Adventitious roots and shoots can be regenerated from wounded or detached plant organs via de novo organogenesis. This study reveals the cellular and molecular framework of de novo root organogenesis from leaf explants of Arabidopsis thaliana and shows that auxin-induced WOX11 expression marks the first-step cell fate transition in this process.
Abstract
De novo organogenesis is a process through which wounded or detached plant tissues or organs regenerate adventitious roots and shoots. Plant hormones play key roles in de novo organogenesis, whereas the mechanism by which hormonal actions result in the first-step cell fate transition in the whole process is unknown. Using leaf explants of Arabidopsis thaliana, we show that the homeobox genes WUSCHEL RELATED HOMEOBOX11 (WOX11) and WOX12 are involved in de novo root organogenesis. WOX11 directly responds to a wounding-induced auxin maximum in and surrounding the procambium and acts redundantly with its homolog WOX12 to upregulate LATERAL ORGAN BOUNDARIES DOMAIN16 (LBD16) and LBD29, resulting in the first-step cell fate transition from a leaf procambium or its nearby parenchyma cell to a root founder cell. In addition, our results suggest that de novo root organogenesis and callus formation share a similar mechanism at initiation.
INTRODUCTION
Unlike animals, many plants have remarkable abilities to regenerate and form an entire plant body from various tissues or organs, or even from a single somatic cell (Birnbaum and Sánchez Alvarado, 2008; Sugimoto et al., 2011; Xu and Huang, 2014). Among the different types of plant regeneration, de novo organogenesis, in which adventitious roots and shoots form from wounded or detached plant tissues or organs, is frequently used in basic research and biotechnological breeding as it is a simple and robust in vitro method for plant culture (De Klerk et al., 1999; Duclercq et al., 2011). In recent decades, the regulation of de novo organogenesis in plants has been studied extensively, and phytohormones are considered to be the critical factors affecting this process (Skoog and Miller, 1957; Sangwan et al., 1997; De Klerk et al., 1999; Duclercq et al., 2011; Ikeuchi et al., 2013).
Physiological studies have shown that the phytohormones auxin and cytokinin play major roles in cell fate determination during de novo organogenesis. Auxin is the main hormone inducing de novo root organogenesis, while cytokinin promotes de novo shoot organogenesis (Skoog and Miller, 1957; Duclercq et al., 2011; Correa Lda et al., 2012). Recent research has identified a number of the factors mediating de novo organogenesis, including hormone receptors, proteins involved in hormone signaling and transport, and several transcription factors (Duclercq et al., 2011; Su and Zhang, 2014).
In traditional in vitro tissue cultures, adventitious roots and shoots are usually induced from a pluripotent cell mass known as callus. Previously, it was thought that callus was a group of dedifferentiated cells, but recent research has revealed that it is a group of root meristem tip cells and that the formation of callus and lateral roots is very similar (Sugimoto et al., 2010). The fact that callus formation resembles root formation suggests that this process is actually a type of de novo organogenesis. It was reported that callus is initiated from xylem-pole pericycle cells of root explants and pericycle-like cells of aerial organs, although the exact cell type of pericycle-like cells in aerial organs is not yet known (Che et al., 2007; Atta et al., 2009; Sugimoto et al., 2010). Genetic approaches by gain-of- or loss-of-function analyses in Arabidopsis thaliana led to the identification of genes involved in callus formation. The aberrant lateral root formation4 (alf4) mutant, which is defective in forming lateral roots (Celenza et al., 1995), almost completely failed to form callus (Sugimoto et al., 2010). Mutants with loss of function of the Polycomb Repressive Complex 2, which catalyzes genome-wide histone H3 lysine 27 trimethylation (H3K27me3) on chromatin, also showed a block in callus formation from leaf explants, but not from root explants (He et al., 2012). The lateral organ boundaries domain (LBD) transcription factors were recently reported to be important in regulating callus formation (Fan et al., 2012). The LBD16, LBD17, LBD18, and LBD29 genes were rapidly induced in explants cultured on callus-inducing medium (CIM), and ectopic expression of each of these four genes in Arabidopsis was sufficient to trigger callus formation without supplied phytohormones. In addition, suppression of the LBD function resulted in defects in callus formation (Fan et al., 2012). LBD genes were shown to be the direct targets of AUXIN RESPONSE FACTOR7 (ARF7) and ARF19 during lateral root formation (Okushima et al., 2007), and the arf7 arf19 double mutant is defective in producing lateral roots (Okushima et al., 2005).
The results of these previous studies greatly advanced our knowledge of de novo organogenesis. However, a long unanswered question in this field still remains elusive: What is the mechanism guiding the first-step cell fate transition in de novo organogenesis. Here, we report the elucidation of the regulatory mechanism underlying the first-step cell fate transition, which links the upstream wounding and hormonal signaling to the downstream organ formation, using our simple de novo root organogenesis system that mimics the natural conditions without added hormones. We also show that the mechanism of the first-step cell fate transition for callus initiation is similar to that for de novo root organogenesis.
RESULTS
Auxin Accumulation Near Wounds Initiates Adventitious Root Formation from Leaf Explants
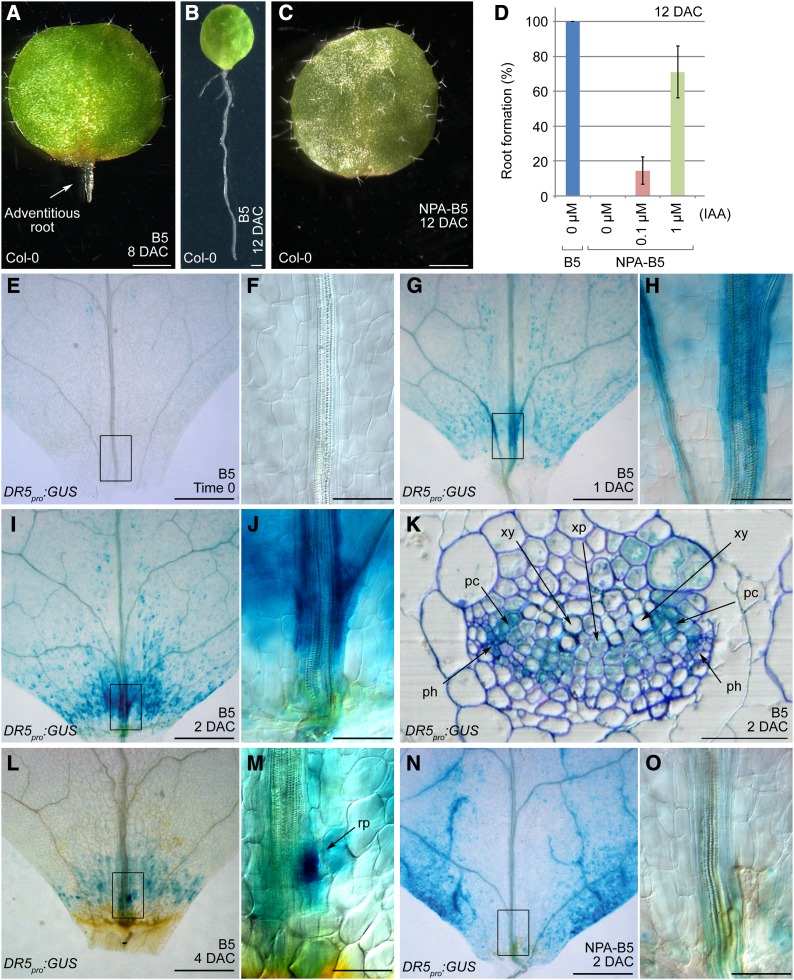
In natural conditions, detached organs from some plant species, such as members of the Crassulaceae and Cactaceae, are able to regenerate a new plant. Different from in vitro tissue culture in which explants regenerate on medium containing phytohormones, plant regeneration in natural conditions relies on the endogenous hormones in the detached tissues or organs. To investigate the molecular mechanisms guiding this process, we attempted to imitate natural conditions by culturing Arabidopsis leaf explants on B5 medium (Gamborg et al., 1968) without additional phytohormones. Leaves were cut at the region between the blade and petiole, and this simple method resulted in de novo root organogenesis that regenerated adventitious roots. Usually one adventitious root per leaf explant was visible on the proximal part at 8 d after culture (DAC) (Figure 1A). Roots continued to grow on the medium, and almost every leaf explant had regenerated roots by 12 DAC, with some explants having two or three roots (Figure 1B). On B5 medium containing 1 μM naphthylphthalamic acid (a polar auxin transport inhibitor), hereafter referred to as NPA-B5, rooting from leaf explants was blocked completely (Figure 1C). This phenotype could be rescued by culturing leaf explants on NPA-B5 with exogenous indole-3-acetic acid (IAA) (Figure 1D). These results suggest that not only auxin but also its transport are required for adventitious root formation from leaf explants.
