GA and JA regulate diverse aspects of plant growth, development, and defense. This work reveals a mechanism for GA and JA signaling synergy and identifies a signaling complex for the GA pathway: DELLAs and JAZs interact with and repress the WD-repeat/bHLH/MYB complex to mediate the synergistic action between GA and JA signaling in regulating trichome development.
Abstract
Integration of diverse environmental and endogenous signals to coordinately regulate growth, development, and defense is essential for plants to survive in their natural habitat. The hormonal signals gibberellin (GA) and jasmonate (JA) antagonistically and synergistically regulate diverse aspects of plant growth, development, and defense. GA and JA synergistically induce initiation of trichomes, which assist seed dispersal and act as barriers to protect plants against insect attack, pathogen infection, excessive water loss, and UV irradiation. However, the molecular mechanism underlying such synergism between GA and JA signaling remains unclear. In this study, we revealed a mechanism for GA and JA signaling synergy and identified a signaling complex of the GA pathway in regulation of trichome initiation. Molecular, biochemical, and genetic evidence showed that the WD-repeat/bHLH/MYB complex acts as a direct target of DELLAs in the GA pathway and that both DELLAs and JAZs interacted with the WD-repeat/bHLH/MYB complex to mediate synergism between GA and JA signaling in regulating trichome development. GA and JA induce degradation of DELLAs and JASMONATE ZIM-domain proteins to coordinately activate the WD-repeat/bHLH/MYB complex and synergistically and mutually dependently induce trichome initiation. This study provides deep insights into the molecular mechanisms for integration of different hormonal signals to synergistically regulate plant development.
INTRODUCTION
To survive, plants must integrate diverse environmental and endogenous signals to regulate growth, development, and defense. The hormonal signals gibberellin (GA) and jasmonate (JA) regulate many aspects of plant growth, development, and defense. GA plays essential roles in promotion of plant growth and development (Ueguchi-Tanaka et al., 2007; Harberd et al., 2009; Sun, 2011; Hauvermale et al., 2012; Davière and Achard, 2013), including root growth (Fu and Harberd, 2003; Ubeda-Tomás et al., 2008), seed germination (Kahn et al., 1957; Piskurewicz et al., 2008), hypocotyl elongation (Silk and Jones, 1975), leaf expansion (Achard et al., 2009), stem elongation (Kato, 1956), flower development (Cheng et al., 2004), trichome initiation (Chien and Sussex, 1996; Gan et al., 2006; Gan et al., 2007), and in repression of plant defense (Navarro et al., 2008). The GA receptors GA-INSENSITIVE DWARF1a/b/c perceive GA signals (Griffiths et al., 2006; Murase et al., 2008) and recruit DELLA proteins, including RGA (Silverstone et al., 1997), GAI (Peng et al., 1997), RGL1 (Wen and Chang, 2002), RGL2 (Lee et al., 2002), and RGL3 (Wild et al., 2012), for ubiquitination and subsequent degradation (Willige et al., 2007; Ariizumi et al., 2008). Degradation of the DELLAs releases various DELLA-interacting transcription factors to activate their respective GA responses, including hypocotyl elongation, fruit patterning, and hook formation (Oh et al., 2007; de Lucas et al., 2008; Feng et al., 2008; Arnaud et al., 2010; Heo et al., 2011; Zhang et al., 2011; An et al., 2012; Bai et al., 2012; Gallego-Bartolomé et al., 2012; Li et al., 2012; Park et al., 2013; Sarnowska et al., 2013).
JAs are a class of lipid-derived hormone molecules (Howe and Jander, 2008; Browse, 2009; Gfeller et al., 2010; Wasternack and Hause, 2013) that control diverse developmental processes, including stamen development (Feys et al., 1994; McConn and Browse, 1996; Huang et al., 2014), root growth (Staswick et al., 1992; Pauwels et al., 2010), trichome formation (Yoshida et al., 2009; Qi et al., 2011), and secondary metabolism (Hong et al., 2012; Pollier et al., 2013), and regulate various defense responses against pathogen infection (Thomma et al., 1998; Vijayan et al., 1998; Melotto et al., 2006; Yang et al., 2008; Rowe et al., 2010; Zheng et al., 2012) and insect attack (Howe et al., 1996; McConn et al., 1997; Moreno et al., 2009; Hu et al., 2013a; Mousavi et al., 2013; Schweizer et al., 2013; Song et al., 2014). The F-box protein CORONATINE INSENSITIVE1 (COI1) (Xie et al., 1998; Yan et al., 2013) perceives JA signals (Yan et al., 2009; Sheard et al., 2010) and recruits Jasmonate ZIM-domain (JAZ) proteins for ubiquitination and subsequent degradation through the 26S proteasomes (Chini et al., 2007; Thines et al., 2007; Yan et al., 2007), leading to release of the downstream signaling cascades required for various JA responses (Cheng et al., 2011; Fernández-Calvo et al., 2011; Niu et al., 2011; Song et al., 2011; Zhu et al., 2011; Shan et al., 2012; Hu et al., 2013b; Nakata et al., 2013; Song et al., 2013b).
GA and JA exhibit antagonistic actions in regulating hypocotyl elongation, root growth, flowering, and defense against necrotrophic pathogens and hemibiotrophic bacterial pathogens (Navarro et al., 2008; Hou et al., 2010, 2013; Wild et al., 2012; Yang et al., 2012). It was speculated that interaction between DELLAs and JAZs would attenuate their repression on their respective downstream transcription factors. GA signals would induce DELLAs degradation, abolish the DELLA-JAZ interactions, and release JAZs to bind and attenuate transcription factor MYC2, resulting in inhibition of JA-regulated root growth (Hou et al., 2010). Reciprocally, JA signals would promote JAZ degradation to release DELLAs, which further interact with and repress their downstream transcription factor PIF3, leading to inhibition of hypocotyl elongation (Yang et al., 2012).
In addition to antagonistic regulation, GA and JA also act synergistically to mediate many important developmental processes: Both GA and JA regulate development of stamens (Feys et al., 1994; McConn and Browse, 1996; Cheng et al., 2004; Mandaokar et al., 2006; Song et al., 2011) and induce initiation of trichomes (Chien and Sussex, 1996; Perazza et al., 1998; Traw and Bergelson, 2003; Li et al., 2004; Yoshida et al., 2009), which assist seed dispersal and protect plants against insect attack, pathogen infection, excessive water loss, and UV irradiation (Wagner et al., 2004; Ramsay and Glover, 2005; Ishida et al., 2008; Tissier, 2012). However, the molecular mechanism underlying the synergism between GA and JA signaling remains unclear. In this study, we reveal a mechanism for GA and JA signaling synergy and show that the WD-repeat/bHLH/MYB complex acts as a direct target of DELLAs in the GA pathway. We also show that DELLAs and JAZs interact with essential components of the WD-repeat/bHLH/MYB complex to modulate GA and JA signaling synergy in regulating trichome development. Identification of the WD-repeat/bHLH/MYB complex as a DELLA- and JAZ-interacting target, whose activation demands both GA and JA signaling, suggests a general mechanism for integration of different hormone signals to synergistically regulate plant development and growth.
RESULTS
GA Promotes Trichome Initiation in a WD-Repeat/bHLH/MYB Complex-Dependent Manner
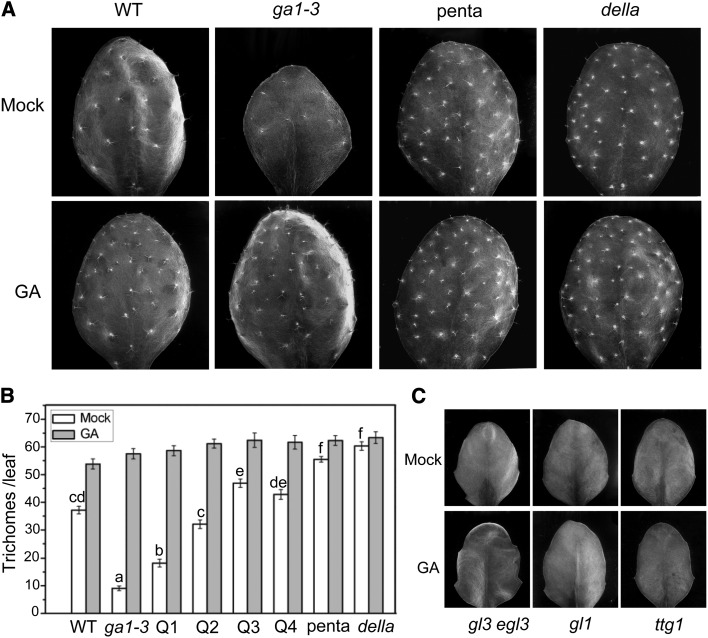
GA is required for trichome formation (Chien and Sussex, 1996; Perazza et al., 1998; Gan et al., 2007). Consistent with this, we also noticed that the GA biosynthetic mutant ga1-3 exhibited a nearly trichome-less phenotype (Figures 1A and 1B) and that the penta mutant (ga1-3 gai-t6 rga-t2 rgl1-1 rgl2-1, wild-type for RGL3) rescued the trichome-less phenotype (Figures 1A and 1B). In addition, we found that the della mutant (gai-t6 rga-t2 rgl1-1 rgl2-1 rgl3-1) displayed maximum trichome density (Figures 1A and 1B), suggesting that the DELLA proteins repress trichome initiation.
Figure 1.
GA Promotes Trichome Formation in a WD-Repeat/bHLH/MYB Complex–Dependent Manner.
(A) Trichome images of wild-type (WT) Landsberg erecta (Ler), ga1-3, penta (ga1-3 rga gai rgl1 rgl2), and della (rga gai rgl1 rgl2 rgl3) plants treated without (Mock) or with GA3 (GA). The fifth true leaves were selected to count trichome numbers using an environmental scanning electron microscope.
(B) Statistical analysis of total trichome numbers in the fifth true leaves of Ler (WT), ga1-3, Q1 (ga1-3 gai rgl1 rgl2), Q2 (ga1-3 rga rgl1 rgl2), Q3 (ga1-3 rga gai rgl1), Q4 (ga1-3 rga gai rgl2), penta, and della plants treated without (Mock) or with GA3 (GA). Eight leaves for each genotype were used for trichome number measurement in each biological experiment. Data are means (±se) of three biological replicates. Lowercase letters indicate significant differences by one-way ANOVA analysis with SAS software (P < 0.05).
(C) Trichome images of Arabidopsis gl3 egl3, gl1, and ttg1 mutants treated without (Mock) or with GA3 (GA). The fifth true leaves were selected to count trichome numbers. No trichome was observed in gl3 egl3, gl1, and ttg1 mutants.
