Abstract
Background & objectives:
Updating information on response (susceptible / resistant status) of vectors to the insecticides in use is essential to formulate and introduce appropriate resistance management strategy. Therefore, a study was undertaken in the 10 southern districts of Odisha State, which are endemic for Plasmodium falciparum malaria, to determine the insecticide susceptibility/ resistance status of Anopheles fluviatilis and An. culicifacies, the vectors of malaria.
Methods:
Mosquitoes were collected during September 2010 - February 2012 from 60 randomly selected villages in the 10 districts and blood-fed females were exposed to the diagnostic dosage of DDT (4.0%), malathion (5.0%) and deltamethrin (0.05%) for one hour. Mortality was recorded at 24 h after the exposure. The test mortality was corrected to the control mortality.
Results:
An. fluviatilis was susceptible to the three insecticides tested while, An. culicifacies was resistant to DDT and malathion in all the 10 districts except in two, where its response against malathion was under ‘verification required’ category. Against deltamethrin, An. culicifacies was susceptible in two districts; while in the other eight districts its response was under ‘verification required’ category.
Interpretation & conclusions:
Since An. fluviatilis the vector species primarily associated with transmission of malaria, was still susceptible to DDT, indoor residual spraying with DDT could be continued in the 10 districts. Also, in view of the large scale implementation of long lasting insecticidal nets and the signs of development of resistance in An. culicifacies to deltamethrin, response of the vectors to synthetic pyrethroids needs to be periodically monitored.
Keywords: Anopheles culicifacies, Anopheles fluviatilis, India, insecticide resistance, insecticide susceptibility, Odisha
Odisha State in India is afflicted with high incidence of malaria, predominantly of Plasmodium falciparum infection, for the last many years, and during 2010 it contributed to around 45 per cent of the total P. falciparum cases recorded in the country. Deaths due to cerebral malaria caused by this Plasmodium species are high and the data for 2005 to 2010 showed that more than 20 per cent (n = 1417) of the total deaths due to malaria in India (n = 6947) occurred in the State, although Odisha State represents only about 4 per cent of the population of the country1. Of the total 30 districts of Odisha State, the 10 southern districts: Rayagada, Nowrangpur, Kalahandi, Nuapada, Bolangir, Kandhamal, Gajapati, Ganjam, Malkangiri and Koraput have seriously been affected by malaria contributing more than 70 per cent of the total positive cases and more than 64 per cent of the total malaria deaths in the State during 20112. These highly malarious districts have been under the influence of two vectors, viz. Anopheles fluviatilis and An. culicifacies3,4. Despite various control measures, malaria continues to be a major public health problem with high morbidity and mortality even today. Currently, indoor residual spraying with DDT or synthetic pyrethroids (deltamethrin) has been carried out as the major vector control measure. In addition, long lasting synthetic pyrethroid (deltamethrin) treated mosquito nets (LLINs) have been distributed in these districts.
Development of resistance by malaria vectors to insecticides has been reported from various parts of India. DDT resistance in Odisha State in An. culicifacies was first reported during 19665. Subsequently, there were reports of DDT and HCH resistance in this species from Koraput during 1990 and 19956,7 and from Sundargarh district during 19918. Sharma et al3 studied the susceptibility status of An. fluviatilis and An. culicifacies in eight northern and western districts of Odisha State. However, there is no information on the susceptibility status of the malaria vectors, particularly of An. fluviatilis, in the other highly malarious districts of the State. Since, such information is essential to formulate appropriate vector control strategy or to redesign the ongoing strategy in these districts, a study was undertaken to assess the current status of insecticide susceptibility of An. fluviatilis and An. culicifacies to DDT and deltamethrin, the insecticides in use in malaria control programme and also to malathion for cross resistance, if any, in the 10 southern districts of Odisha State.