Figure 1.
Wound-Induced Auxin Accumulation and Polar Transport Are Essential for Adventitious Root Formation.
(A) and (B) Leaf explants at 8 (A) and 12 DAC (B) on B5 medium.
(C) The 12-DAC leaf explant on NPA-B5. A total of 30 leaf explants were analyzed, and they all failed to form adventitious roots.
(D) Addition of IAA to NPA-B5 could rescue the NPA-caused rooting defect. Bars show sd with three biological repeats. n = 30 in each individual repeat.
(E) to (J) GUS staining at time 0 ([E] and [F]), 1 DAC ([G] and [H]), and 2 DAC ([I] and [J]) of DR5pro:GUS leaf explants cultured on B5 medium.
(K) Transverse section through the GUS-staining region of a 2-DAC DR5pro:GUS leaf explant grown on B5 medium. Note that GUS staining was mainly concentrated in the procambium and the nearby parenchyma cells. See the similar section without toluidine blue staining in Supplemental Figure 1.
(L) and (M) GUS staining of a 4-DAC DR5pro:GUS leaf explant on B5 medium. Arrow in (M) indicates an emerging root primordium.
(N) and (O) GUS staining of a 2-DAC DR5pro:GUS leaf explant cultured on NPA-B5.
(F), (H), (J), (M), and (O) are close-ups of the boxed regions in (E), (G), (I), (L), and (N), respectively. rp, root primordium; xy, xylem; xp, xylem parenchyma cell; pc, procambium; ph, phloem. Bars = 1 mm in (A) to (C), 500 μm in (E), (G), (I), (L), and (N), 100 μm in (F), (H), (J), (M), and (O), and 50 μm in (K).
Next, we used the DR5pro:GUS (β-glucuronidase) line (Ulmasov et al., 1997) to monitor the level of endogenous free auxin. Leaf explants from DR5pro:GUS plants showed only very faint GUS staining prior to culturing (time 0) (Figures 1E and 1F). In 1-DAC explants, GUS staining was visible in both mesophyll and vascular cells near wounds (Figures 1G and 1H) and was even stronger in the 2-DAC leaf explants (Figures 1I and 1J). Analysis of transverse sections showed that the strongest GUS staining in the 2-DAC leaf explants was in the procambium cells, although GUS signals were also visible in some parenchyma cells near the procambium (Figure 1K; Supplemental Figure 1). The regenerating root primordium was visible under a microscope in 4-DAC explants, and the GUS staining became further concentrated in the region in which the new root primordium was forming (Figures 1L and 1M). Compared with that in 2-DAC leaf explants on B5 medium, GUS staining was not detected in similar regions of the same-age leaf explants grown on NPA-B5, although some mesophyll cells of explants grown on NPA-B5 displayed much stronger GUS signals than those in the explants grown on B5 medium (Figures 1N and 1O). These results indicate that regeneration of adventitious roots requires an auxin maximum, and free auxin production, polar transport, and auxin signaling may all be involved.
Callus and Adventitious Roots Share a Similar Genetic Pathway for Initiation
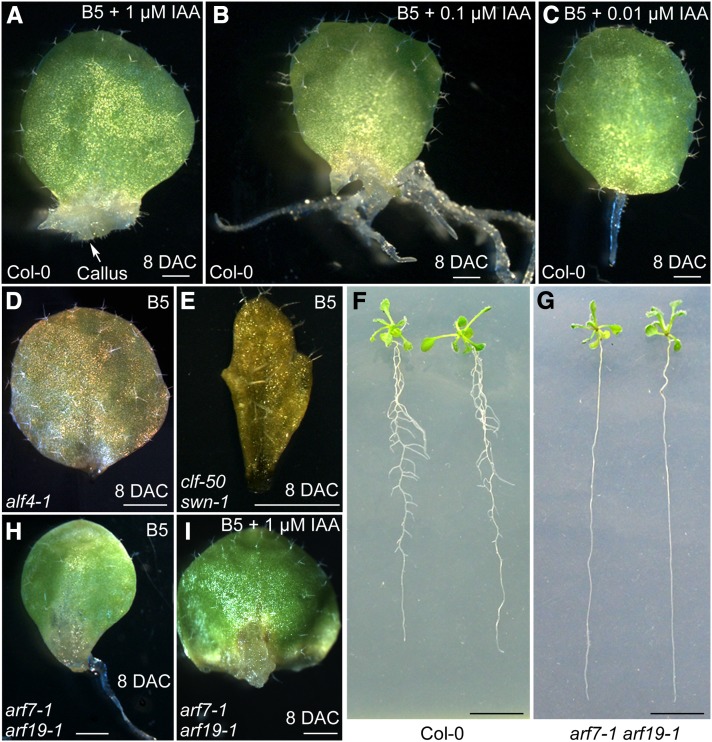
To analyze the effect of auxin on adventitious root formation from leaf explants, we cultured leaf explants on B5 medium containing different concentrations of IAA. Leaf explants cultured on B5 medium with 1 μM IAA produced callus (Figure 2A), whereas those cultured on B5 medium with 0.1 μM IAA formed several thickened adventitious roots (Figure 2B). Explants cultured on B5 medium with 0.01 μM IAA formed only one root (Figure 2C), mimicking those on B5 medium without phytohormones (Figure 1A). These results indicate that formation of callus or adventitious roots from a leaf explant highly depends on the auxin concentration in the medium. This observation is consistent with a previous model in which callus was proposed to resemble the tip of the root meristem (Sugimoto et al., 2010).
Figure 2.
Callus Formation Resembles Adventitious Root Formation.
(A) to (C) Phenotypes of the 8-DAC leaf explants on B5 medium containing 1 μM (A), 0.1 μM (B), and 0.01 μM (C) IAA.
(D) and (E) Leaf explants of alf4-1 (D) and clf-50 swn-1 (E) mutants, which are defective in adventitious root formation. A total of 30 leaf explants from each mutant were analyzed, and the results were consistent.
(F) and (G) Lateral root formation differs between wild-type Col-0 (F) and arf7-1 arf19-1 (G). Seedlings were grown on half-strength MS medium for14 d.
(H) and (I) Leaf explants from the arf7-1 arf19-1 double mutant on B5 medium (H) or B5 medium containing 1 μm IAA (I). Note that seedlings of the arf7-1 arf19-1 double mutant produce no lateral roots but can generate adventitious roots normally without IAA or callus with 1 μM IAA. A total of 30 leaf explants were tested, and the results were consistent.
Bars = 1 mm.
To study the genetic pathways involved in the formation of callus, adventitious roots and lateral roots, we analyzed Arabidopsis mutants defective in callus and/or lateral root formation. Leaf explants of both alf4-1 and clf-50 swn-1 are defective in callus formation (Sugimoto et al., 2010; He et al., 2012), and they also failed to form adventitious roots on B5 medium (Figures 2D and 2E). Whereas the arf7-1 arf19-1 double mutant produced no lateral roots (Figure 2G) (Okushima et al., 2005) unlike the wild type (Figure 2F), its leaf explants were capable of forming both adventitious roots (Figure 2H) and callus (Figure 2I). These results suggest that adventitious root formation may share similar regulatory mechanisms with callus formation.