Furthermore, we showed that exogenous application of GA induces maximum trichome density in the ga1-3 mutant (wild-type for five DELLAs) and the mutants with various DELLA mutations, such as Q1 (ga1-3 gai-t6 rgl1-1 rgl2-1, wild-type for RGA and RGL3), Q2 (ga1-3 rga-t2 rgl1-1 rgl2-1, wild-type for GAI and RGL3), Q3 (ga1-3 gai-t6 rgl1-1 rga-t2, wild-type for RGL2 and RGL3), Q4 (ga1-3 gait6 rga-t2 rgl2-1, wild-type for RGL1 and RGL3), and penta (ga1-3 gai-t6 rga-t2 rgl1-1 rgl2-1, wild-type for RGL3) (Figures 1A and 1B). Quantitative analysis of trichome density demonstrated that, among these five DELLA proteins, RGA and GAI play the major roles in repressing trichome formation, RGL1 and RGL2 play moderate roles, and RGL3 plays a minor role (Figures 1A and 1B).
Previous studies have shown that the WD-repeat (TRANSPARENT TESTA GLABRA1 [TTG1])/bHLH (GLABRA3 [GL3] or ENHANCER OF GLABRA3 [EGL3])/MYB (GLABRA3 [GL1] or MYB23) complex mediates trichome development (Zhang et al., 2003; Ishida et al., 2008; Pesch and Hülskamp, 2009; Grebe, 2012). We further found that exogenous application of GA was unable to induce trichome initiation in gl3 egl3, gl1, and ttg1, the mutants of WD-repeat/bHLH/MYB complex (Figure 1C). As expected, the gl3 egl3, gl1, and ttg1 mutants showed trichome-deficient phenotypes (Figure 1C) (Zhang et al., 2003). Taken together (Figure 1), these results indicated that GA induces the degradation of DELLAs to activate trichome formation in a WD-repeat/bHLH/MYB complex-dependent manner.
DELLA Proteins Interact with the bHLH Transcription Factors GL3/EGL3 and the MYB Factor GL1
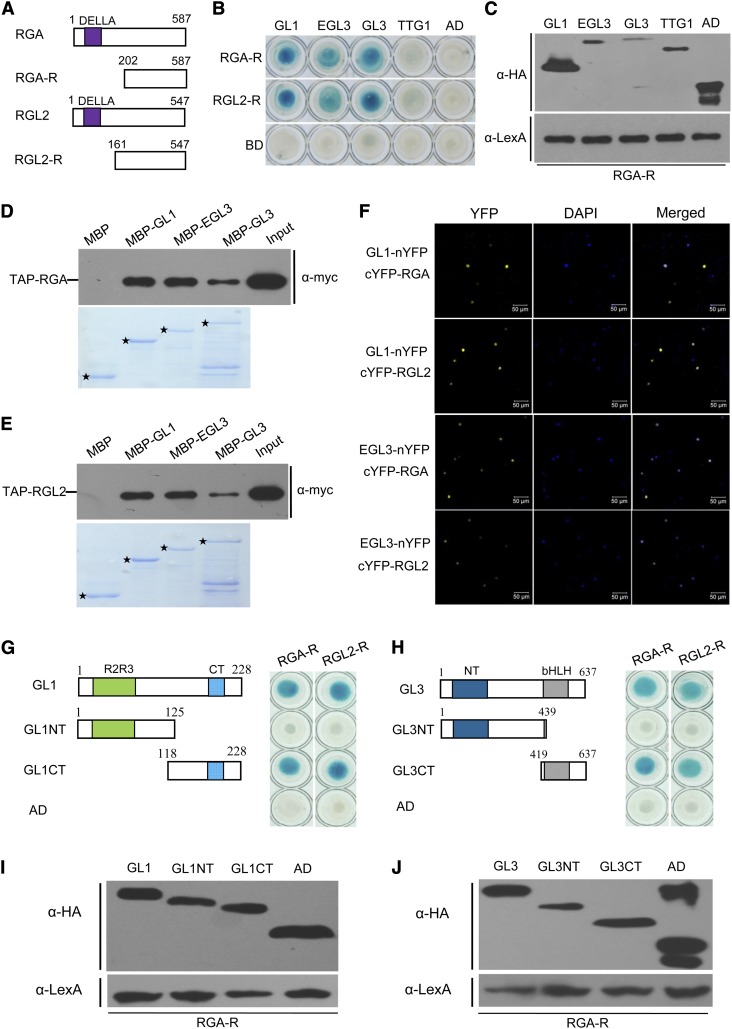
Having shown that the WD-repeat/bHLH/MYB complex is required for GA-induced trichome initiation (Figure 1), we then investigated whether the DELLA repressors interact with the WD-repeat/bHLH/MYB complex to attenuate trichome initiation. The full-length DELLAs displayed strong autoactivation when fused with the LexA DNA binding domain (BD) in our yeast two-hybrid (Y2H) assay; therefore, the truncated C-terminal part of RGA (RGA-R), a representative DELLA protein with the strongest repression of trichome formation (Figure 1B), was fused with BD domain to test its interaction with TTG1, GL1, EGL3, and GL3, the major components of the WD-repeat/bHLH/MYB complex (Figures 2A and 2B).
Figure 2.
Interactions of DELLAs with the WD-Repeat/bHLH/MYB Complex.
(A) Schematic diagrams show domain constructs of RGA and RGL2. The diagrams display the conserved DELLA domain. The numbers indicate positions of the first and the last amino acid of the domain constructs.
(B) Y2H assays to test the interactions of DELLAs with GL1, EGL3, GL3, and TTG1. The RGA-R (202 to 587 amino acids) and RGL2-R (161 to 547 amino acids) were fused with the LexA DNA BD. GL1, EGL3, GL3, and TTG1 were fused with the activation domain (AD).
(C) Expression of GL1, EGL3, GL3, TTG1, and RGA-R in the yeast strains shown in the top panel of (B). GL1, EGL3, GL3, and TTG1 were detected with anti-HA antibody (top). RGA-R was detected with anti-LexA antibody (bottom).
(D) and (E) In vitro pull-down assay to verify the interactions of RGA (D) or RGL2 (E) with GL1, EGL3, and GL3. Purified MBP, MBP-GL1, MBP-EGL3, and MBP-GL3 fusion proteins were incubated with the total proteins extracted from the Arabidopsis seedlings transgenic for TAP-RGA (D) or TAP-RGL2 (E). Bound proteins were washed, separated on SDS-PAGE, and immunoblotted with the anti-c-myc antibody (a-myc; top panel). The input lane shows the expression level of myc-RGA (D) or myc-RGL2 (E) in the transgenic plant. The positions of purified MBP, MBP-GL1, MBP-EGL3, and MBP-GL3 separated on SDS-PAGE are indicated with an asterisk (bottom panel; stained by Coomassie blue).
(F) BiFC assay to detect the interactions of RGA and RGL2 (fused with C-terminal fragment of YFP) with GL1 and EGL3 (fused with N-terminal fragment of YFP). Construct pairs indicated were coinfiltrated into leaves of N. benthamiana. YFP fluorescence was detected 50 h after infiltration. The nuclei are indicated by 4′,6-diamidino-2-phenylindole (DAPI) staining.
(G) and (H) Y2H assays to detect the interactions of RGA-R and RGL2-R with different domains of GL1 (G) and GL3 (H). The schematic diagram showed the AD domain–fused GL1NT and GL1CT, the conserved R2R3 domain and CT domain of GL1 (G), the AD domain–fused GL3NT and GL3CT, and the conserved NT domain and bHLH domain of GL3 (H). The numbers indicate the positions of amino acids.
(I) Expression of GL1, GL1NT, GL1CT, and RGA-R in the yeast strains used in (G). GL1, GL1NT, GL1CT, and AD were detected with anti-HA antibody (top). RGA-R was detected with anti-LexA antibody (bottom).
(J) Expression of GL3, GL3NT, GL3CT, and RGA-R in yeast strains used in (H). GL3, GL3NT, GL3CT, and AD were detected with anti-HA antibody (top). RGA-R was detected with anti-LexA antibody (bottom).
We found that RGA-R interacts with GL1, EGL3, and GL3, but not with TTG1, in the Y2H system (Figures 2B and 2C). Similarly, the BD domain–fused C terminus of RGL2 (RGL2-R), another representative DELLA, also interacts with GL1, EGL3, and GL3, but not with TTG1 (Figure 2B). These results implied that DELLA proteins physically interact with the bHLH and MYB members of the WD-repeat/bHLH/MYB complex.
We next adopted a pull-down assay to verify the interaction of GL1, EGL3, and GL3 with DELLA proteins. The purified GL1, EGL3, or GL3 proteins, fused with the maltose binding protein (MBP), were incubated with total proteins from transgenic Arabidopsis thaliana plants expressing the DELLA proteins RGA or RGL2 fused with tandem affinity purification (TAP) tag (TAP-RGA or TAP-RGL2). Immunoblot analysis showed that all the MBP-fused GL1, EGL3, and GL3 could retain TAP-RGA, whereas MBP alone could not (Figure 2D). We observed a similar result for TAP-RGL2 when incubated with MBP-fused GL1, EGL3, and GL3 (Figure 2E). These results verified the physical interaction of DELLAs with GL1/EGL3/GL3 in vitro.
We further performed bimolecular fluorescence complementation (BiFC) assays to verify the interactions of DELLA with GL1 and EGL3. RGA and RGL2 were fused with the C-terminal part of yellow fluorescent protein (cYFP) individually to generate cYFP-RGA and cYFP-RGL2, while GL1 and EGL3 were ligated with the N-terminal fragment of YFP (nYFP) to generate GL1-nYFP and EGL3-nYFP, respectively. We found that coexpression of GL1-nYFP with cYFP-RGA or cYFP-RGL2 in Nicotiana benthamiana leaves produced strong YFP fluorescence in the nucleus (Figure 2F), whereas no YFP signal was observed in negative controls (Supplemental Figure 1). YFP fluorescence was also observed when EGL3-nYFP was coexpressed with cYFP-RGA or cYFP-RGL2 (Figure 2F). The BiFC results further demonstrated that the MYB (GL1) and bHLH factor (EGL3) interact with DELLAs in vivo.
To study which domains of the MYB and bHLH members are responsible for interaction with DELLA proteins, we further divided GL1 into N-terminal fragment (GL1NT) containing the R2R3 DNA BD and C-terminal fragment (GL1CT) (Figure 2G). GL3 was also divided into an N-terminal fragment (GL3NT) and a C-terminal fragment (GL3CT) containing the bHLH DNA BD (Figure 2H). As shown in Figures 2G to 2J, RGA-R and RGL2-R interact with both the C-terminal fragments of GL1 and GL3, but not with GL1NT and GL3NT, suggesting that C-terminal parts of MYB factor GL1 and bHLH factor GL3 are required for the interaction with DELLA proteins.
In summary, the Y2H, pull-down, and BiFC assays demonstrated that the DELLA repressors interact with the MYB (GL1) and bHLH (EGL3 and GL3) members of the WD-repeat/bHLH/MYB complex.