Material & Methods
Study area: The study was carried out in the 10 southern districts of Odisha State during September 2010 - February 2012. Most of the districts are hilly and forested. Dry summer (March-June), wet rainy (July-September) and dry winter (October-February) are the three prevailing seasons. The districts have been hyper-endemic for malaria for many decades. P. falciparum is the predominant species, contributing to >90 per cent of the total malaria cases2. Malaria incidence peaks during two seasons, one during July to September and the other during November to December. An. fluviatilis has been incriminated as the major malaria vector4. Streams and terraced paddy fields are the major breeding habitats of An. fluviatilis9. An. culicifacies, which is a secondary vector, prevalent during summer and rainy seasons4, prefers to breed in riverbed pools, terraced paddy fields and ponds9.
There are altogether 115 community health centres (CHCs) in the 10 districts. Among these, 20 CHCs, two from each district, were randomly selected. In each CHC, three villages were selected randomly for collection of the vector mosquitoes to determine their susceptibility status. The study protocol was approved by the Ethical Committee of Vector Control Research Centre (VCRC), Puducherry, India.
Mosquito collections and susceptibility test: The required number of female mosquitoes of An. fluviatilis and An. culicifacies were collected from cattle sheds and human dwellings in the morning hours using mouth aspirator and flash light in the selected villages. Susceptibility tests were performed on the wild caught blood-fed females using WHO kits10. The field collected mosquitoes were provided with 10 per cent glucose solution soaked in cotton pads and brought to the camp laboratory in 1ft5 mosquito cages wrapped with a wet towel. The temperature and relative humidity in the camp laboratory was maintained at 25 ± 2 °C and 70-85 per cent, respectively. Insecticide impregnated papers of DDT 4 per cent, malathion 5 per cent and deltamethrin 0.05 per cent were obtained from the University Sains Malaysia, Penang, Malaysia. Female mosquitoes were exposed for one hour in 3 to 4 replicates, each replicate with 15 to 20 mosquitoes, to the diagnostic dosage of the insecticides. Parallel controls for comparison were maintained. Number knocked down was recorded after one hour exposure and after the exposure the mosquitoes were maintained for 24 h with glucose food at the same temperature and relative humidity. Mortality was scored after 24 h holding period and if the control mortality remained within 5-20 per cent, the test mortality was corrected using Abbott's formula and expressed as corrected per cent mortality11. In case, the control mortality is >20 per cent, the tests were discarded11. According to the WHO criteria10, a corrected mortality of >98 per cent is ‘susceptible’, <80 per cent is ‘resistant’ and 80-98 per cent is ‘verification required’.
Results
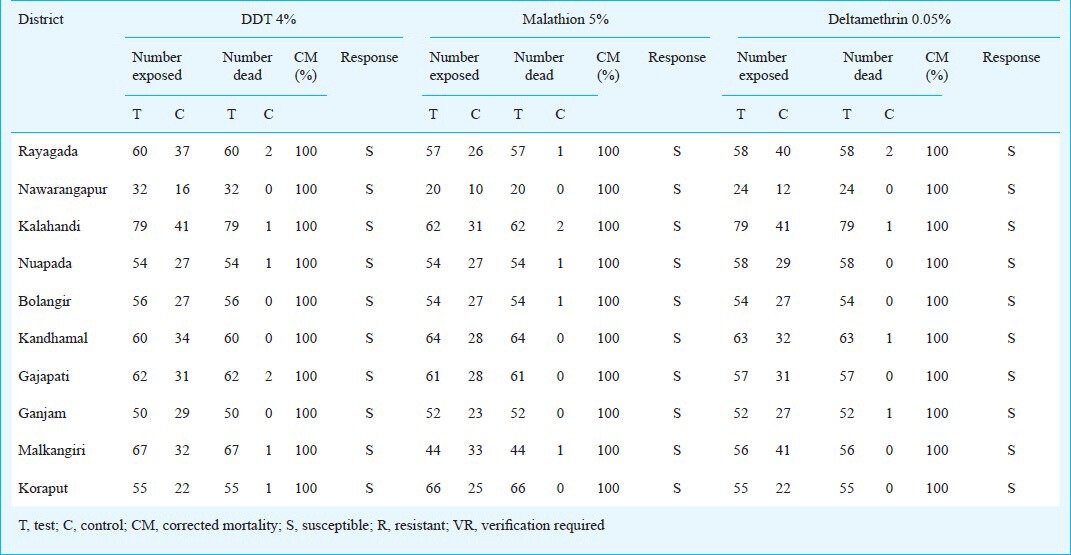
The corrected mortality of An. fluviatilis on exposure to DDT 4 per cent, malathion 5 per cent and deltamethrin 0.05 per cent are given in Table I. Adequate number (minimum of 100) of An. fluviatilis could not be exposed to each of the three insecticides, because of its relatively lower density in the study area. The results indicated that An. fluviatilis was susceptible to DDT, malathion and deltamethrin in all the 10 southern districts.