WOX11 Is Directly Induced by Auxin for Stem Cell Fate Transition
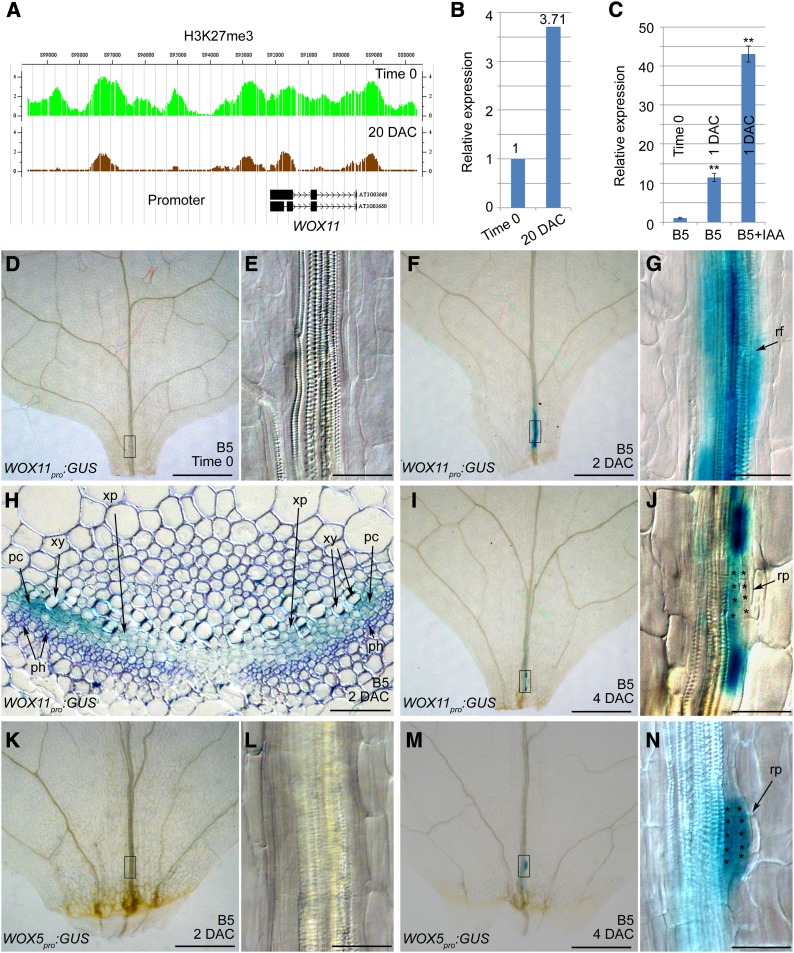
Based on the hypothesis that there are similar regulatory mechanisms in the formation of adventitious roots and callus, we expected that key genes for initiation of adventitious roots might be identified by analyzing our previously established databases of gene expression and histone methylation during the leaf-to-callus process (He et al., 2012). We selected genes that were differentially expressed between leaf explants and callus, and the identified genes were further evaluated in terms of their IAA responsiveness. WUSCHEL RELATED HOMEOBOX11 (WOX11) was one of the genes in the target category as it showed differential expression between leaf explants and callus (Figures 3A and 3B), and its expression was induced by IAA (Figure 3C). Since a previous report showed that the rice (Oryza sativa) homolog of WOX11 is required for the development of the crown root (a kind of adventitious root) (Zhao et al., 2009), we characterized WOX11 in more detail.
Figure 3.
WOX11 and WOX5 Are Differentially Expressed during Adventitious Root Formation.
(A) The level of epigenetic marker H3K27me3 at the WOX11 locus was dramatically reduced in the 20-DAC leaf explants that produce callus (brown) compared with that in the time-0 leaf explants (green). Note that the high level of H3K27me3 at a locus usually marks repression of the corresponding gene (Schatlowski et al., 2008).
(B) WOX11 expression was upregulated in the 20-DAC leaf explants cultured on CIM compared with that in the time-0 explants. The data in (A) and (B) were generated from previous ChIP-chip and microarray analyses, respectively (He et al., 2012).
(C) qRT-PCR analysis of WOX11 expression in the 1-DAC leaf explants on B5 media without IAA or with 2 μM IAA. The value of time-0 leaf explants was arbitrarily fixed at 1.0. Bars show se with three technical repeats. **P < 0.01 in two-sample t test compared with time-0 leaf explants.
(D) to (G) GUS staining of time-0 ([D] and [E]) and 2-DAC ([F] and [G]) leaf explants from WOX11pro:GUS plants, cultured on B5 medium.
(H) Transverse section of a 2-DAC WOX11pro:GUS leaf explant grown on B5 medium. Note that GUS staining appeared mainly in the procambium cells and some xylem parenchyma cells. See the similar section without toluidine blue staining in Supplemental Figure 1.
(I) and (J) GUS staining of a 4-DAC WOX11pro:GUS leaf explant cultured on B5 medium.
(K) to (N) GUS staining of 2-DAC ([K] and [L]) and 4-DAC ([M] and [N]) WOX5pro:GUS leaf explants cultured on B5 medium.
(E), (G), (J), (L), and (N) are close-ups of the boxed regions in (D), (F), (I), (K), and (M), respectively. rf, root founder cell; rp, root primordium; xy, xylem; xp, xylem parenchyma cell; pc, procambium; ph, phloem. Asterisks indicate the dividing cells that are in the process of forming a root primordium. Bars = 500 μm in (D), (F), (I), (K), and (M) and 50 μm in (E), (G), (H), (J), (L), and (N).
Analysis of GUS staining from leaf explants of the WOX11pro:GUS marker lines revealed that there was no GUS signal in time-0 leaf explants (Figures 3D and 3E), but a GUS signal was present in vascular tissues near wounds in 2-DAC explants on B5 medium (Figures 3F and 3G), coincident with the region in which auxin was highly accumulated (Figures 1I and 1J). To better understand the WOX11 expression pattern, we analyzed transverse sections of WOX11pro:GUS leaf explants. GUS staining was mainly concentrated in the procambium cells and sometimes could also be observed in some xylem parenchyma cells near the procambium (Figure 3H; Supplemental Figure 1). These results were consistent with the quantitative RT-PCR (qRT-PCR) results (Figure 3C), further indicating that WOX11 expression is induced by auxin.
In plants, the procambium or cambium is a meristematic tissue that contains adult stem cell populations (Lachaud et al., 1999). Several previous histological observations revealed that both adventitious roots and shoots initiate from the procambium or cambium (Greenwood et al., 2001; Ahkami et al., 2009, 2013; de Almeida et al., 2012; Correa Lda et al., 2012), and callus also initiates from procambium cells (Yu et al., 2010). Thus, it is possible that procambium cells, perhaps together with some parenchyma cells near the procambium in leaf explants, function like the xylem-pole pericycle cells in roots to produce callus, adventitious roots, and adventitious shoots.
We speculated that the expression of WOX11 marked the transition of stem cell fate from a procambium cell to a root founder cell because these cells initiated divisions to form the root primordium in 4-DAC leaf explants (Figures 3I and 3J). The intensity of GUS staining, which reflected the WOX11 expression level, was markedly decreased in these small root primordium cells (Figure 3J). To monitor cell fate transition in the newly formed adventitious root primordium, we analyzed leaf explants of the WOX5pro:GUS transgenic plants because WOX5 is known to be expressed in cells of the root quiescent center (Gonzali et al., 2005; Sarkar et al., 2007). GUS staining was not detected in root founder cells in 2-DAC leaf explants of WOX5pro:GUS transgenic plants (Figures 3K and 3L), whereas it was clearly visible in root primordium cells of 4-DAC leaf explants (Figures 3M and 3N), indicating the completion of fate transition from root founder cells to root primordium cells. The expression of WOX5 in the small dividing root primordium cells suggested that these cells may have some quiescent center cell features. Interestingly, the neighboring root founder cells flanking the dividing small root primordium cells continually expressed high levels of WOX11 (Figure 3J).