DELLAs Repress the Transcriptional Function of the WD-Repeat/bHLH/MYB Complex
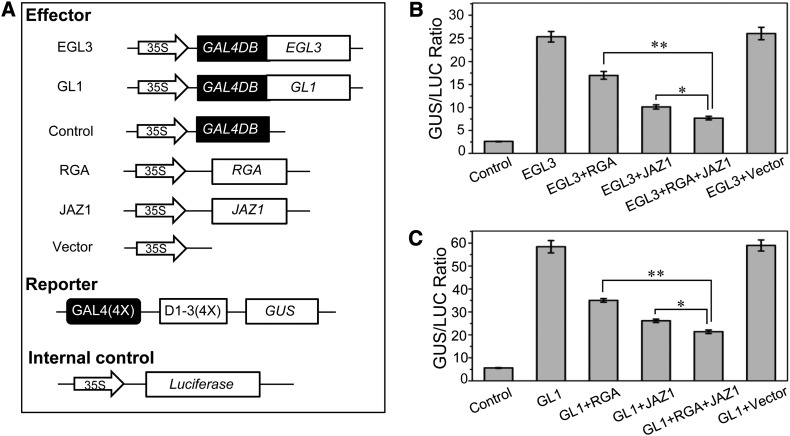
To explore the significance of the interaction between DELLA proteins and these MYB/bHLH factors, we tested whether DELLAs can affect the transcriptional function of these MYB and bHLH factors. To do this, we used an Arabidopsis protoplast transient expression system based on the GAL4 DNA binding domain (GAL4DB) and its binding sites [GAL4(4X)-D1-3(4X)] (Tiwari et al., 2001).
The R2R3-MYB transcription factor GL1 and the two bHLH factors EGL3 and GL3 were fused with the 35S promoter–driven GAL4DB to serve as effectors. The GUS (β-glucuronidase) gene under control of four upstream GAL4 DNA binding sites [GAL4(4x)-D1-3(4x)] was used as a reporter, whereas the 35S promoter–driven LUC (luciferase) was the internal control (Figure 3A). The DELLA genes RGA and RGL2 were inserted into pGreen62 vector under control of the 35S promoter (Figure 3A). As shown in Figure 3B, expression of GAL4DB-GL1 with the GUS reporter largely increased the GUS/LUC ratio, indicating that GL1 functions as a transcription activator. Coexpression of RGA or RGL2 with GAL4DB-GL1 significantly reduced the GUS/LUC ratio (Figure 3B), indicating that the GL1 transcription activity was inhibited by RGA or RGL2. Similar experiments also showed that RGA and RGL2 inhibit the transcription activity of EGL3 and GL3 (Figures 3A, 3C, and 3D).
Figure 3.
DELLA Proteins Inhibit Transcriptional Function of the WD-Repeat/bHLH/MYB Complex.
(A) The schematic diagram shows the constructs used in the transient expression assays of (B) to (D).
(B) Transient expression assay shows that RGA and RGL2 inhibit transcriptional function of GL1. The GUS reporter and the internal control LUC were cotransformed with the indicated constructs. Data are means (±se) of three biological replicates. Asterisks represent Student’s t test significance compared with GL1 (**P < 0.01).
(C) Transient expression assay shows that RGA and RGL2 inhibit transcriptional function of EGL3. Data are means (±se) of three biological replicates. Asterisks represent Student’s t test significance compared with EGL3 (**P < 0.01).
(D) Transient expression assay shows that RGA and RGL2 inhibit transcriptional function of GL3. Data are means (±se) of three biological replicates. Asterisks represent Student’s t test significance compared with GL3 (**P < 0.01).
(E) The schematic diagram shows the constructs used in the transient expression assays of (F).
(F) Transient expression assay shows that activation of GL2 promoter by GL3/GL1 is repressed by RGA. The PGL2-LUC reporter was cotransformed with the indicated constructs. Data are means (±se) of three biological replicates. Asterisks represent Student’s t test significance compared with GL1+GL3 (**P < 0.01).
(G) and (H) Real-time PCR analysis for GL2 (G) and MYB23 (H) in Arabidopsis Ler wild-type (WT), ga1-3, and penta plants. ACTIN8 was used as the internal control. Data are means (±se) of three biological replicates. Asterisks indicate significant differences compared with the wild type by one-way ANOVA analysis with SAS software (**P < 0.01).
As GL2 is a direct target of GL1 and GL3 (Zhang et al., 2003; Morohashi et al., 2007), we used the GL2 promoter–driven LUC reporter (PGL2-LUC) system to further verify the inhibitory effect of DELLAs on transcriptional function of the WD-repeat/bHLH/MYB (Figure 3E). Consistent with a previous study (Wang and Chen, 2008), our results showed that expression of GL3 and GL1 induced PGL2-LUC activity (Figure 3F). Furthermore, we observed that coexpression of RGA repressed GL3/GL1-induced PGL2-LUC expression (Figure 3F).
Taken together (Figures 2 and 3), our results suggested that the DELLA proteins interact with GL1, EGL3, and GL3 to attenuate their transcriptional functions. The quantitative real-time PCR assay on GA-related mutants (ga1-3 and penta) further demonstrated that endogenous DELLA proteins in planta attenuated the transcription function of the WD-repeat/bHLH/MYB complex and repressed expression of its downstream target genes, including GL2 and MYB23 (Zhang et al., 2003; Kang et al., 2009; Morohashi and Grotewold, 2009) (Figures 3G and 3H). Compared with the wild type, the GA-deficient mutant ga1-3, in which DELLA proteins accumulate (Silverstone et al., 2001), exhibited reduced expression of GL2 and MYB23 (Figures 3G and 3H), while the expression level of GL2 and MYB23 was fully rescued in the penta mutant (Figures 3G and 3H).
DELLAs Act through the WD-Repeat/bHLH/MYB Complex to Repress GA-Mediated Trichome Formation
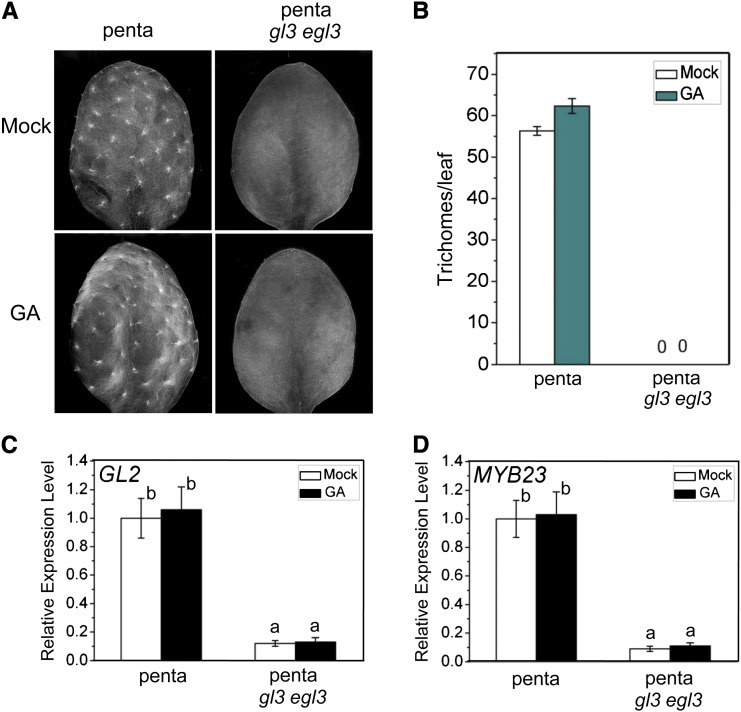
To investigate whether the WD-repeat/bHLH/MYB complex acts downstream of DELLAs to regulate GA-induced trichome formation, we crossed the penta mutant with the gl3 egl3 double mutant to produce the heptuple mutant (penta gl3 egl3). Comparison of trichome numbers in penta and penta gl3 egl3 mutants showed that trichome initiation in the penta mutant was abolished by the gl3 egl3 double mutations (Figure 4A). The penta mutant produced high density of trichomes (Figure 4B); however, the penta gl3 egl3 heptuple mutant exhibited a no-trichome phenotype (Figures 4A and 4B), which is similar to the glabrous phenotype of the gl3 egl3 mutant (Figure 1C). Consistent with this, the expression levels of GL2 and MYB23, targets of the WD-repeat/bHLH/MYB complex, were significantly decreased in the penta gl3 egl3 mutant (Figures 4C and 4D). These results demonstrated that the DELLAs act upstream of the WD-repeat/bHLH/MYB complex to regulate GA-mediated trichome formation.
Figure 4.
Double Mutations in GL3 and EGL3 Block Trichome Initiation in the penta Mutant.
(A) Images of the fifth true leaves of penta and penta gl3 egl3 heptuple mutant treated without (Mock) or with GA3 (GA).
(B) Statistical analysis of total trichome numbers per leaf indicated in (A). Eight leaves for each genotype were used for trichome number measurement in each biological experiment. Data are means (±se) of three biological replicates.
(C) and (D) Real-time PCR analysis for GL2 (C) and MYB23 (D) in penta and penta gl3 egl3 mutants treated without (Mock) or with GA3 (GA). ACTIN8 was used as the internal control. Data are means (±se) of three biological replicates. Lowercase letters indicate significant differences by one-way ANOVA analysis with SAS software (P < 0.05).
[See online article for color version of this figure.]
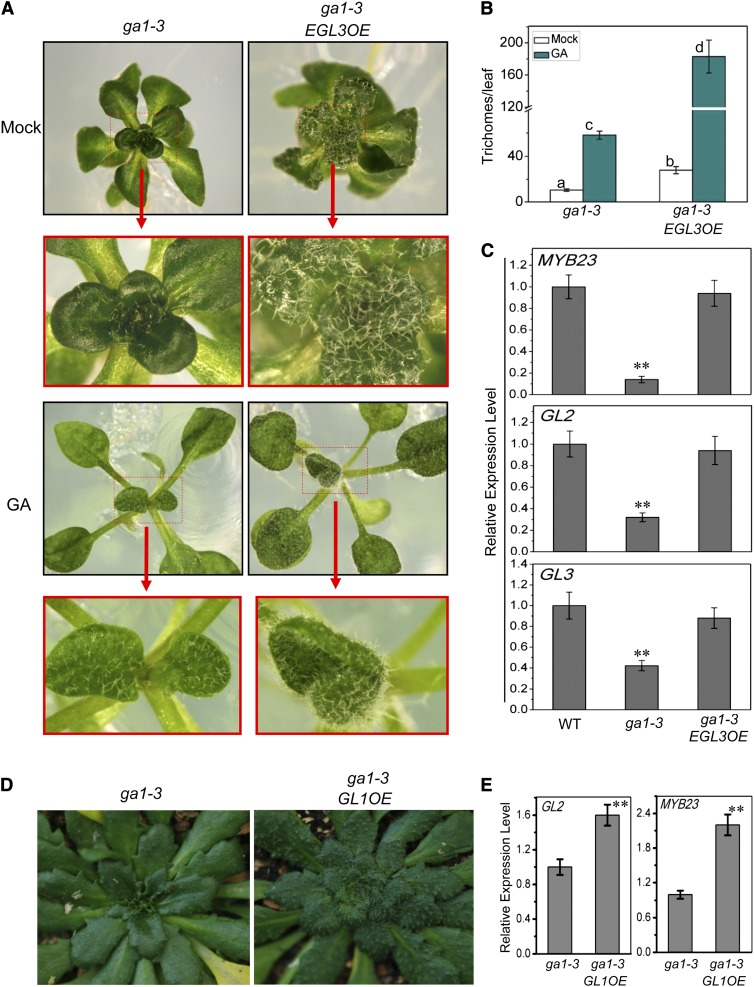
To further verify that DELLAs act through the WD-repeat/bHLH/MYB complex to repress GA-mediated trichome formation, we examined whether overexpression of the bHLH factor EGL3 or MYB factor GL1 could escape the inhibition of DELLAs to rescue trichome formation in the GA-deficient mutant ga1-3. Trichome density analysis showed that the transgenic ga1-3 plants overexpressing EGL3 (ga1-3 EGL3OE) developed high density of trichomes (Figures 5A and 5B), suggesting that overexpression of EGL3 could restore the trichome formation in ga1-3. Furthermore, we found that EGL3 overexpression in ga1-3 could rescue expression of MYB23, GL2, and GL3 (target genes or component of the WD-repeat/bHLH/MYB complex) (Figure 5C).