Table I.
Response of An. fluviatilis to DDT, malathion and deltamethrin in the 10 southern districts of Odisha State
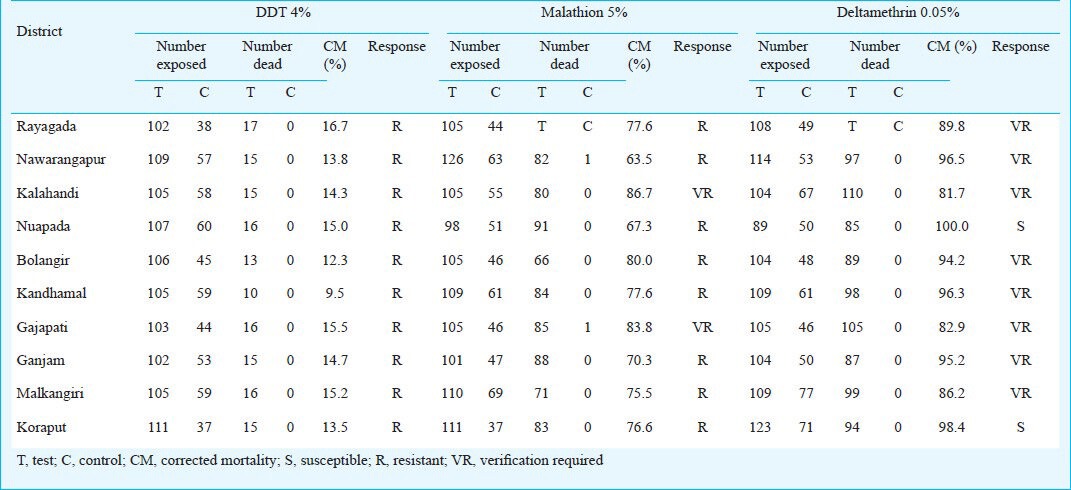
Tables II summarizes the susceptibility status of An. culicifacies to DDT, malathion and deltamethrin. The corrected mortality of this species ranged between 9.5 and 16.7 per cent against DDT 4, 63.5 and 86.7 per cent against malathion 5 and 81.7 per cent and 100 per cent against deltamethrin 0.05 per cent. The results thus showed that An. culicifacies was resistant to DDT and malathion in all the 10 districts except in Kalahandi and Gajapati, where the response of this species against malathion was under ‘verification required’ category and further monitoring at periodical intervals could confirm the susceptibility/ resistance status of this species against malathion in these districts. To deltamethrin, An. culicifacies was susceptible in two districts viz. Nuapada and Koraput, while, in the remaining eight districts, its status was under ‘verification required’ category. Overall, the results indicated that An. culicifacies developed resistance to DDT and malathion, and showed an increased tolerance to deltamethrin, as the corrected mortality of this species fell under ‘verification required’ category in most of the districts.
Table II.