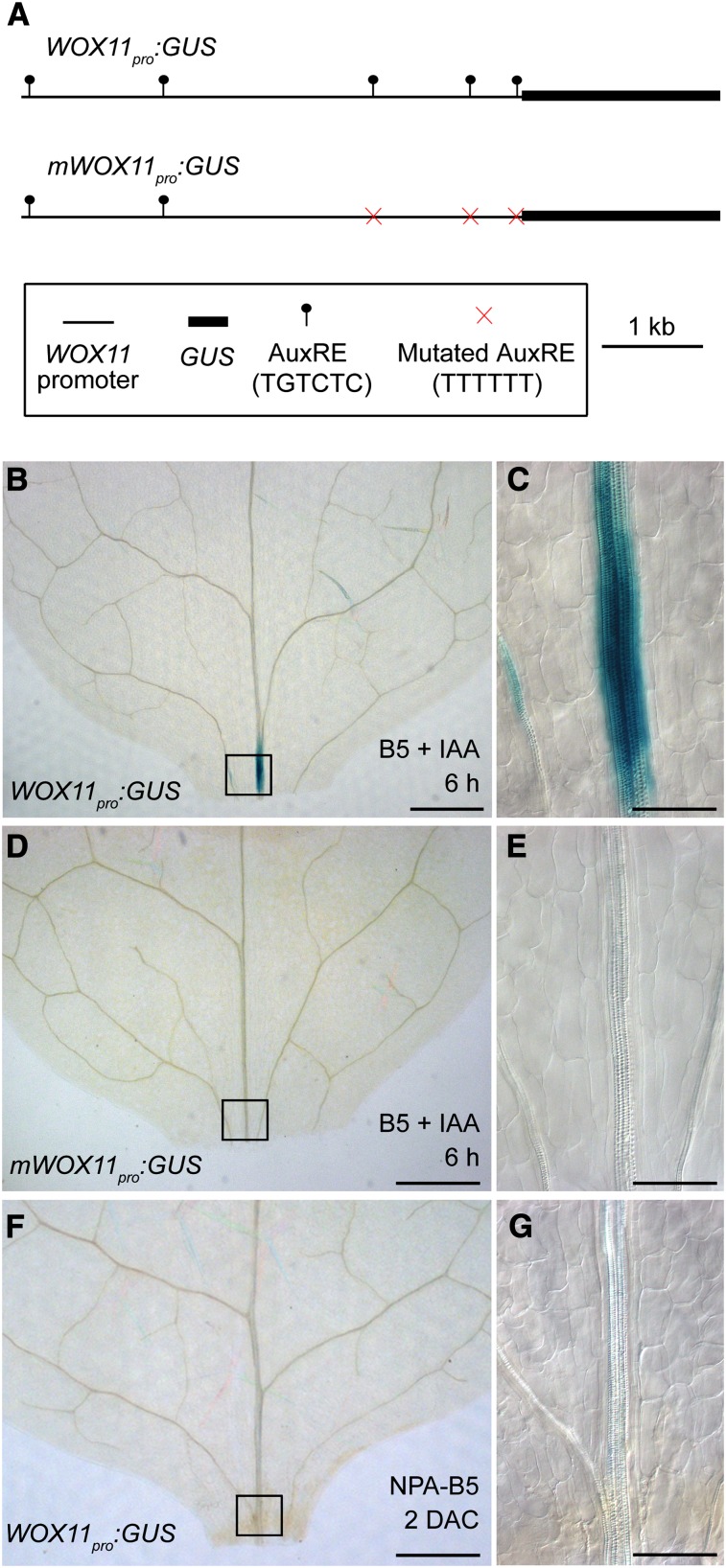
To test whether WOX11 expression was directly induced by auxin, we analyzed the WOX11 promoter. The promoter harbored at least five auxin response elements (AuxREs) (Figure 4A). Remarkably, WOX11 expression could be detected in leaf explants of the WOX11pro:GUS line after ∼6 h on B5 medium containing 1 μM IAA (Figures 4B and 4C). By contrast, GUS staining of leaf explants from the mWOX11pro:GUS line, in which three out of the five AuxREs were mutated (Figure 4A), was undetectable after 6 h of IAA induction (Figures 4D and 4E). In addition, no GUS staining was detected in leaf explants of the WOX11pro:GUS line cultured on NPA-B5 (Figures 4F and 4G). These data indicate that WOX11 is directly regulated by the auxin signaling pathway.
Figure 4.
Auxin Directly Induces WOX11 Expression.
(A) Diagram of structures of the WOX11pro:GUS and mWOX11pro:GUS constructs.
(B) to (E) GUS staining of WOX11pro:GUS ([B] and [C]) and mWOX11pro:GUS ([D] and [E]) leaf explants after 6 h cultured on B5 medium containing 1 μM IAA. Two independent WOX11pro:GUS or mWOX11pro:GUS lines were analyzed and the results were consistent.
(F) and (G) GUS staining was undetectable in 2-DAC leaf plants from WOX11pro:GUS plants cultured on NPA-B5.
(C), (E), and (G) are close-ups of the boxed regions in (B), (D), and (F), respectively. Bars = 500 μm in (B), (D), and (F) and 100 μm in (C), (E), and (G).
Molecular Evidence for Similar Mechanisms Regulating Adventitious Root and Callus Initiation
WOX11 and WOX5 expressions reflect different stages of adventitious root formation from leaf explants. Therefore, if initiation of adventitious roots and of callus share the same regulatory mechanism, the expression patterns of these two genes during callus formation should resemble those during adventitious root formation. Thus, we analyzed the expressions of WOX11 and WOX5 during callus formation and compared them with those during adventitious root formation. In 2-DAC leaf explants of the WOX11pro:GUS line grown on CIM, there were GUS signals in the vascular bundle of the midvein near wounds (Figure 5A), similar to that observed during the rooting process on B5 medium (Figures 3F and 3G), suggesting that there was cell fate transition to root founder cells. In the 4-DAC explants, small proliferating callus cells with decreased WOX11 expression appeared (Figures 5B and 5C). WOX5 was not expressed in 2-DAC leaf explants on CIM (Figure 5D), but it was strongly expressed in the small dividing callus cells in 4-DAC leaf explants (Figures 5E and 5F), suggesting that the cells undergo fate transition from root founder cells to callus cells, which are actually a group of fast-dividing root primordium cells continuously stimulated by the high level of hormones in CIM. Our data showed that the pattern of WOX11 and WOX5 expression in callus formation was similar to that during adventitious root formation. The only difference was that both WOX11 and WOX5 were expressed more strongly in leaf explants during callus induction on CIM than during adventitious root induction on B5 medium. These results provide further evidence at the cellular and molecular levels that initiation of callus and of adventitious roots share the same genetic pathway.
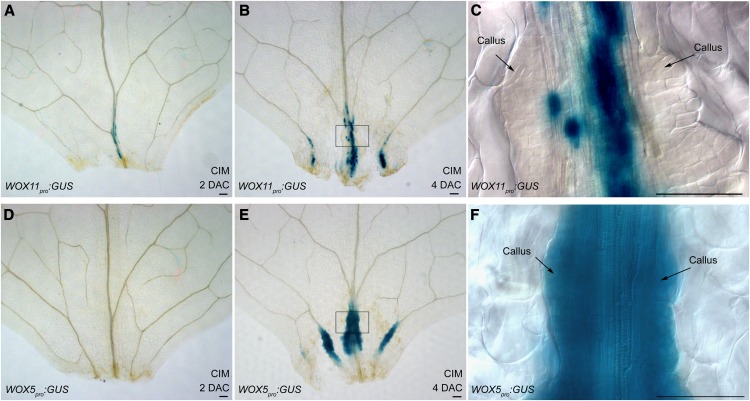
Figure 5.
WOX11 and WOX5 Expression Patterns in Callus Formation.
(A) to (C) GUS staining of the 2-DAC (A) and 4-DAC ([B] and [C]) leaf explants from WOX11pro:GUS plants on CIM. GUS staining was found in the vascular tissues near wounds in 2-DAC leaf explants (A) and became stronger at 4 DAC ([B] and [C]). However, GUS staining was absent in the rapidly proliferating callus cells in the 4-DAC explants (C).
(D) to (F) GUS staining of the 2-DAC (D) and 4-DAC ([E] and [F]) WOX5pro:GUS leaf explants cultured on CIM. GUS staining of WOX5pro:GUS was not detected in the 2-DAC leaf explants (D) but was found in the proliferating callus cells in the 4-DAC explants ([E] and [F]).
(C) and (F) are close-ups of the boxed regions in (B) and (E), respectively. Bars = 100 μm.
WOX11 and WOX12 Act Redundantly in Adventitious Root Formation
To better understand the role of WOX11 in de novo root organogenesis, we tested the ability of leaf explants from both gain-of-function and loss-of-function WOX11 plants to regenerate adventitious roots. Compared with that in wild-type Columbia-0 (Col-0), rooting was accelerated in 35Spro:WOX11 leaf explants (Figure 6A). In addition, the 35Spro:WOX11 leaf explants produced more adventitious roots (Figures 6B and 6H) than did the explants from wild-type Col-0 (Figure 1A). Interestingly, 7.8% of leaf explants from the 35Spro:WOX11 lines produced callus instead of roots on B5 medium (Figures 6C and 6H), similar to the wild-type explants cultured on B5 medium containing 1 μM IAA (Figure 2A). Furthermore, a proportion of 35Spro:WOX11 leaf explants regenerated adventitious roots on NPA-B5 (Figure 6D), suggesting that overexpression of WOX11 bypassed the block in auxin signaling. This supports the proposal that WOX11 is a major response gene of auxin signaling in adventitious root formation. Finally, WOX11 overexpression caused rapid callus formation on CIM (Figures 6E and 6F). For wild-type Col-0, the 8-DAC explants only produced small pieces of callus at their proximal parts (Figure 6E), whereas calli formed everywhere on the 35Spro:WOX11 explants (Figure 6F). All these results indicate that WOX11 plays a role in promoting adventitious root and callus formation.