Figure 5.
Overexpression of EGL3 or GL1 Rescues Trichome Initiation in ga1-3 Mutant.
(A) Plant images of ga1-3 and EGL3 overexpression transgenic plant in ga1-3 background (ga1-3 EGL3OE) supplied without (Mock) or with GA3 (GA).
(B) Statistical analysis of total trichome numbers in the fifth true leaves of indicated plants in (A). Eight leaves for each genotype were used for trichome number measurement in each biological experiment. Data are means (±se) of three biological replicates. Asterisks indicate significant differences by one-way ANOVA analysis with SAS software (**P < 0.05).
(C) Real-time PCR analysis for GL2, MYB23, and GL3 in Arabidopsis wild-type Ler (WT), ga1-3, and ga1-3 EGL3OE plants plated on MS medium. ACTIN8 was used as the internal control. Data are means (±se) of three biological replicates. Asterisks indicate significant differences compared with ga1-3 EGL3OE by one-way ANOVA analysis with SAS software (**P < 0.01).
(D) Plant images of 3-month-old ga1-3 and ga1-3 GL1OE transgenic plants. GL1 overexpression obviously increased trichome formation in ga1-3 background.
(E) Real-time PCR analysis for GL2 and MYB23 in ga1-3 and ga1-3 GL1OE plants in (D). ACTIN8 was used as the internal control. Data are means (±se) of three biological replicates. Asterisks indicate significant differences compared with ga1-3 by one-way ANOVA analysis with SAS software (**P < 0.01).
In addition to overexpression of the bHLH factor EGL3, we also examined whether overexpression of MYB factor GL1 restored trichome initiation in the ga1-3 mutant. As shown in Figure 5D, overexpression of GL1 in ga1-3 increased trichome numbers. Consistent with this, overexpression of GL1 in ga1-3 enhanced the expression of its target genes GL2 and MYB23 (Figure 5E). Taken together, we demonstrated that overexpression of EGL3 or GL1 can restore trichome formation in ga1-3 mutants, suggesting that the WD-repeat/bHLH/MYB complex acts downstream of DELLAs to regulate GA-induced trichome formation. These results collectively demonstrated that the WD-repeat/bHLH/MYB complex acts downstream of DELLAs to regulate GA-mediated trichome formation.
GA and JA Synergistically and Mutually Dependently Regulate Trichome Formation
Consistent with our previous studies (Qi et al., 2011), we further found that trichome initiation is induced by JA and attenuated by disruption of JA signaling (e.g., coi1-1) or biosynthesis (e.g., aos) (Supplemental Figures 2A and 2B) and that the WD-repeat/bHLH/MYB complex acts as a direct target of JAZ proteins to modulate trichome initiation (Qi et al., 2011) (Supplemental Figures 2 and 3).
As both DELLAs and JAZs interact with the WD-repeat/bHLH/MYB transcription complex to repress its transcription function (Figures 2 and 3; Supplemental Figure 2), we further investigated whether DELLAs and JAZs regulate the transcription function of the WD-repeat/bHLH/MYB complex synergistically. As expected, RGA or JAZ1 alone repressed the transcription activity of the bHLH factor EGL3 of the WD-repeat/bHLH/MYB complex (Figures 6A and 6B). We further found that coexpression of RGA and JAZ1 displayed a stronger repression of the transcriptional activity of EGL3 compared with RGA or JAZ1 alone in the transient expression assay (Figures 6A and 6B). We observed similar results for the repression of RGA and JAZ1 on the MYB factor GL1 (Figures 6A and 6C). These results demonstrated that DELLAs and JAZ proteins synergistically repressed the transcription activity of the WD-repeat/bHLH/MYB complex.
Figure 6.
RGA and JAZ1 Coordinately Inhibit Transcriptional Function of EGL3 and GL1.
(A) Schematic diagram of the constructs used in transient expression assays in (B) and (C).
(B) and (C) Transient expression assay showed that both RGA and JAZ1 proteins could significantly repress transcriptional function of EGL3 (B) and GL1 (C). The GUS/LUC ratios were measured in Arabidopsis leaf protoplasts after transiently transfected with reporter, internal control, and indicated effectors. Data are means (±se) of three biological replicates. Asterisks represent Student’s t test significance between pairs indicated with brackets (*P < 0.05; **P < 0.01).
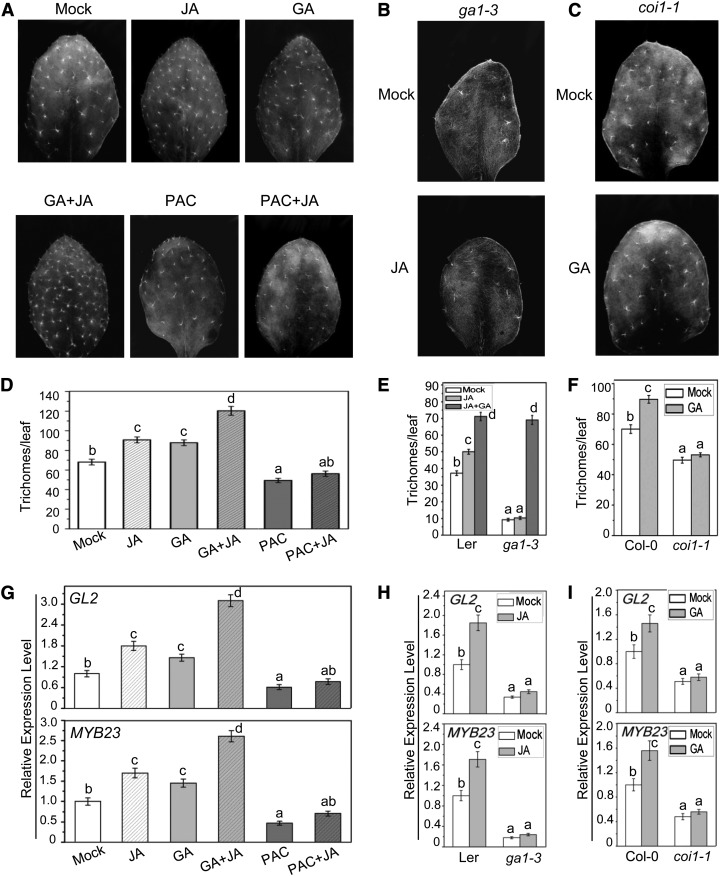
We further investigated whether GA and JA synergistically regulated trichome formation in planta. The wild-type plants were treated with GA, JA, or a combination of GA and JA and subjected for quantitative trichome analysis. As shown in Figures 7A and 7D, the wild-type plants displayed much more trichome numbers under treatment with both GA and JA, compared with treatment with GA or JA alone. Consistent with this, the expression of GL2 and MYB23, the target genes of the WD-repeat/bHLH/MYB complex, was also highly induced by combination of GA and JA (Figure 7G). These results demonstrated that GA and JA synergistically induce trichome initiation in planta.
Figure 7.
GA and JA Function Synergistically to Promote Trichome Initiation.
(A) Trichome images of the fifth true leaves from wild-type Columbia-0 (Col-0) treated without (Mock) or with MeJA (JA), GA3 (GA), GA3 plus MeJA (GA+JA), paclobutrazol (PAC), or paclobutrazol plus MeJA (PAC+JA).
(B) Trichome images of the fifth true leave from ga1-3 treated without (Mock) or with MeJA (JA).
(C) Trichome images of the fifth true leave from coi1-1 treated without (Mock) or with GA3 (GA).
(D) Statistical analysis of total trichome numbers in the fifth true leaves of Col-0 with the indicated treatments in (A). Eight leaves for each genotype were used for trichome number measurement in each biological experiment. Data are means (±se) of three biological replicates. Lowercase letters indicate significant differences by one-way ANOVA analysis with SAS software (P < 0.05).
(E) and (F) Statistical analysis of total trichome numbers in the fifth true leaves of the indicated genotype with the indicated treatments. Eight leaves for each genotype were used for trichome number measurement in each biological experiment. Data are means (±se) of three biological replicates. Lowercase letters indicate significant differences by one-way ANOVA analysis with SAS software (P < 0.05).
(G) Real-time PCR analysis for GL2 and MYB23 in Col-0 plants indicated in (A). ACTIN8 was used as the internal control. Data are means (±se) of three biological replicates. Lowercase letters indicate significant differences by one-way ANOVA analysis with SAS software (P < 0.05).
(H) and (I) Real-time PCR analysis for GL2 (H) and MYB23 (I) in the indicated genotype with the indicated treatment in (B) and (C), respectively. ACTIN8 was used as the internal control. Data are means (±se) of three biological replicates. Lowercase letters indicate significant differences by one-way ANOVA analysis with SAS software (P < 0.05).
Interestingly, we found that paclobutrazol, a GA biosynthetic inhibitor that causes DELLAs accumulation in plant (Feng et al., 2008), repressed JA-induced trichome initiation (Figures 7A and 7D) and the expression of GL2 and MYB23 (Figure 7G). Furthermore, we observed that the GA biosynthesis mutant ga1-3 (with high level of DELLAs) clearly suppressed the JA-induced trichome initiation and the expression of GL2 and MYB23 (Figures 7B, 7E, and 7H) and that the combination of GA and JA significantly induced trichome initiation in ga1-3 (Figure 7E). These observations of DELLA suppression on JA-induced trichome formation suggested that GA signaling is required for JA-regulated trichome development.
Similarly, we found that the GA-induced trichome formation was attenuated in the JA signaling-deficient mutant coi1-1 (with high level of JAZs) (Figures 7C and 7F). The GA-induced expression of GL2 and MYB23 in coi1-1 was also inhibited (Figure 7I). These results suggested that GA-induced trichome formation requires JA signaling. Taken together, our results demonstrated that plant hormones GA and JA synergistically induce trichome initiation and that JA and GA signaling regulate trichome development in a mutually dependent fashion.