Response of An. culicifacies to DDT, malathion and deltamethrin in the 10 southern districts of Odisha State
Discussion
Chemical control of vectors continues to be the mainstay of the malaria control programme in India. Monitoring vector susceptibility to the insecticides at regular interval has become imperative to ensure judicious and effective use of insecticides in the control programme. The present findings highlight the current status of insecticide susceptibility / resistance status of malaria vectors in the southern districts of Odisha State, which are predominantly inhabited by tribes and hyperendemic for P. falciparum malaria. The primary role of An. fluviatilis and the secondary importance of An. culicifacies in transmission of malaria in the study area were established during nineties3,4. The earlier studies have reported that An. fluviatilis was susceptible to DDT, malathion and deltamethrin in Koraput6,7 and Sundergarh district8 of Odisha State. Sharma et al3 also reported its susceptibility to the three insecticides in five other districts of Odisha State including Kalahandi and Phulabani (presently called as Kandhamal) which are among the 10 southern districts covered by the current study. However, resistance to DDT in this vector species has been reported from Puri and Balasore districts of Odisha12. Kumari et al12 reported development of resistance by this species to DDT in 11 districts from eight States in India. Recently, DDT resistance in An. fluviatilis has been reported from Jharkhand13,14. In the current study, An. fluviatilis was found to be still susceptible to DDT, malathion and deltamethrin in all the 10 southern districts of Odisha. Further, An. fluviatilis is mainly distributed in hill-top, foot-hill and forested villages in India and its preferential breeding habitat is streams15, which are less likely to be exposed to agriculture pesticides.
An. culicifacies was resistant to DDT and malathion in all the 10 southern districts except in two, where its mortality against malathion fell under ‘verification required’ category. To deltamethrin, this species was found susceptible in two districts while in the other eight districts, its response was under ‘verification required’ category indicating that this species was tending to develop resistance to deltamethrin. The earlier studies in Koraput district showed that An. culicifacies was resistant to DDT but susceptible to malathion 5 per cent and deltamethrin 0.025 per cent6,7. Subsequent studies carried out during 2004 in eight districts of Odisha State including the five districts covered in the current study reported that An. culicifacies was resistant to DDT in all the eight districts, to malathion in four districts (Mayurbhanj, Bolangir, Nuapada and Kalahandi) and was showing signs of development of multiple resistance to DDT, malathion and deltamethrin in three districts (Bolangir, Nuapada and Kalahandi)3.
Double or triple resistance to DDT, dieldrin and malathion was reported in An. culicifacies from 30 districts of Maharashtra16. Malathion resistance in this species was first observed in the adjoining State of Gujarat17. Subsequent report of resistance to malathion came from Andhra Pradesh18. In Dhanora taluka of Gadchiroli district in Maharashtra, An. culicifacies was resistant to DDT, but found susceptible to malathion and deltamethrin19. In Murumgaon PHC area of Gadchiroli district in Maharastra An. culicifacies was found resistant only to DDT, while it was tolerant to malathion and deltamethrin20. There are also reports of An. culicifacies showing resistance to synthetic pyrethroids in Tamil Nadu and Gujarat21,22, indicating the possibility of widespread resistance to other related compounds of this group. Synthetic pyrethroids are the potent insecticide most commonly used for indoor residual spraying, space spraying and for impregnating bednets under vector control programme. Synthetic pyrethroids are highly effective, if optimally applied, but development of resistance to these insecticides reduces their impact23. Although, An. culicifacies is only a secondary vector in the study area, the sign of development of resistance by this species to deltamethrin may pose certain amount of threat to the ongoing vector control programme. Therefore, regular monitoring is required for early detection of development of resistance by this species to deltamethrin and for assessing its epidemiological impact. The density of An. fluviatilis in the study area was low and, therefore, as per the WHO criteria10, the minimum number of 100 mosquitoes of this species could not be exposed to the insecticides in each district due to non-availability of adequate number in the field.
