium handling regulation may not be excluded by a negative association study. Hypertension. 1999;34:649–54. doi: 10.1161/01.hyp.34.4.649. [DOI] [PubMed] [Google Scholar]
- 26.Niu WQ, Zhang Y, Ji KD, Gao PJ, Zhu DL. Lack of association between alpha adducin G460W polymorphism and hypertension: evidence from a case-control study and a meta-analysis. J Hum Hypertens. 2010;24:467–74. doi: 10.1038/jhh.2009.88. [DOI] [PubMed] [Google Scholar]
- 27.Castejon AM, Alfieri AB, Hoffmann IS, Rathinavelu A, Cubeddu LX. Alpha adduein polymorphism, salt sensitivity, nitric oxide excretion, and cardiovascular risk factors in normotensive Hispanics. Am J Hypertens. 2003;16:1018–24. doi: 10.1016/j.amjhyper.2003.07.022. [DOI] [PubMed] [Google Scholar]