DISCUSSION
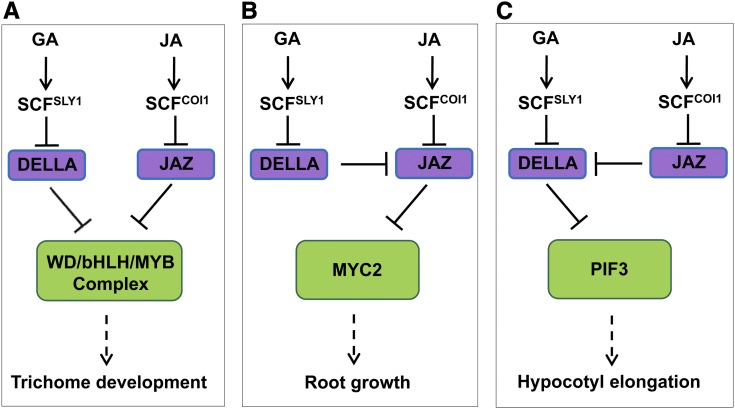
Plants integrate diverse environmental and endogenous signals to coordinately regulate growth, development, and defense to improve their survival in natural habitats. This study reveals a key molecular mechanism for integration of different hormonal signals to synergistically regulate plant development. Previous studies demonstrated that the GA-signaling repressors DELLAs interact with the JA-signaling repressors JAZs to modulate the antagonistic action between GA and JA in regulating root growth and hypocotyl elongation (Hou et al., 2010; Yang et al., 2012) (Figures 8B and 8C). The results reported here demonstrate that the WD-repeat/bHLH/MYB complex acts as a direct target of DELLAs and that both DELLAs and JAZs interact with the WD-repeat/bHLH/MYB complex to mediate the synergistic and mutually dependent action between GA and JA signaling in regulating plant trichome development. DELLAs and JAZs interact with bHLH factors (EGL3 and GL3) and MYB factor (GL1), essential components of the WD-repeat/bHLH/MYB complex, to repress their transcription activity, while GA and JA induce degradation of DELLAs and JAZs, respectively, to coordinately activate the WD-repeat/bHLH/MYB complex and synergistically and mutually dependently regulate trichome development (Figure 8A).
Figure 8.
A Simplified Model for the Crosstalk between GA and JA.
(A) Both DELLAs and JAZs interact with and attenuate WD-repeat/bHLH/MYB complex to inactivate downstream genes and repress trichome formation. GA and JA signal induce degradation of DELLAs and JAZs, respectively, to derepress WD-repeat/bHLH/MYB complex and synergistically and mutually dependently modulate trichome development in Arabidopsis.
(B) A previous study showed that the Arabidopsis DELLAs interact with and repress JAZs to release MYC2 that mediates JA-regulated root growth (Hou et al., 2010).
(C) A previous study showed that JAZs interact with and repress DELLAs to release PIF3 that regulates GA-mediated hypocotyl elongation in Arabidopsis (Yang et al., 2012).
[See online article for color version of this figure.]
Consistent with this synergistic regulatory mechanism, in the mutants deficient in GA biosynthesis (e.g., gal-3), DELLA proteins accumulate to high levels to interact with and repress the WD-repeat/bHLH/MYB complex and inhibit trichome initiation. Similarly, in the mutants deficient in JA biosynthesis or signaling (e.g., aos and coi1-1), JAZ proteins accumulated to interact with and repress WD-repeat/bHLH/MYB and attenuate trichome initiation. GA and JA signals are mutually indispensable for activation of WD-repeat/bHLH/MYB, and disruption of either GA or JA signaling would lead to significant accumulation of either DELLAs or JAZs, which interact with and repress nearly all members of the WD-repeat/bHLH/MYB complex and attenuate trichome formation (Figures 1, 2, 3, 6, and 7; Supplemental Figure 2). Also, exogenous JA treatment of the GA-biosynthetic mutant gal-3 did not rescue trichome formation (Figures 7B and 7E) as the high levels of DELLAs in gal-3 are sufficient to repress WD-repeat/bHLH/MYB; reciprocally, GA treatment of the JA-related mutant coi1-1, which has high levels of JAZs, failed to restore trichome development (Figures 7C and 7F).
In response to developmental cues or environmental challenges, wild-type plants generate GA and/or JA signals to coordinately control levels of DELLAs and JAZs via SCFSLY1 and SCFCOI1-mediated 26S proteolysis. This maintains the appropriate transcriptional activity of WD-repeat/bHLH/MYB to activate expression of its downstream transcription factors (e.g., GL2 and MYB23) and mediate proper trichome formation (Figures 7A, 7D, 7G, and 8A). Treatment of exogenous GA and/or JA further induces degradation of the remaining DELLAs and/or JAZs in the wild type to derepress WD-repeat/bHLH/MYB, leading to excessive trichome development in wild-type plants (Figures 1A, 1B, 7A, 7D, 7G, and 8A; Supplemental Figures 2A and 2B). The coordinated regulation of trichome development by both growth-related hormones (GA) and stress-triggered signals (JA) may benefit plant growth and survival in nature.
Our results and previous studies showed that the C-terminal part of DELLAs can bind to different transcriptional factors, including EGL3/GL3/GL1 (Figure 2), BZR1 (Bai et al., 2012), and EIN3/EIL1 (An et al., 2012). Similarly, the C-terminal Jas domain of JAZ proteins interact with various transcriptional factors, such as EGL3/GL3/TT8/GL1/MYB75 (Qi et al., 2011), MYC2/MYC3/MYC4 (Chini et al., 2009; Cheng et al., 2011), IIId bHLH factors (Song et al., 2013b), ICE1/ICE1 (Hu et al., 2013b), and MYB21/MYB24 (Song et al., 2011). As JAZs and DELLAs both interact with the bHLH and MYB components of the WD-repeat/bHLH/MYB complex (EGL3, GL3, and GL1) (Figure 2) (Qi et al., 2011), it will be interesting to investigate whether JAZ and DELLA proteins simultaneously bind to the WD-repeat/bHLH/MYB complex for synergistic repression.
In addition to trichome development, GA and JA also synergistically regulate sesquiterpene synthesis (Hong et al., 2012) and stamen development (Ma, 2005; Plackett et al., 2011; Song et al., 2013a). The R2R3 MYB transcription factors MYB21, MYB24, and MYB57 constitute a class of master regulators essential for stamen development (Mandaokar et al., 2006; Reeves et al., 2012). Our previous study demonstrated that JA modulates the interaction of JAZs with MYB21 and MYB24 to control stamen development (Song et al., 2011) and that GA promotes the expression of JA biosynthesis genes and induces JA accumulation in the flower to activate the COI1-JAZ-MYB21/MYB24 pathway for stamen development (Cheng et al., 2009). It would be very interesting to investigate, in addition to the GA-regulated JA biosynthesis in flower, whether both DELLAs and JAZs also interact with MYB21/MYB24 to exert a synergistic and mutually indispensable effect in regulation of stamen development.
METHODS
Plant Materials and Growth Conditions
The Arabidopsis thaliana mutants ga1-3, penta, della, Q1, Q2, Q3, Q4, gl3 egl3, gl1, ttg1, coi1-1, and aos and the transgenic plants TAP-RGA and TAP-RGL2 were as described previously (Feys et al., 1994; Park et al., 2002; Feng et al., 2008; Cheng et al., 2009; Qi et al., 2011). The heptuple mutant penta gl3 egl3 was generated by crossing penta with gl3 egl3.
After surface sterilization with 20% bleach for 10 min, Arabidopsis seeds were plated on Murashige and Skoog (MS) medium (Sigma-Aldrich) supplied with 3% Suc, chilled at 4°C in the dark for 3 d, and then transferred to growth house at 19 to 23°C with 16-h-light/8-h-dark photoperiod. Seeds with ga1-3 background were immerged in 100 µM GA3 at 4°C for 7 d before sowing. Nicotiana benthamiana was cultivated at 22 to 28°C under a 16-h-light/8-h-dark photoperiod.
Y2H Assays
The C-terminal fragments of RGA or RGL2 (RGA-R or RGL2-R) and coding regions of GL1, EGL3, GL3, TTG1, and their truncated forms were inserted into pLexA or pB42AD vectors. Y2H assays were performed following the manufacturer's instructions (Clontech) according to Qi et al. (2011). The EGY48 yeast cotransformed with the indicated constructs were selected on solid SD/-His/-Trp/-Ura medium. Surviving yeast transformants were then cultured with liquid SD/-His/-Trp/-Ura medium, harvested by centrifugation, and resuspended with 200 μL of distilled water, of which 5 μL were dropped onto a well of 96-well plates containing the induction medium (SD/Gal/Raf/X-gal/-Ura/-His/Trp/-Leu). Photos were taken after incubation at 30°C for 2 to 3 d. The expression of proteins fused with AD and BD domain were detected by immunoblots with anti-HA and anti-LexA antibodies, respectively. Primers for making these constructs are listed in Supplemental Table 1. The experiment was repeated for three independent biological replicates.
Trichome Measurement
For detecting the effect of GA on trichome formation, seeds were disinfected, chilled, and cultivated on MS medium containing 100 µM GA3 for 6 d and then transferred to MS medium for another 2 weeks before trichome observation. The fifth true leaves were further used for trichome observation by environmental scanning electron microscopy (FEI Quanta 200). For exploring the interaction effect of GA and JA on trichome formation, seeds were disinfected, chilled, and plated on MS medium with or without 100 µM GA3 for 6 d and then transferred to MS medium supplemented with indicated combinations of 0.2 µM paclobutrazol and 200 nM methyl jasmonate (MeJA) in a 15-cm-diameter plate. GA3 and paclobutrazol were applied in the MS medium. For MeJA treatment, an Eppendorf tube cap with a piece of filter paper in it was placed in the center of plate. MeJA was dropped onto the filter paper for evaporation. After 2 weeks, the total trichome numbers of the fifth true leaves were calculated using environmental scanning electron microscopy.
BiFC Assay and Luciferase Complementation Imaging Assay
For BiFC assays, the full-length coding sequences of RGA, RGL2, GL1, and EGL3 were first inserted into the pDONR207 vector and subsequently recombined into binary cYFP or nYFP vector through the Gateway reaction system (Invitrogen) (Qi et al., 2011). Primers used are presented in Supplemental Table 1. These vectors were subsequently transformed into Agrobacterium tumefaciens strain GV3101 and coexpressed in N. benthamiana leaves as described previously (Qi et al., 2011). YFP fluorescence signal was detected using confocal microscopy (LSM710; Zeiss) 50 h after infiltration. The nuclei were indicated by staining with 4′,6-diamidino-2-phenylindole. The experiment was repeated for three independent biological replicates.
For the firefly LUC complementation imaging assays, the full length coding sequences of JAZ1 and JAZ11 were fused with N-terminal fragment of luciferase (nLUC) to form JAZ1-nLUC and JAZ11-nLUC, respectively. The full-length coding sequence of EGL3 was ligated with C-terminal fragment of luciferase (cLUC) to form cLUC-EGL3. Primers used are presented in Supplemental Table 1. These vectors were subsequently transformed into Agrobacterium strain GV3101 and coexpressed in N. benthamiana leaves. The LUC complementation images were taken with a low-light cooled charge-coupled device imaging apparatus (Andor iXon) as described previously (Song et al., 2011). The experiment was repeated for three independent biological replicates.