Two important conclusions could be derived from the current study. The first one is that An. fluviatilis, the major vector of malaria in the study area was susceptible to DDT and synthetic pyrethroids, the presently used insecticides; while DDT has been used for indoor residual spraying, synthetic pyrethroids are used both for indoor residual spraying and impregnating bednets under public health programme in these districts. Therefore, indoor residual spraying with DDT could be continued but by ensuring adequate coverage and quality through strengthening the advance information system. Also, compliance of bednet use by the community should be enhanced. While advocating use of bednets, it is necessary to insist upon regular use and to focus malaria prevention as the benefit of using nets, because, people who used bed nets as protection against mosquito bites were more likely not to use these when mosquitoes were few than those who used bed nets for malaria protection24,25. The second conclusion is that in view of resistance developed by An. culicifacies to DDT and malathion and of its ‘verification required’ status to deltamethrin, monitoring susceptibility of this species to synthetic pyrethroids, which are currently being used in the malaria control programme, is essential for a rationalized use of insecticides for vector control.
References
- 1.National Vector Borne Disease Control Programme, Directorate General of Health Services, Ministry of Health & Family Welfare, Govt. of India; 2013. [accessed on September 16, 2013]. National Vector Borne Disease Control Programme. Malaria situation in India. Availabe from: www.nvbdcp.gov.in . [Google Scholar]
- 2.Bhuwaneshwar: National Vector Borne Disease Control Programme, Ministry of Health & Family Welfare, Govt. of Odisha; 2013. Annual Report 2011-2012; p. 36. [Google Scholar]
- 3.Sharma SK, Upadhyay AK, Haque MA, Singh OP, Adak T, Subbarao SK. Insecticide susceptibility status of malaria vectors in some hyper-endemic tribal districts of Odisha. Curr Sci. 2004;87:1718–26. [Google Scholar]
- 4.Gunasekaran K, Sahu SS, Jambulingam P, Das PK. DDT Indoor residual spray, still an effective tool to control Anopheles fluviatilis - transmitted Plasmodium falciparum malaria in India. Trop Med Int Health. 2005;10:1–9. doi: 10.1111/j.1365-3156.2004.01369.x. [DOI] [PubMed] [Google Scholar]
- 5.Das M. A note on susceptibility status of some Anopheles to chlorinated hydrocarbon insecticides in Odisha. Bull Indian Soc Malaria Commun Dis. 1966;3:323–9. [Google Scholar]
- 6.Sahu SS, Gunasekaran K, Jambulingam P, Das PK. Susceptibility status of Anopheles fluviatilis, An. annularis and An. culicifacies to insecticides in Koraput district, Odisha. Indian J Malariol. 1990;27:51–3. [PubMed] [Google Scholar]
- 7.Sahu SS, Patra KP. A study on insecticides resistance in Anopheles fluviatilis and An. culicifacies to HCH and DDT in Malkangiri district of Odisha. Indian J Malariol. 1995;32:112–8. [PubMed] [Google Scholar]
- 8.Chand SK, Yadav RS. Insecticide susceptibility of mosquito vectors in Sundargarh district, Odisha. Indian J Malariol. 1991;28:65–8. [PubMed] [Google Scholar]
- 9.Sahu SS, Parida SK, Sadanandane C, Gunasekaran K, Jambulingam P, Das PK. Breeding habitats of malaria vectors: A. fluviatilis, A. annularis and A. culicifacies in Koraput district, Orissa. Indian J Malariol. 1990;27:209–16. [PubMed] [Google Scholar]