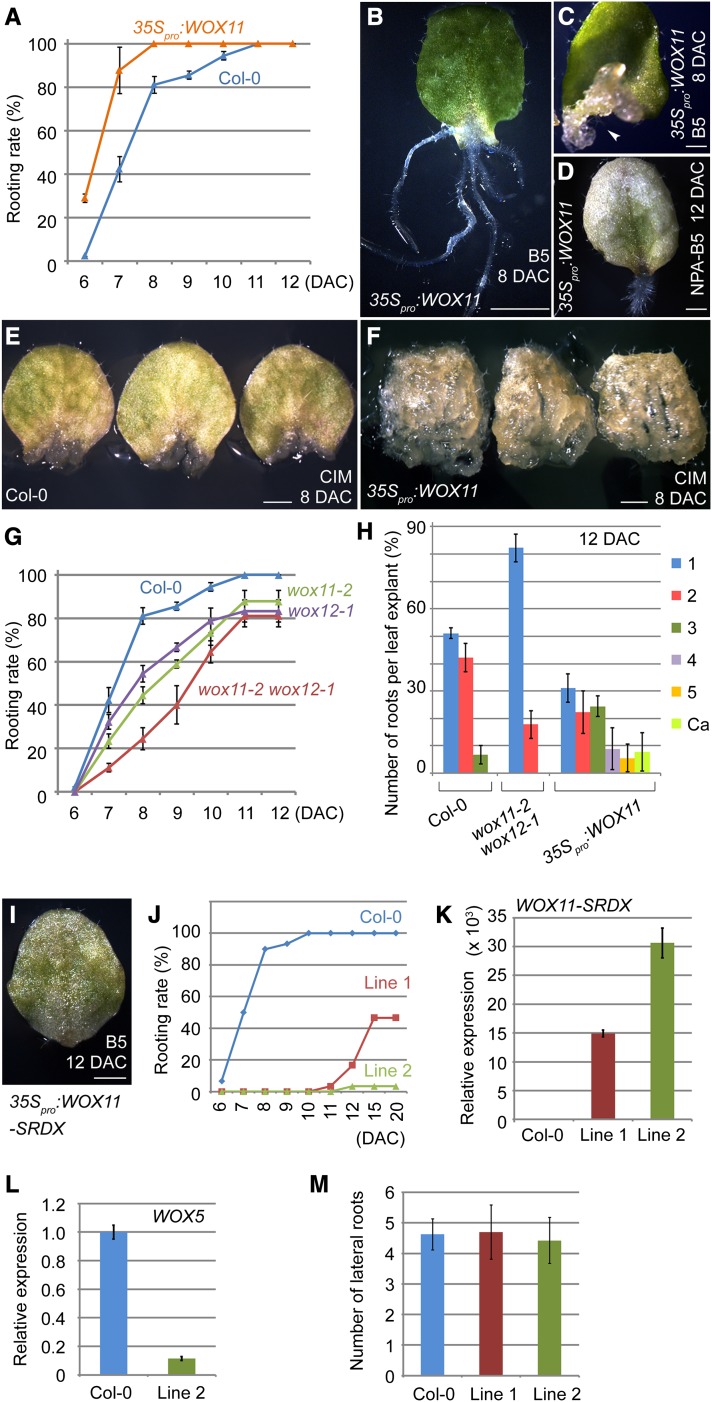
Figure 6.
WOX11 and WOX12 Are Involved in Adventitious Root and Callus Formation.
(A) Overexpression of WOX11 accelerated adventitious root formation on B5 medium. Bars show sd with three biological repeats. n = 30 in each repeat.
(B) The 8-DAC leaf explant of 35Spro:WOX11, showing five regenerated adventitious roots on B5 medium.
(C) The 8-DAC leaf explant of 35Spro:WOX11, showing callus formation (arrowhead) on B5 medium.
(D) Defective adventitious root formation in 10% of leaf explants (three out of 30 from two independent 35Spro:WOX11 lines) on NPA-B5 could be rescued by overexpression of WOX11. Shown is a 12-DAC leaf explant.
(E) and (F) Leaf explants from wild-type (E) and 35Spro:WOX11 (F) plants were cultured on CIM. Note that the callus formation in the 35Spro:WOX11 leaf explants was markedly accelerated compared with that in the wild-type leaf explants. A total of 30 leaf explants from two independent 35Spro:WOX11 lines were tested.
(G) WOX11 and WOX12 functions are involved in de novo root organogenesis. Both wox11-2 and wox12-1 displayed delayed adventitious root formation, and the wox11-2 wox12-1 double mutant showed even slower rooting on B5 medium. Bars show sd with three biological repeats. n = 30 in each repeat.
(H) Quantitative analyses of the adventitious root number per 12-DAC leaf explant grown on B5 medium. Bars show sd with three biological repeats. n = 30 in each repeat.
(I) The 12-DAC leaf explant of the 35Spro:WOX11-SRDX plant Line 2, showing rooting defect on B5 medium.
(J) Quantitative analyses of the adventitious root formation using leaf explants from two independent 35Spro:WOX11-SRDX transgenic lines, Line 1 and Line 2. A total of 30 leaf explants from each line were analyzed.
(K) qRT-PCR analyses of WOX11-SRDX expression levels in Line 1 and Line 2 of the 35Spro:WOX11-SRDX transgenic plants. Note that the higher WOX11-SRDX expression level in Line 2 explants is consistent with its stronger defect in rooting.
(L) qRT-PCR analysis showed that WOX5 was only weakly expressed in 4-DAC leaf explants of Line 2, compared with that in wild-type Col-0. In (K) and (L), the value of wild-type leaf explants was arbitrarily fixed at 1.0, and bars show se from three technical repeats.
(M) Quantitative analyses of the lateral root number per 1 cm in length of the primary root from 12-d-old Line 1 and Line 2 grown on half-strength MS medium. Bars show sd from three biological repeats. n = 10 seedlings in each individual repeat.
Bars = 2 mm in (B) and 1 mm in (C) to (F) and (I).
Compared with those of the wild type, leaf explants from the wox11-2 mutant showed a slight delay in rooting (Figure 6G). WOX12, another member of the WOX family in Arabidopsis, shares high protein sequence similarity with WOX11 (Supplemental Figure 2), and a phylogenetic analysis indicated that WOX11 and WOX12 belong to the same clade (Haecker et al., 2004). Like WOX11, WOX12 expression was strongly induced during adventitious root formation, and leaf explants of 35Spro:WOX12 also produced more adventitious roots than did the wild-type leaf explants (Supplemental Figure 3). However, like that in wox11-2, the rooting process in the wox12-1 single mutant was mildly affected (Figure 6G). To test the possible functional redundancy of WOX11 and WOX12, we constructed a wox11-2 wox12-1 double mutant (Supplemental Figure 2). Our data showed that the rooting time of wox11-2 wox12-1 was more strongly delayed (Figure 6G), and wox11-2 wox12-1 explants produced fewer roots than did the wild-type Col-0 (Figure 6H). The fact that rooting of the wox11-2 wox12-1 leaf explants were only partly affected suggests that the wox12-1 allele may not be strong (Supplemental Figure 2); alternatively, other as yet unknown redundant factor(s) may exist.
Fusion of the repression domain SRDX to a transcription factor can specifically suppress the expression of target genes, resulting in disruption of genetic pathways regulated by the transcription factor, even in the presence of redundant transcription factors (Hiratsu et al., 2003). Therefore, we constructed 35Spro:WOX11-SRDX lines to analyze the role of WOX11. The leaf explants from the 35Spro:WOX11-SRDX plants displayed severe rooting defects. In a strong WOX11-SRDX–expressing line (Line 2), leaf explants barely formed adventitious roots (Figures 6I to 6K), while in a relatively weak WOX11-SRDX–expressing line (Line 1), rooting occurred at a low frequency (Figures 6J and 6K) even with a prolonged culture time. In addition, only very low WOX5 expression was detected in the Line 2 leaf explants at DAC 4, when they were cultured on B5 medium (Figure 6L). This result is consistent with the fact that rooting in the 35Spro:WOX11-SRDX leaf explants was defective. Interestingly, lateral root initiation on the primary roots was normal in the Line 1 and Line 2 seedlings (Figure 6M). Analyses of the 35Spro:WOX11-SRDX transgenic plants indicate the possibility that WOX11 may act redundantly with some other WOX family members in regulating adventitious root formation.
WOX11 Promotes Expression of LBD16 and LBD29
Several LBD family genes, such as LBD16, 18, 29, and 33, are known to promote callus formation from multiple detached plant tissues (Fan et al., 2012). In an attempt to elucidate the genetic pathway regulating adventitious root formation, we analyzed the expressions of these four genes. During rooting from leaf explants, they were all upregulated, albeit to different levels (Figure 7A). We then determined the expression levels of these LBD genes in the leaves of 35Spro:WOX11 transgenic plants and found that LBD16 and 29 were ectopically expressed (Figure 7B).