Pull-Down Assay
The full-length coding sequences of EGL3, GL1, and GL3 were inserted into the pMAL-c2x (NEB) vector to produce MBP-EGL3, MBP-GL1, and MBP-GL3, respectively. The MBP and MBP-fused EGL3, GL3, and GL1 were expressed in Escherichia coli and purified by amylose resin beads. Total proteins was extracted from 5 g of TAP-RGA and TAP-RGL2 transgenic plants using RB buffer (100 mM NaCl, 50 mM Tris-Cl, pH 7.8, 25 mM imidazole, 0.1% [v/v] Tween 20, 10% [v/v] glycerol, EDTA-free complete mini protease inhibitor cocktail, and 20 mM 2-mercaptoethanol), and subsequently concentrated to the volume of 400 μL. The procedure of pull-down assay was performed according to Qi et al. (2011). About 50 mg of purified MBP, MBP-EGL3, MBP-GL1, and MBP-GL3 proteins were incubated with 100 μL of amylose resin for 2 h at 4°C with gentle rocking and subsequently washed with 1 mL of RB buffer five times, then further incubated with 100 μL concentrated total proteins containing TAP-RGA and TAP-RGL2 for 2 h at 4°C. The proteins binding to amylose resin was further washed for five times with 1 mL RB buffer, resuspended in SDS loading buffer, and immunoblotted using anti-c-myc antibody (Roche). Coomassie Brilliant Blue was applied to determine protein levels. The experiment was repeated for three independent biological replicates.
Protoplast Transfection Assay
For transient expression assays using the GUS reporter, the coding sequences of EGL3, GL3, and GL1 were fused with the GAL4DB under control of the 35S promoter. The coding sequences of RGA, RGL2, and JAZ1 were inserted into the pGreenII 62-SK under control of the 35S promoter (Hellens et al., 2005). Primers used for plasmid construction are listed in Supplemental Table 1. The GUS reporter contains four copies of upstream GAL4DB binding sites [GAL4(4x)-D1-3(4x)] followed by subsequent GUS gene (Tiwari et al., 2001). The internal control was a firefly LUC gene under control of 35S promoter. Arabidopsis mesophyll protoplasts preparation and subsequent transfection were performed according to the protocols reported previously (Yoo et al., 2007). The GUS:LUC ratio was presented. For Figures 3B to 3D, 10 µg for all the constructs were used in these experiments. For Figures 6B and 6C, 4 µg JAZ1 and 5 µg RGA were used, respectively, while for other reporters and effectors, 10 μg plasmid was individually used.
To perform transient transcription activity assay using the LUC reporter, a −2045-bp GL2 promoter was amplified from genomic DNA and inserted into pGreenII 0800-LUC to generate the PGL2-LUC reporter construct (Hellens et al., 2005). The coding sequences of GL3 and GL1 were amplified and inserted into pGreenII 62-SK vector under control of the 35S promoter. All primers used for these constructs are listed in Supplemental Table 1. After protoplasts preparation and subsequent transfection, firefly LUC and renillia luciferase (REN) activities were measured using the dual-luciferase reporter assay system (Promega). The LUC:REN ratio was presented. For Figure 3F and Supplemental Figure 2F, 10 µg for all the constructs were used in these experiments.
Real-Time PCR Analysis
The indicated Arabidopsis seedlings were used for quantitative real-time PCR analysis of gene expression. Total RNAs were extracted using Trizol reagent (Invitrogen) and reverse transcribed using M-MLV (Takara). Real-time PCR was performed with the ABi7500 real-time PCR system using the SYBR Prime Script RT-PCR kit (Takara). The relative gene expression level was calculated by normalizing against the internal control ACTIN8. Primers used for real-time PCR analysis are listed in Supplemental Table 2.
Generation of EGL3 and GL1 Transgenic Plants
To generate Arabidopsis EGL3 and GL1 overexpression transgenic plants, the coding sequences of EGL3 and GL1 were amplified and inserted into the modified pCAMBIA1300 vector (Cambia) under control of 35S promoter. These vectors were transformed into Agrobacterium strain GV3101 and subsequently introduced into the Arabidopsis wild-type plants. EGL3 overexpression transgenic plants were crossed with ga1-3 and coi1-1 to produce ga1-3 EGL3OE and coi1-1 GL1OE plants. GL1 overexpression transgenic plants were crossed with ga1-3 to generate ga1-3 GL1OE. Three individual transgenic lines for each gene (EGL3 or GL1) were identified, and one transgenic line for EGL3 (or GL1) was representatively displayed in the article.
Accession Numbers
The Arabidopsis Genome Initiative numbers for the genes mentioned in this article are as follows: GAI (AT4G02780), RGA (AT2G01570), RGL1 (AT1G66350), RGL2 (AT3G03450), RGL3 (AT5G17490), GA1 (AT4G02780), COI1 (AT2G39940), AOS (AT5G42650), JAZ1 (AT1G19180), JAZ8 (AT1G30135), JAZ10 (AT5G13220), JAZ11 (AT3G43440), GL3 (AT5G41315), EGL3 (AT1G63650), GL1 (AT3G27920), MYB23 (AT5G40330), GL2 (AT1G79840), TTG1 (AT5G24520), and ACTIN8 (AT1G49240).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Negative Controls of BiFC Assay in N. benthamiana Leaves.
Supplemental Figure 2. JAZs Interact with the WD-Repeat/bHLH/MYB Complex to Modulate Jasmonate-Regulated Trichome Development.
Supplemental Figure 3. EGL3 Overexpression Restores Trichome Formation in coi1-1.
Supplemental Table 1. Primers Used for Vector Construction.
Supplemental Table 2. Primers Used for Quantitative Real-Time PCR Analysis.
Supplementary Material
Acknowledgments
We thank Xingwang Deng, Kang Chong, and Hongwei Guo for TAP-RGA/RGL2 seeds and vectors. The work was supported by the National Science Foundation of China (31230008, 31200214, and 91017012).
AUTHOR CONTRIBUTIONS
T.Q., S.S., and D.X. designed the research. T.Q., H.H., D.W., and S.S. performed research. T.Q., J.Y., Y.Q., S.S., and D.X. analyzed data. T.Q., S.S., and D.X. wrote the article.
Glossary
- GA
gibberellin
- JA
jasmonate
- BD
binding domain
- Y2H
yeast two-hybrid
- BiFC
bimolecular fluorescence complementation
- MS
Murashige and Skoog
- MeJA
methyl jasmonate
- Ler
Landsberg erecta
- Col-0
Columbia-0
Footnotes
Some figures in this article are displayed in color online but in black and white in the print edition.
Online version contains Web-only data.
References
- Achard P., Gusti A., Cheminant S., Alioua M., Dhondt S., Coppens F., Beemster G.T., Genschik P. (2009). Gibberellin signaling controls cell proliferation rate in Arabidopsis. Curr. Biol. 19: 1188–1193. [DOI] [PubMed] [Google Scholar]
- An F., Zhang X., Zhu Z., Ji Y., He W., Jiang Z., Li M., Guo H. (2012). Coordinated regulation of apical hook development by gibberellins and ethylene in etiolated Arabidopsis seedlings. Cell Res. 22: 915–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariizumi T., Murase K., Sun T.P., Steber C.M. (2008). Proteolysis-independent downregulation of DELLA repression in Arabidopsis by the gibberellin receptor GIBBERELLIN INSENSITIVE DWARF1. Plant Cell 20: 2447–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaud N., Girin T., Sorefan K., Fuentes S., Wood T.A., Lawrenson T., Sablowski R., Østergaard L. (2010). Gibberellins control fruit patterning in Arabidopsis thaliana. Genes Dev. 24: 2127–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai M.Y., Shang J.X., Oh E., Fan M., Bai Y., Zentella R., Sun T.P., Wang Z.Y. (2012). Brassinosteroid, gibberellin and phytochrome impinge on a common transcription module in Arabidopsis. Nat. Cell Biol. 14: 810–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browse J. (2009). Jasmonate passes muster: A receptor and targets for the defense hormone. Annu. Rev. Plant Biol. 60: 183–205. [DOI] [PubMed] [Google Scholar]
- Cheng H., Qin L., Lee S., Fu X., Richards D.E., Cao D., Luo D., Harberd N.P., Peng J. (2004). Gibberellin regulates Arabidopsis floral development via suppression of DELLA protein function. Development 131: 1055–1064. [DOI] [PubMed] [Google Scholar]
- Cheng H., Song S., Xiao L., Soo H.M., Cheng Z., Xie D., Peng J. (2009). Gibberellin acts through jasmonate to control the expression of MYB21, MYB24, and MYB57 to promote stamen filament growth in Arabidopsis. PLoS Genet. 5: e1000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Z., Sun L., Qi T., Zhang B., Peng W., Liu Y., Xie D. (2011). The bHLH transcription factor MYC3 interacts with the Jasmonate ZIM-domain proteins to mediate jasmonate response in Arabidopsis. Mol. Plant 4: 279–288. [DOI] [PubMed] [Google Scholar]
- Chien J.C., Sussex I.M. (1996). Differential regulation of trichome formation on the adaxial and abaxial leaf surfaces by gibberellins and photoperiod in Arabidopsis thaliana (L.) Heynh. Plant Physiol. 111: 1321–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chini A., Fonseca S., Chico J.M., Fernández-Calvo P., Solano R. (2009). The ZIM domain mediates homo- and heteromeric interactions between Arabidopsis JAZ proteins. Plant J. 59: 77–87. [DOI] [PubMed] [Google Scholar]
- Chini A., Fonseca S., Fernández G., Adie B., Chico J.M., Lorenzo O., García-Casado G., López-Vidriero I., Lozano F.M., Ponce M.R., Micol J.L., Solano R. (2007). The JAZ family of repressors is the missing link in jasmonate signalling. Nature 448: 666–671. [DOI] [PubMed] [Google Scholar]
- Davière J.M., Achard P. (2013). Gibberellin signaling in plants. Development 140: 1147–1151. [DOI] [PubMed] [Google Scholar]
- de Lucas M., Davière J.M., Rodríguez-Falcón M., Pontin M., Iglesias-Pedraz J.M., Lorrain S., Fankhauser C., Blázquez M.A., Titarenko E., Prat S. (2008). A molecular framework for light and gibberellin control of cell elongation. Nature 451: 480–484. [DOI] [PubMed] [Google Scholar]
- Feng S., et al. (2008). Coordinated regulation of Arabidopsis thaliana development by light and gibberellins. Nature 451: 475–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Calvo P., et al. (2011). The Arabidopsis bHLH transcription factors MYC3 and MYC4 are targets of JAZ repressors and act additively with MYC2 in the activation of jasmonate responses. Plant Cell 23: 701–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feys B.J.F., Benedetti C.E., Penfold C.N., Turner J.G. (1994). Arabidopsis mutants selected for resistance to the phytotoxin coronatine are male-sterile, insensitive to methyl jasmonate, and resistant to a bacterial pathogen. Plant Cell 6: 751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X., Harberd N.P. (2003). Auxin promotes Arabidopsis root growth by modulating gibberellin response. Nature 421: 740–743. [DOI] [PubMed] [Google Scholar]