- 10.Geneva, Switzerland: WHO; 1998. World Health Organization. Report of the WHO informal consultation. Test procedures for insecticide resistance monitoring in malaria vectors, bio-efficacy and persistence of monitoring insecticides on treated surfaces. WHO/CDS/CPC/MAL/98/12; p. 43. [Google Scholar]
- 11.Abbott WS. A method of computing the effectiveness of an insecticide. J Econ Entomol. 1925;18:265–7. [Google Scholar]
- 12.Kumari R, Thapar BR, Dasgupta RK, Kaul SM, Lal S. Susceptibility status of malaria vectors to insecticides in India. J Commun Dis. 1998;30:179–85. [PubMed] [Google Scholar]
- 13.Singh RK, Dhiman RC, Mittal PK, Das MK. Susceptibility of malaria vectors to insecticides in Gumla district, Jharkhand state, India. J Vector Borne Dis. 2010;47:116–8. [PubMed] [Google Scholar]
- 14.Singh RK, Dhiman RC, Kumar G, Sinha ATS, Dua VK. Susceptibility status of malaria vectors to insecticides in Koderma, Jharkhand. J Commun Dis. 2011;43:273–6. [PubMed] [Google Scholar]
- 15.Singh N, Khare KK. Forest malaria in Madhya Pradesh: changing scenario of disease and its vectors. Indian J Parasit Dis. 1999;23:105–12. [Google Scholar]
- 16.Vittal M, Deshpande LB. Development of malathion resistance in a DDT, HCH resistant Anopheles culicifacies population in Thane district (Maharashtra) J Commun Dis. 1983;15:144–5. [PubMed] [Google Scholar]
- 17.Rajagopal R. Malathion resistance in Anopheles culicifacies in Gujarat. Indian J Med Res. 1977;66:27–8. [PubMed] [Google Scholar]
- 18.Raghavendra K, Vasantha K, Subbarao SK, Pillai MKK, Sharma VP. Resistance in Anopheles culicifacies sibling species B and C to malathion in Andhra Pradesh and Gujarat states in India. J Am Mosq Control Assoc. 1991;7:255–9. [PubMed] [Google Scholar]
- 19.Dhiman RC, Shahi B, Sharma SN, Khargiwarkar VN, Subbarao SK. Persistence of malaria transmission in a tribal area in Maharashtra. Curr Sci. 2005;88:754–8. [Google Scholar]
- 20.Singh RK, Mittal PK, Gourshettiwar MP, Pande SJ, Dhiman RC. Susceptibility of malaria vectors to insecticides in Gadchiroli district (Maharashtra), India. J Vector Borne Dis. 2012;49:42–4. [PubMed] [Google Scholar]
- 21.Mittal PK, Adak T, Singh OP, Raghavendra K, Subbarao SK. Reduced susceptibility to deltamethrin in Anopheles culicifacies sensu lato in Ramnathapuram district, Tamil Nadu - Selection of a pyrethroid-resistant strain. Curr Sci. 2002;82:185–8. [Google Scholar]
- 22.Singh OP, Raghavendra K, Nanda N, Mittal PK, Subbarao SK. Pyrethroid resistance in An. culicifacies in Surat district, Gujarat, west India. Curr Sci. 2002;82:547–50. [Google Scholar]
- 23.N’Guessan R, Corbel V, Akogbeto M, Rowland M. Reduced efficacy of insecticide-treated nets and indoor residual spraying for malaria control in pyrethroid resistance area, Benin. Emerg Infect Dis. 2007;13:199–206. doi: 10.3201/eid1302.060631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yohannes K, Dulhunty JM, Kourleoutov C, Manuopangai VT, Polyn M.K, Parks WJ, et al. Malaria control in central Malaita, Solomon Islands. 1. The use of insecticide impregnated bed nets. Acta Trop. 2000;75:173–83. doi: 10.1016/s0001-706x(00)00055-3. [DOI] [PubMed] [Google Scholar]
- 25.Gunasekaran K, Sahu SS, Vijayakumar KN, Jambulingam P. Acceptability, willing to purchase and use long lasting insecticide treated mosquito nets in Orissa State, India. Acta Trop. 2009;112:149–55. doi: 10.1016/j.actatropica.2009.07.013. [DOI] [PubMed] [Google Scholar]