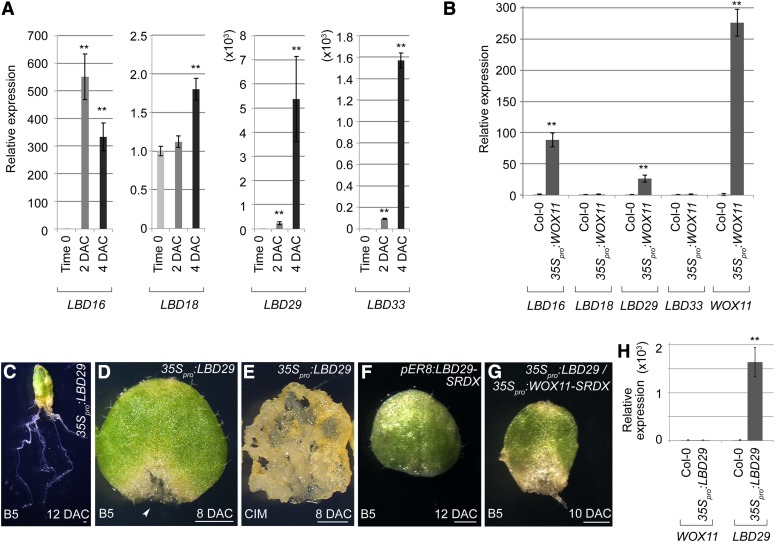
Figure 7.
WOX11 Upregulates LBD16 and LBD29.
(A) qRT-PCR analyses showed that expression levels of the analyzed LBD genes were elevated during the culture of leaf explants on B5 medium.
(B) qRT-PCR analyses of LBD and WOX11 expression using rosette leaves of 12-d-old 35Spro:WOX11 seedlings. Two independent 35Spro:WOX11 lines were analyzed, and the results were consistent. Values of time-0 leaf explants in (A) and wild-type leaves in (B) were arbitrarily fixed at 1.0. Bars show se from three technical repeats. **P < 0.01 in two-sample t test comparing with time-0 leaf explants (A) or wild-type leaves (B).
(C) The 12-DAC 35Spro:LBD29 leaf explant on B5 medium, showing multiple regenerated adventitious roots.
(D) The 8-DAC 35Spro:LBD29 leaf explant cultured on B5 medium, showing callus formation (arrowhead). A total of 40 leaf explants from two independent 35Spro:LBD29 lines were analyzed on B5 medium. Among them, eight showed callus growth (D), while the rest regenerated multiple adventitious roots (C).
(E) The 8-DAC leaf explant of 35Spro:LBD29 cultured on CIM, showing robust callus growth. A total of 30 leaf explants from two independent 35Spro:LBD29 lines were tested, and all of them showed similar phenotypes.
(F) The 12-DAC leaf explant of pER8:LBD29-SRDX, showing defective adventitious root formation on B5 medium. A total of 70 leaf explants from three independent lines were tested, and 47 of them showed the regeneration defect.
(G) The 10-DAC leaf explant of 35Spro:LBD29/35Spro:WOX11-SRDX, in which the regeneration defect caused by 35Spro:WOX11-SRDX was partly rescued. The 35Spro:LBD29/35Spro:WOX11-SRDX plants were constructed by crossing a phenotypically tested 35Spro:LBD29 line to Line 1 of 35Spro:WOX11-SRDX. The F1 seedlings, after PCR verification, were used for regeneration analysis. Among a total of 16 leaf explants analyzed, 12 formed adventitious roots or callus on B5 medium.
(H) qRT-PCR analyses of WOX11 and LBD29 expression using rosette leaves from 12-d-old 35Spro:LBD29 seedlings. Two independent 35Spro:LBD29 lines were analyzed, and the results were consistent. Values of wild-type Col-0 leaves were arbitrarily fixed at 1.0, and bars show se from three technical repeats. **P < 0.01 in two-sample t test comparing with wild-type leaves.
Bars = 1 mm in (C) to (G).
To confirm the functions of the LBD genes in regeneration, we analyzed the phenotypes of the 35Spro:LBD29 plants during adventitious root and callus formation. Leaf explants of 35Spro:LBD29 lines on B5 medium showed an enhanced ability to regenerate adventitious roots and formed more roots than did the wild type (Figure 7C). A proportion of the leaf explants from 35Spro:LBD29 lines was also able to form callus on B5 medium (Figure 7D). In addition, 35Spro:LBD29 explants on CIM showed accelerated callus formation (Figure 7E). To analyze the LBD functions in callus and adventitious root formation, we constructed pER8:LBD29-SRDX transgenic plants, which harbored a chimeric LBD29-SRDX fusion under the control of a β-estradiol–inducible promoter. Our results showed that 67% of leaf explants from the pER8:LBD29-SRDX transgenic lines failed to form adventitious roots by 12 DAC (Figure 7F). These phenotypes are similar to those observed in the corresponding analyses for WOX11. In addition, overexpression of LBD29 in the 35Spro:WOX11-SRDX background was able to partly rescue the regeneration defect caused by loss of WOX functions (Figure 7G). By contrast, upregulation of WOX11 expression was not detected in leaves of the 35Spro:LBD29 transgenic plants (Figure 7H). Taken together, these results indicate that WOX11 acts upstream of LBDs, and WOX11 regulates adventitious root and callus formation at least partly through the LBD pathway.
DISCUSSION
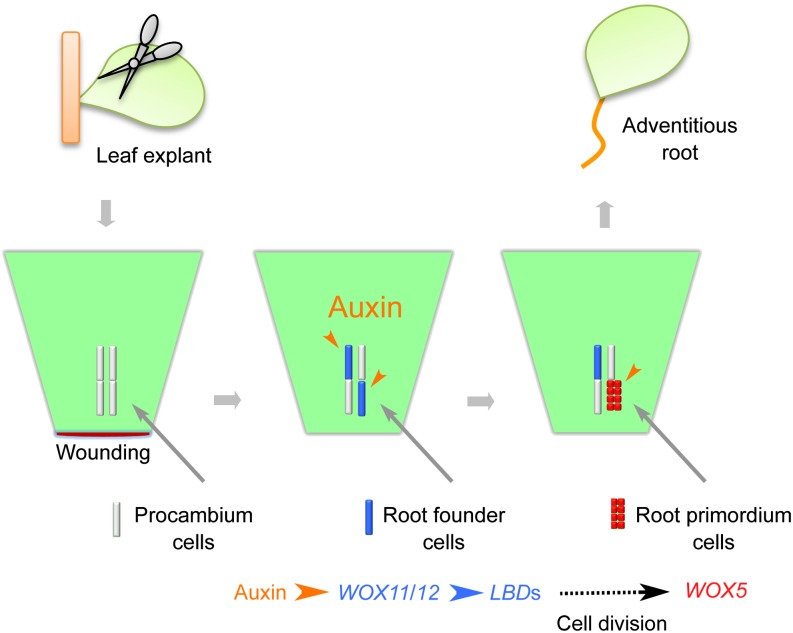
Our study revealed the cell lineage and molecular mechanisms of de novo root organogenesis. Our data support the following model for the mechanism guiding the first-step cell fate transition (Figure 8). Wounding induces the production of free auxin, which is then highly concentrated in procambium stem cells and their surrounding parenchyma cells via polar auxin transport. The auxin maximum formed in the procambium and parenchyma cells directly induces WOX11 expression. WOX11 and WOX12 then act redundantly to upregulate LBD16 and LBD29. The actions of WOX11 and WOX12 together mediate the first-step stem cell fate transition from procambium cells to root founder cells.
Figure 8.
A Model for de Novo Root Organogenesis.
A cellular and molecular framework of de novo root organogenesis from Arabidopsis leaf explants is shown in the model. Note that there are two steps of cell fate transition during adventitious root formation.
It was previously reported that several LBD genes participate in lateral root formation (Berckmans et al., 2011; Feng et al., 2012a, 2012b; Lee et al., 2012). During the initiation of root primordia, LBD18 was found to regulate the cell cycle–controlling gene E2Fa and the cell wall–loosening factor gene EXPANSIN14 (Berckmans et al., 2011; Lee et al., 2012). These findings suggested a possible role for LBDs in promoting cell division and in preparation for the second-step cell fate transition from root founder cells to root primordium cells (see the model in Figure 8). In addition, AuxREs are also present in the promoter regions of the LBD genes (Okushima et al., 2007), suggesting that expression of these LBDs may also depend on the auxin signaling pathway.