- Gallego-Bartolomé J., Minguet E.G., Grau-Enguix F., Abbas M., Locascio A., Thomas S.G., Alabadí D., Blázquez M.A. (2012). Molecular mechanism for the interaction between gibberellin and brassinosteroid signaling pathways in Arabidopsis. Proc. Natl. Acad. Sci. USA 109: 13446–13451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan Y., Kumimoto R., Liu C., Ratcliffe O., Yu H., Broun P. (2006). GLABROUS INFLORESCENCE STEMS modulates the regulation by gibberellins of epidermal differentiation and shoot maturation in Arabidopsis. Plant Cell 18: 1383–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan Y., Yu H., Peng J., Broun P. (2007). Genetic and molecular regulation by DELLA proteins of trichome development in Arabidopsis. Plant Physiol. 145: 1031–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gfeller A., Dubugnon L., Liechti R., Farmer E.E. (2010). Jasmonate biochemical pathway. Sci. Signal. 3: cm3. [DOI] [PubMed] [Google Scholar]
- Grebe M. (2012). The patterning of epidermal hairs in Arabidopsis—updated. Curr. Opin. Plant Biol. 15: 31–37. [DOI] [PubMed] [Google Scholar]
- Griffiths J., Murase K., Rieu I., Zentella R., Zhang Z.L., Powers S.J., Gong F., Phillips A.L., Hedden P., Sun T.P., Thomas S.G. (2006). Genetic characterization and functional analysis of the GID1 gibberellin receptors in Arabidopsis. Plant Cell 18: 3399–3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harberd N.P., Belfield E., Yasumura Y. (2009). The angiosperm gibberellin-GID1-DELLA growth regulatory mechanism: How an “inhibitor of an inhibitor” enables flexible response to fluctuating environments. Plant Cell 21: 1328–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauvermale A.L., Ariizumi T., Steber C.M. (2012). Gibberellin signaling: A theme and variations on DELLA repression. Plant Physiol. 160: 83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellens R.P., Allan A.C., Friel E.N., Bolitho K., Grafton K., Templeton M.D., Karunairetnam S., Gleave A.P., Laing W.A. (2005). Transient expression vectors for functional genomics, quantification of promoter activity and RNA silencing in plants. Plant Methods 1: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo J.O., Chang K.S., Kim I.A., Lee M.H., Lee S.A., Song S.K., Lee M.M., Lim J. (2011). Funneling of gibberellin signaling by the GRAS transcription regulator scarecrow-like 3 in the Arabidopsis root. Proc. Natl. Acad. Sci. USA 108: 2166–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong G.J., Xue X.Y., Mao Y.B., Wang L.J., Chen X.Y. (2012). Arabidopsis MYC2 interacts with DELLA proteins in regulating sesquiterpene synthase gene expression. Plant Cell 24: 2635–2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou X., Ding L., Yu H. (2013). Crosstalk between GA and JA signaling mediates plant growth and defense. Plant Cell Rep. 32: 1067–1074. [DOI] [PubMed] [Google Scholar]
- Hou X., Lee L.Y., Xia K., Yan Y., Yu H. (2010). DELLAs modulate jasmonate signaling via competitive binding to JAZs. Dev. Cell 19: 884–894. [DOI] [PubMed] [Google Scholar]
- Howe G.A., Jander G. (2008). Plant immunity to insect herbivores. Annu. Rev. Plant Biol. 59: 41–66. [DOI] [PubMed] [Google Scholar]
- Howe G.A., Lightner J., Browse J., Ryan C.A. (1996). An octadecanoid pathway mutant (JL5) of tomato is compromised in signaling for defense against insect attack. Plant Cell 8: 2067–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P., Zhou W., Cheng Z., Fan M., Wang L., Xie D. (2013a). JAV1 controls jasmonate-regulated plant defense. Mol. Cell 50: 504–515. [DOI] [PubMed] [Google Scholar]
- Hu Y., Jiang L., Wang F., Yu D. (2013b). Jasmonate regulates the inducer of cbf expression-C-repeat binding factor/DRE binding factor1 cascade and freezing tolerance in Arabidopsis. Plant Cell 25: 2907–2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H., Wang C., Tian H., Sun Y., Xie D., Song S. (2014). Amino acid substitutions of GLY98, LEU245 and GLU543 in COI1 distinctively affect jasmonate-regulated male fertility in Arabidopsis. Sci. China Life Sci. 57: 145–154. [DOI] [PubMed] [Google Scholar]
- Ishida T., Kurata T., Okada K., Wada T. (2008). A genetic regulatory network in the development of trichomes and root hairs. Annu. Rev. Plant Biol. 59: 365–386. [DOI] [PubMed] [Google Scholar]
- Kahn A., Goss J.A., Smith D.E. (1957). Effect of gibberellin on germination of lettuce seed. Science 125: 645–646. [DOI] [PubMed] [Google Scholar]
- Kang Y.H., Kirik V., Hulskamp M., Nam K.H., Hagely K., Lee M.M., Schiefelbein J. (2009). The MYB23 gene provides a positive feedback loop for cell fate specification in the Arabidopsis root epidermis. Plant Cell 21: 1080–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato J. (1956). Effect of gibberellin on elongation, water uptake, and respiration of pea stem sections. Science 123: 1132. [DOI] [PubMed] [Google Scholar]
- Lee S., Cheng H., King K.E., Wang W., He Y., Hussain A., Lo J., Harberd N.P., Peng J. (2002). Gibberellin regulates Arabidopsis seed germination via RGL2, a GAI/RGA-like gene whose expression is up-regulated following imbibition. Genes Dev. 16: 646–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Zhao Y., McCaig B.C., Wingerd B.A., Wang J., Whalon M.E., Pichersky E., Howe G.A. (2004). The tomato homolog of CORONATINE-INSENSITIVE1 is required for the maternal control of seed maturation, jasmonate-signaled defense responses, and glandular trichome development. Plant Cell 16: 126–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q.F., Wang C., Jiang L., Li S., Sun S.S., He J.X. (2012). An interaction between BZR1 and DELLAs mediates direct signaling crosstalk between brassinosteroids and gibberellins in Arabidopsis. Sci. Signal. 5: ra72. [DOI] [PubMed] [Google Scholar]
- Ma H. (2005). Molecular genetic analyses of microsporogenesis and microgametogenesis in flowering plants. Annu. Rev. Plant Biol. 56: 393–434. [DOI] [PubMed] [Google Scholar]
- Mandaokar A., Thines B., Shin B., Lange B.M., Choi G., Koo Y.J., Yoo Y.J., Choi Y.D., Choi G., Browse J. (2006). Transcriptional regulators of stamen development in Arabidopsis identified by transcriptional profiling. Plant J. 46: 984–1008. [DOI] [PubMed] [Google Scholar]
- McConn M., Browse J. (1996). The critical requirement for linolenic acid is pollen development, not photosynthesis, in an Arabidopsis mutant. Plant Cell 8: 403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConn M., Creelman R.A., Bell E., Mullet J.E., Browse J. (1997). Jasmonate is essential for insect defense in Arabidopsis. Proc. Natl. Acad. Sci. USA 94: 5473–5477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melotto M., Underwood W., Koczan J., Nomura K., He S.Y. (2006). Plant stomata function in innate immunity against bacterial invasion. Cell 126: 969–980. [DOI] [PubMed] [Google Scholar]
- Moreno J.E., Tao Y., Chory J., Ballaré C.L. (2009). Ecological modulation of plant defense via phytochrome control of jasmonate sensitivity. Proc. Natl. Acad. Sci. USA 106: 4935–4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morohashi K., Grotewold E. (2009). A systems approach reveals regulatory circuitry for Arabidopsis trichome initiation by the GL3 and GL1 selectors. PLoS Genet. 5: e1000396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morohashi K., Zhao M., Yang M., Read B., Lloyd A., Lamb R., Grotewold E. (2007). Participation of the Arabidopsis bHLH factor GL3 in trichome initiation regulatory events. Plant Physiol. 145: 736–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousavi S.A., Chauvin A., Pascaud F., Kellenberger S., Farmer E.E. (2013). GLUTAMATE RECEPTOR-LIKE genes mediate leaf-to-leaf wound signalling. Nature 500: 422–426. [DOI] [PubMed] [Google Scholar]
- Murase K., Hirano Y., Sun T.P., Hakoshima T. (2008). Gibberellin-induced DELLA recognition by the gibberellin receptor GID1. Nature 456: 459–463. [DOI] [PubMed] [Google Scholar]
- Nakata M., Mitsuda N., Herde M., Koo A.J., Moreno J.E., Suzuki K., Howe G.A., Ohme-Takagi M. (2013). A bHLH-type transcription factor, ABA-INDUCIBLE BHLH-TYPE TRANSCRIPTION FACTOR/JA-ASSOCIATED MYC2-LIKE1, acts as a repressor to negatively regulate jasmonate signaling in Arabidopsis. Plant Cell 25: 1641–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro L., Bari R., Achard P., Lisón P., Nemri A., Harberd N.P., Jones J.D. (2008). DELLAs control plant immune responses by modulating the balance of jasmonic acid and salicylic acid signaling. Curr. Biol. 18: 650–655. [DOI] [PubMed] [Google Scholar]
- Niu Y., Figueroa P., Browse J. (2011). Characterization of JAZ-interacting bHLH transcription factors that regulate jasmonate responses in Arabidopsis. J. Exp. Bot. 62: 2143–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh E., Yamaguchi S., Hu J., Yusuke J., Jung B., Paik I., Lee H.S., Sun T.P., Kamiya Y., Choi G. (2007). PIL5, a phytochrome-interacting bHLH protein, regulates gibberellin responsiveness by binding directly to the GAI and RGA promoters in Arabidopsis seeds. Plant Cell 19: 1192–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J., Nguyen K.T., Park E., Jeon J.S., Choi G. (2013). DELLA proteins and their interacting RING Finger proteins repress gibberellin responses by binding to the promoters of a subset of gibberellin-responsive genes in Arabidopsis. Plant Cell 25: 927–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.H., Halitschke R., Kim H.B., Baldwin I.T., Feldmann K.A., Feyereisen R. (2002). A knock-out mutation in allene oxide synthase results in male sterility and defective wound signal transduction in Arabidopsis due to a block in jasmonic acid biosynthesis. Plant J. 31: 1–12. [DOI] [PubMed] [Google Scholar]
- Pauwels L., et al. (2010). NINJA connects the co-repressor TOPLESS to jasmonate signalling. Nature 464: 788–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J., Carol P., Richards D.E., King K.E., Cowling R.J., Murphy G.P., Harberd N.P. (1997). The Arabidopsis GAI gene defines a signaling pathway that negatively regulates gibberellin responses. Genes Dev. 11: 3194–3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perazza D., Vachon G., Herzog M. (1998). Gibberellins promote trichome formation by Up-regulating GLABROUS1 in Arabidopsis. Plant Physiol. 117: 375–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesch M., Hülskamp M. (2009). One, two, three...models for trichome patterning in Arabidopsis? Curr. Opin. Plant Biol. 12: 587–592. [DOI] [PubMed] [Google Scholar]
- Piskurewicz U., Jikumaru Y., Kinoshita N., Nambara E., Kamiya Y., Lopez-Molina L. (2008). The gibberellic acid signaling repressor RGL2 inhibits Arabidopsis seed germination by stimulating abscisic acid synthesis and ABI5 activity. Plant Cell 20: 2729–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plackett A.R., Thomas S.G., Wilson Z.A., Hedden P. (2011). Gibberellin control of stamen development: A fertile field. Trends Plant Sci. 16: 568–578. [DOI] [PubMed] [Google Scholar]
- Pollier J., et al. (2013). The protein quality control system manages plant defence compound synthesis. Nature 504: 148–152. [DOI] [PubMed] [Google Scholar]
- Qi T., Song S., Ren Q., Wu D., Huang H., Chen Y., Fan M., Peng W., Ren C., Xie D. (2011). The Jasmonate-ZIM-domain proteins interact with the WD-Repeat/bHLH/MYB complexes to regulate Jasmonate-mediated anthocyanin accumulation and trichome initiation in Arabidopsis thaliana. Plant Cell 23: 1795–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay N.A., Glover B.J. (2005). MYB-bHLH-WD40 protein complex and the evolution of cellular diversity. Trends Plant Sci. 10: 63–70. [DOI] [PubMed] [Google Scholar]
- Reeves P.H., et al. (2012). A regulatory network for coordinated flower maturation. PLoS Genet. 8: e1002506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe H.C., Walley J.W., Corwin J., Chan E.K., Dehesh K., Kliebenstein D.J. (2010). Deficiencies in jasmonate-mediated plant defense reveal quantitative variation in Botrytis cinerea pathogenesis. PLoS Pathog. 6: e1000861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnowska E.A., et al. (2013). DELLA-interacting SWI3C core subunit of switch/sucrose nonfermenting chromatin remodeling complex modulates gibberellin responses and hormonal cross talk in Arabidopsis. Plant Physiol. 163: 305–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer F., Fernández-Calvo P., Zander M., Diez-Diaz M., Fonseca S., Glauser G., Lewsey M.G., Ecker J.R., Solano R., Reymond P. (2013). Arabidopsis basic helix-loop-helix transcription factors MYC2, MYC3, and MYC4 regulate glucosinolate biosynthesis, insect performance, and feeding behavior. Plant Cell 25: 3117–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan X., Yan J., Xie D. (2012). Comparison of phytohormone signaling mechanisms. Curr. Opin. Plant Biol. 15: 84–91. [DOI] [PubMed] [Google Scholar]
- Sheard L.B., et al. (2010). Jasmonate perception by inositol-phosphate-potentiated COI1-JAZ co-receptor. Nature 468: 400–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk W.K., Jones R.L. (1975). Gibberellin response in lettuce hypocotyl sections. Plant Physiol. 56: 267–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstone A.L., Jung H.S., Dill A., Kawaide H., Kamiya Y., Sun T.P. (2001). Repressing a repressor: gibberellin-induced rapid reduction of the RGA protein in Arabidopsis. Plant Cell 13: 1555–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstone A.L., Mak P.Y., Martínez E.C., Sun T.P. (1997). The new RGA locus encodes a negative regulator of gibberellin response in Arabidopsis thaliana. Genetics 146: 1087–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S., Huang H., Gao H., Wang J., Wu D., Liu X., Yang S., Zhai Q., Li C., Qi T., Xie D. (2014). Interaction between MYC2 and ETHYLENE INSENSITIVE3 modulates antagonism between jasmonate and ethylene signaling in Arabidopsis. Plant Cell 26: 263–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S., Qi T., Fan M., Zhang X., Gao H., Huang H., Wu D., Guo H., Xie D. (2013b). The bHLH subgroup IIId factors negatively regulate jasmonate-mediated plant defense and development. PLoS Genet. 9: e1003653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S., Qi T., Huang H., Ren Q., Wu D., Chang C., Peng W., Liu Y., Peng J., Xie D. (2011). The Jasmonate-ZIM domain proteins interact with the R2R3-MYB transcription factors MYB21 and MYB24 to affect Jasmonate-regulated stamen development in Arabidopsis. Plant Cell 23: 1000–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S., Qi T., Huang H., Xie D. (2013a). Regulation of stamen development by coordinated actions of jasmonate, auxin, and gibberellin in Arabidopsis. Mol. Plant 6: 1065–1073. [DOI] [PubMed] [Google Scholar]
- Staswick P.E., Su W.P., Howell S.H. (1992). Methyl jasmonate inhibition of root growth and induction of a leaf protein are decreased in an Arabidopsis thaliana mutant. Proc. Natl. Acad. Sci. USA 89: 6837–6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T.P. (2011). The molecular mechanism and evolution of the GA-GID1-DELLA signaling module in plants. Curr. Biol. 21: R338–R345. [DOI] [PubMed] [Google Scholar]
- Thines B., Katsir L., Melotto M., Niu Y., Mandaokar A., Liu G., Nomura K., He S.Y., Howe G.A., Browse J. (2007). JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signalling. Nature 448: 661–665. [DOI] [PubMed] [Google Scholar]
- Thomma B.P., Eggermont K., Penninckx I.A., Mauch-Mani B., Vogelsang R., Cammue B.P., Broekaert W.F. (1998). Separate jasmonate-dependent and salicylate-dependent defense-response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens. Proc. Natl. Acad. Sci. USA 95: 15107–15111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissier A. (2012). Glandular trichomes: What comes after expressed sequence tags? Plant J. 70: 51–68. [DOI] [PubMed] [Google Scholar]
- Tiwari S.B., Wang X.J., Hagen G., Guilfoyle T.J. (2001). AUX/IAA proteins are active repressors, and their stability and activity are modulated by auxin. Plant Cell 13: 2809–2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traw M.B., Bergelson J. (2003). Interactive effects of jasmonic acid, salicylic acid, and gibberellin on induction of trichomes in Arabidopsis. Plant Physiol. 133: 1367–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubeda-Tomás S., Swarup R., Coates J., Swarup K., Laplaze L., Beemster G.T., Hedden P., Bhalerao R., Bennett M.J. (2008). Root growth in Arabidopsis requires gibberellin/DELLA signalling in the endodermis. Nat. Cell Biol. 10: 625–628. [DOI] [PubMed] [Google Scholar]
- Ueguchi-Tanaka M., Nakajima M., Motoyuki A., Matsuoka M. (2007). Gibberellin receptor and its role in gibberellin signaling in plants. Annu. Rev. Plant Biol. 58: 183–198. [DOI] [PubMed] [Google Scholar]
- Vijayan P., Shockey J., Lévesque C.A., Cook R.J., Browse J. (1998). A role for jasmonate in pathogen defense of Arabidopsis. Proc. Natl. Acad. Sci. USA 95: 7209–7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner G.J., Wang E., Shepherd R.W. (2004). New approaches for studying and exploiting an old protuberance, the plant trichome. Ann. Bot. (Lond.) 93: 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Chen J.G. (2008). Arabidopsis transient expression analysis reveals that activation of GLABRA2 may require concurrent binding of GLABRA1 and GLABRA3 to the promoter of GLABRA2. Plant Cell Physiol. 49: 1792–1804. [DOI] [PubMed] [Google Scholar]
- Wasternack C., Hause B. (2013). Jasmonates: Biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann. Bot. (Lond.) 111: 1021–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen C.K., Chang C. (2002). Arabidopsis RGL1 encodes a negative regulator of gibberellin responses. Plant Cell 14: 87–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild M., Davière J.M., Cheminant S., Regnault T., Baumberger N., Heintz D., Baltz R., Genschik P., Achard P. (2012). The Arabidopsis DELLA RGA-LIKE3 is a direct target of MYC2 and modulates jasmonate signaling responses. Plant Cell 24: 3307–3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willige B.C., Ghosh S., Nill C., Zourelidou M., Dohmann E.M., Maier A., Schwechheimer C. (2007). The DELLA domain of GA INSENSITIVE mediates the interaction with the GA INSENSITIVE DWARF1A gibberellin receptor of Arabidopsis. Plant Cell 19: 1209–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie D.X., Feys B.F., James S., Nieto-Rostro M., Turner J.G. (1998). COI1: An Arabidopsis gene required for jasmonate-regulated defense and fertility. Science 280: 1091–1094. [DOI] [PubMed] [Google Scholar]
- Yan J., Li H., Li S., Yao R., Deng H., Xie Q., Xie D. (2013). The Arabidopsis F-box protein CORONATINE INSENSITIVE1 is stabilized by SCFCOI1 and degraded via the 26S proteasome pathway. Plant Cell 25: 486–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J., Zhang C., Gu M., Bai Z., Zhang W., Qi T., Cheng Z., Peng W., Luo H., Nan F., Wang Z., Xie D. (2009). The Arabidopsis CORONATINE INSENSITIVE1 protein is a jasmonate receptor. Plant Cell 21: 2220–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y.X., Stolz S., Chételat A., Reymond P., Pagni M., Dubugnon L., Farmer E.E. (2007). A downstream mediator in the growth repression limb of the jasmonate pathway. Plant Cell 19: 2470–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D.L., et al. (2012). Plant hormone jasmonate prioritizes defense over growth by interfering with gibberellin signaling cascade. Proc. Natl. Acad. Sci. USA 109: E1192–E1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J.Y., Iwasaki M., Machida C., Machida Y., Zhou X., Chua N.H. (2008). betaC1, the pathogenicity factor of TYLCCNV, interacts with AS1 to alter leaf development and suppress selective jasmonic acid responses. Genes Dev. 22: 2564–2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo S.D., Cho Y.H., Sheen J. (2007). Arabidopsis mesophyll protoplasts: A versatile cell system for transient gene expression analysis. Nat. Protoc. 2: 1565–1572. [DOI] [PubMed] [Google Scholar]
- Yoshida Y., Sano R., Wada T., Takabayashi J., Okada K. (2009). Jasmonic acid control of GLABRA3 links inducible defense and trichome patterning in Arabidopsis. Development 136: 1039–1048. [DOI] [PubMed] [Google Scholar]
- Zhang F., Gonzalez A., Zhao M., Payne C.T., Lloyd A. (2003). A network of redundant bHLH proteins functions in all TTG1-dependent pathways of Arabidopsis. Development 130: 4859–4869. [DOI] [PubMed] [Google Scholar]
- Zhang Z.L., Ogawa M., Fleet C.M., Zentella R., Hu J., Heo J.O., Lim J., Kamiya Y., Yamaguchi S., Sun T.P. (2011). Scarecrow-like 3 promotes gibberellin signaling by antagonizing master growth repressor DELLA in Arabidopsis. Proc. Natl. Acad. Sci. USA 108: 2160–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X.Y., Spivey N.W., Zeng W., Liu P.P., Fu Z.Q., Klessig D.F., He S.Y., Dong X. (2012). Coronatine promotes Pseudomonas syringae virulence in plants by activating a signaling cascade that inhibits salicylic acid accumulation. Cell Host Microbe 11: 587–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z., et al. (2011). Derepression of ethylene-stabilized transcription factors (EIN3/EIL1) mediates jasmonate and ethylene signaling synergy in Arabidopsis. Proc. Natl. Acad. Sci. USA 108: 12539–12544. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.