Based on the genetic pathways involved, we propose that initiation of adventitious roots shares similar regulatory mechanisms with that of callus, and whether leaf explants produce adventitious roots or callus mainly depends on free auxin levels. Since the processes of lateral root and adventitious root formation after their initiation are rather similar developmentally, it is likely that they share some similar regulation mechanisms, such as those involving LBD and ALF4 genes and others involved in the late root developmental stages. The only possible difference between adventitious root/callus formation and lateral root formation that we found in this study was in the genes involved in the first-step cell fate transition. In particular, adventitious root and callus formation require WOX11 and WOX12, whereas lateral root initiation does not. We showed that lateral roots initiated normally from primary roots of the 35Spro:WOX11-SRDX plants, whereas initiation of adventitious roots was severely defective in leaf explants from the same 35Spro:WOX11-SRDX lines. By contrast, the arf7 arf19 double mutant showed defective lateral root formation, whereas it had the normal adventitious root and callus formation. ARF7 and 19 were previously known to directly activate LBDs during lateral root formation (Okushima et al., 2007). Because de novo root regeneration from a detached organ is not a usual process in the development of Arabidopsis plants, unlike lateral root formation, activation of LBD genes during de novo root regeneration may require additional regulatory processes, such as that involving WOX11/12 action.
The procambium or cambium is thought to be a population of multipotent adult stem cells that are responsible for stem thickening and production of the xylem and phloem in the vascular tissues (Lachaud et al., 1999). Among many different cell types in the leaf explant, procambium cells, probably also including some other parenchyma cells surrounding the procambium, are likely to serve as competent cells that initiate adventitious roots and callus. This is not only supported by several previous studies (Greenwood et al., 2001; Ahkami et al., 2009, 2013; Yu et al., 2010; Correa Lda et al., 2012; de Almeida et al., 2012), but also supported by the specific WOX11 expression patterns upon auxin induction. It was proposed that callus is initiated from the pericycle-like cells surrounding the vascular tissues when aerial organs are used as explants, but the nature of the pericycle-like cells was unknown (Sugimoto et al., 2010, 2011). Based on our current data, we propose that the procambium cells may serve as one type of pericycle-like cells in aerial organs. WOX11 is usually expressed only in the protoxylem cells in the apical region of a root under natural growth conditions. However, WOX11 could be rapidly induced in the xylem-pole pericycle cells after growing seedlings were moved to medium containing auxin (Supplemental Figure 4). Because the xylem-pole pericycle cells are known to have the potential to initiate callus, the similar WOX11 expression pattern in xylem-pole pericycle and procambium cells after auxin induction suggests that these cells have similar features in callus formation.
The WOX11 expression pattern depends on auxin distribution, and auxin induction relies on wounding of explants at the beginning of de novo root organogenesis. Therefore, how wounding induces auxin accumulation and how auxin is specifically transported to certain procambium cells are the next important questions to address.
METHODS
Plant Materials and Tissue Culture
The mutants wox11-2 (SALK_004777), wox12-1 (SALK_087882) (Alonso et al., 2003), alf4-1 (Celenza et al., 1995), and arf7-1 arf19-1 (Okushima et al., 2005) are in the Col-0 background, and clf-50 swn-1 is in the Wassilewskija background (Chanvivattana et al., 2004). For construction of the WOX11pro:GUS transgenic plants, a 4.8-kb fragment upstream to the WOX11 coding region was subcloned into the pBI101 vector, which was then used for plant transformation. The mWOX11pro:GUS construct was made by PCR-based modifications of WOX11pro:GUS. The DR5pro:GUS line in Landsberg erecta and the WOX5pro:GUS line in Col-0 were described previously (Ulmasov et al., 1997; He et al., 2012). 35Spro:WOX11 and 35Spro:LBD29 were constructed by insertion of cDNA fragments encoding the full length of WOX11 and LBD29, respectively, into the pMON530 vector. 35Spro:WOX11-SRDX was constructed by first fusing a sequence encoding the SRDX peptide (Hiratsu et al., 2003) with the WOX11 cDNA. The fusion was then inserted into pMON530. pER8:LBD29-SRDX was constructed by insertion of the LBD29 cDNA-SRDX fusion into the pER8 vector (Zuo et al., 2000). All transgenic lines were obtained by the Agrobacterium tumefaciens–mediated transformation to wild-type Col-0. Primers and restriction sites for cloning are listed in Supplemental Table 1.
For generation of adventitious roots or callus, Arabidopsis thaliana seeds were grown on half-strength Murashige and Skoog (MS) medium (Murashige and Skoog, 1962) at 22°C, with 16 h of light. The first two rosette leaves were cut from 12-d-old seedlings and cultured on B5 medium (Gamborg B5 basal medium with 0.5 g/L MES, 3% Suc, and 0.8% agar, pH 5.7), CIM (B5 medium with 0.2 μmol/L kinetin and 2.2 μmol/L 2,4-D), or B5 medium with additional chemicals at 22°C in the dark to induce adventitious roots or callus for phenotype analysis, GUS staining, and qRT-PCR. Leaves from 25-d-old seedlings were used for sectioning analysis.
GUS Staining, Thin Sectioning, and Microscopy
GUS staining was performed by incubation of leaf explants from the DR5pro:GUS, WOX11pro:GUS, mWOX11pro:GUS, and WOX5pro:GUS lines at 37°C in GUS assay solution (He et al., 2012) for 2, 12, 12, and 2 h, respectively. For differential interference contrast microscopy observation, the stained tissues were incubated in the chloral hydrate solution (200 g chloral hydrate, 20 g glycerol, and 50 mL water) (Tsuge et al., 1996) at 65°C for ∼12 h for tissue transparency. Thin sectioning was performed as previously described (Xu et al., 2003).
RT-PCR and qRT-PCR
RT-PCR and qRT-PCR were performed according to the methods described previously (Xu et al., 2003; He et al., 2012) using gene-specific primers. The qRT-PCR results are shown as the relative expression levels, which were normalized against those produced by the primers for ACTIN. Primers for RT-PCR and qRT-PCR are listed in Supplemental Table 1.
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative under the following accession numbers: WOX11 (At3g03660) and WOX12 (At5g17810).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. DR5 and WOX11 Expression in Leaf Explants during Adventitious Root Formation.
Supplemental Figure 2. Identification of the wox11 and wox12 Mutants.
Supplemental Figure 3. WOX12 Is Involved in Adventitious Root Formation.
Supplemental Figure 4. Auxin Induces WOX11 Expression in Root Pericycle Cells.
Supplemental Table 1. List of Primers Used in This Study.
Supplementary Material
Acknowledgments
We thank T.J. Guilfoyle, J. Goodrich, J. Celenza, and the ABRC for the Arabidopsis seeds used in this study. We also thank X. Chen, S. Liu, and C. He for technical assistance. This work was supported by grants from National Basic Research Program of China (973 Program, 2014CB943500/2012CB910503).
AUTHOR CONTRIBUTIONS
J. Liu, L.S., Y.X., H.H., and L.X. designed the research. J. Liu, L.S., Y.X., and J. Li performed research. All authors analyzed data. H.H. and L.X. wrote the article.
Glossary
- CIM
callus-inducing medium
- DAC
days after culture
- IAA
indole-3-acetic acid
- qRT-PCR
quantitative RT-PCR
- AuxRE
auxin response element
- Col-0
Columbia-0
- MS
Murashige and Skoog
Footnotes
Online version contains Web-only data.
References
- Ahkami A.H., Lischewski S., Haensch K.T., Porfirova S., Hofmann J., Rolletschek H., Melzer M., Franken P., Hause B., Druege U., Hajirezaei M.R. (2009). Molecular physiology of adventitious root formation in Petunia hybrida cuttings: Involvement of wound response and primary metabolism. New Phytol. 181: 613–625. [DOI] [PubMed] [Google Scholar]
- Ahkami A.H., Melzer M., Ghaffari M.R., Pollmann S., Ghorbani Javid M., Shahinnia F., Hajirezaei M.R., Druege U. (2013). Distribution of indole-3-acetic acid in Petunia hybrida shoot tip cuttings and relationship between auxin transport, carbohydrate metabolism and adventitious root formation. Planta 238: 499–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso J.M., et al. (2003). Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657. [DOI] [PubMed] [Google Scholar]
- Atta R., Laurens L., Boucheron-Dubuisson E., Guivarc’h A., Carnero E., Giraudat-Pautot V., Rech P., Chriqui D. (2009). Pluripotency of Arabidopsis xylem pericycle underlies shoot regeneration from root and hypocotyl explants grown in vitro. Plant J. 57: 626–644. [DOI] [PubMed] [Google Scholar]
- Berckmans B., et al. (2011). Auxin-dependent cell cycle reactivation through transcriptional regulation of Arabidopsis E2Fa by lateral organ boundary proteins. Plant Cell 23: 3671–3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum K.D., Sánchez Alvarado A. (2008). Slicing across kingdoms: Regeneration in plants and animals. Cell 132: 697–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celenza J.L., Jr, Grisafi P.L., Fink G.R. (1995). A pathway for lateral root formation in Arabidopsis thaliana. Genes Dev. 9: 2131–2142. [DOI] [PubMed] [Google Scholar]
- Chanvivattana Y., Bishopp A., Schubert D., Stock C., Moon Y.H., Sung Z.R., Goodrich J. (2004). Interaction of Polycomb-group proteins controlling flowering in Arabidopsis. Development 131: 5263–5276. [DOI] [PubMed] [Google Scholar]
- Che P., Lall S., Howell S.H. (2007). Developmental steps in acquiring competence for shoot development in Arabidopsis tissue culture. Planta 226: 1183–1194. [DOI] [PubMed] [Google Scholar]
- Correa, Lda R., Troleis J., Mastroberti A.A., Mariath J.E., Fett-Neto A.G. (2012). Distinct modes of adventitious rooting in Arabidopsis thaliana. Plant Biol. (Stuttg.) 14: 100–109. [DOI] [PubMed] [Google Scholar]
- de Almeida M., de Almeida C.V., Mendes Graner E., Ebling Brondani G., Fiori de Abreu-Tarazi M. (2012). Pre-procambial cells are niches for pluripotent and totipotent stem-like cells for organogenesis and somatic embryogenesis in the peach palm: A histological study. Plant Cell Rep. 31: 1495–1515. [DOI] [PubMed] [Google Scholar]
- De Klerk, G.-J., Van der Krieken, W., and De Jong, J.C. (1999). The formation of adventitious roots: New concepts, new possibilities. In Vitro Cell. Dev. Biol. Plant 35: 189–199. [Google Scholar]
- Duclercq J., Sangwan-Norreel B., Catterou M., Sangwan R.S. (2011). De novo shoot organogenesis: From art to science. Trends Plant Sci. 16: 597–606. [DOI] [PubMed] [Google Scholar]
- Fan M., Xu C., Xu K., Hu Y. (2012). LATERAL ORGAN BOUNDARIES DOMAIN transcription factors direct callus formation in Arabidopsis regeneration. Cell Res. 22: 1169–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z., Sun X., Wang G., Liu H., Zhu J. (2012b). LBD29 regulates the cell cycle progression in response to auxin during lateral root formation in Arabidopsis thaliana. Ann. Bot. (Lond.) 110: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z., Zhu J., Du X., Cui X. (2012a). Effects of three auxin-inducible LBD members on lateral root formation in Arabidopsis thaliana. Planta 236: 1227–1237. [DOI] [PubMed] [Google Scholar]
- Gamborg O.L., Miller R.A., Ojima K. (1968). Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 50: 151–158. [DOI] [PubMed] [Google Scholar]
- Gonzali S., Novi G., Loreti E., Paolicchi F., Poggi A., Alpi A., Perata P. (2005). A turanose-insensitive mutant suggests a role for WOX5 in auxin homeostasis in Arabidopsis thaliana. Plant J. 44: 633–645. [DOI] [PubMed] [Google Scholar]
- Greenwood M.S., Cui X., Xu F. (2001). Response to auxin changes during maturation-related loss of adventitious rooting competence in loblolly pine (Pinus taeda) stem cuttings. Physiol. Plant. 111: 373–380. [DOI] [PubMed] [Google Scholar]
- Haecker A., Gross-Hardt R., Geiges B., Sarkar A., Breuninger H., Herrmann M., Laux T. (2004). Expression dynamics of WOX genes mark cell fate decisions during early embryonic patterning in Arabidopsis thaliana. Development 131: 657–668. [DOI] [PubMed] [Google Scholar]
- He C., Chen X., Huang H., Xu L. (2012). Reprogramming of H3K27me3 is critical for acquisition of pluripotency from cultured Arabidopsis tissues. PLoS Genet. 8: e1002911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiratsu K., Matsui K., Koyama T., Ohme-Takagi M. (2003). Dominant repression of target genes by chimeric repressors that include the EAR motif, a repression domain, in Arabidopsis. Plant J. 34: 733–739. [DOI] [PubMed] [Google Scholar]
- Ikeuchi M., Sugimoto K., Iwase A. (2013). Plant callus: Mechanisms of induction and repression. Plant Cell 25: 3159–3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachaud S., Catesson A.M., Bonnemain J.L. (1999). Structure and functions of the vascular cambium. C. R. Acad. Sci. III 322: 633–650. [DOI] [PubMed] [Google Scholar]
- Lee H.W., Kim M.J., Kim N.Y., Lee S.H., Kim J. (2012). LBD18 acts as a transcriptional activator that directly binds to the EXPANSIN14 promoter in promoting lateral root emergence of Arabidopsis. Plant J. 73: 212–224. [DOI] [PubMed] [Google Scholar]
- Murashige T., Skoog F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 80: 662–668. [Google Scholar]
- Okushima Y., Fukaki H., Onoda M., Theologis A., Tasaka M. (2007). ARF7 and ARF19 regulate lateral root formation via direct activation of LBD/ASL genes in Arabidopsis. Plant Cell 19: 118–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okushima Y., et al. (2005). Functional genomic analysis of the AUXIN RESPONSE FACTOR gene family members in Arabidopsis thaliana: Unique and overlapping functions of ARF7 and ARF19. Plant Cell 17: 444–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangwan R.S., Sangwan-Norreel B., Harada H. (1997). In vitro techniques and plant morphogenesis: Fundamental aspects and practical applications. Plant Biotechnol. 14: 93–100. [Google Scholar]
- Sarkar A.K., Luijten M., Miyashima S., Lenhard M., Hashimoto T., Nakajima K., Scheres B., Heidstra R., Laux T. (2007). Conserved factors regulate signalling in Arabidopsis thaliana shoot and root stem cell organizers. Nature 446: 811–814. [DOI] [PubMed] [Google Scholar]
- Schatlowski N., Creasey K., Goodrich J., Schubert D. (2008). Keeping plants in shape: Polycomb-group genes and histone methylation. Semin. Cell Dev. Biol. 19: 547–553. [DOI] [PubMed] [Google Scholar]
- Skoog F., Miller C.O. (1957). Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Symp. Soc. Exp. Biol. 11: 118–130. [PubMed] [Google Scholar]
- Su Y.H., Zhang X.S. (2014). The hormonal control of regeneration in plants. Curr. Top. Dev. Biol. 108: 35–69. [DOI] [PubMed] [Google Scholar]
- Sugimoto K., Gordon S.P., Meyerowitz E.M. (2011). Regeneration in plants and animals: Dedifferentiation, transdifferentiation, or just differentiation? Trends Cell Biol. 21: 212–218. [DOI] [PubMed] [Google Scholar]
- Sugimoto K., Jiao Y., Meyerowitz E.M. (2010). Arabidopsis regeneration from multiple tissues occurs via a root development pathway. Dev. Cell 18: 463–471. [DOI] [PubMed] [Google Scholar]
- Tsuge T., Tsukaya H., Uchimiya H. (1996). Two independent and polarized processes of cell elongation regulate leaf blade expansion in Arabidopsis thaliana (L.) Heynh. Development 122: 1589–1600. [DOI] [PubMed] [Google Scholar]
- Ulmasov T., Murfett J., Hagen G., Guilfoyle T.J. (1997). Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 9: 1963–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L., Huang H. (2014). Genetic and epigenetic controls of plant regeneration. Curr. Top. Dev. Biol. 108: 1–33. [DOI] [PubMed] [Google Scholar]
- Xu L., Xu Y., Dong A., Sun Y., Pi L., Xu Y., Huang H. (2003). Novel as1 and as2 defects in leaf adaxial-abaxial polarity reveal the requirement for ASYMMETRIC LEAVES1 and 2 and ERECTA functions in specifying leaf adaxial identity. Development 130: 4097–4107. [DOI] [PubMed] [Google Scholar]
- Yu Y., Feng Z., Wang G., Li F., Du X., Zhu J. (2010). Initiation of dedifferentiation and structural changes in in vitro cultured petiole of Arabidopsis thaliana. Protoplasma 241: 75–81. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Hu Y., Dai M., Huang L., Zhou D.X. (2009). The WUSCHEL-related homeobox gene WOX11 is required to activate shoot-borne crown root development in rice. Plant Cell 21: 736–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo J., Niu Q.W., Chua N.H. (2000). Technical advance: An estrogen receptor-based transactivator XVE mediates highly inducible gene expression in transgenic plants. Plant J. 24: 265–273. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.