The capacity of plants to regenerate is impressive. Plant cells can undergo somatic embryogenesis in culture, forming an embryo from a single cell or a group of somatic cells. In addition, plants exhibit de novo organogenesis, in which new organs and even entire plants are produced from other organs upon wounding.
Almost as remarkable as the phenomenon of plant regeneration is how little we know about the underlying processes, despite their widespread exploitation in both basic research and applied settings (reviewed in Xu and Huang, 2014). For example, tissue culture often involves inducing callus, and it has only recently become clear that rather than involving cellular dedifferentiation to create a mass of undifferentiated cells, callus formation actually represents a form of de novo organogenesis (reviewed in Sugimoto et al., 2011; Xu and Huang, 2014). It seems that callus is made up of cells with root meristem characteristics, even when produced from shoot tissues, and it arises from xylem-pole pericycle or pericycle-like cells, which may act as persistent stem cells rather than dedifferentiating to form callus. Lateral roots also initiate from pericycle cells, and in fact, several genes and processes involved in lateral root formation also function in callus formation (Sugimoto et al., 2010).
During tissue culture, adventitious roots or shoots can be induced by transferring the callus to medium containing different ratios of auxin and cytokinin. In some cases in nature, de novo organogenesis from detached organs leads directly to the formation of a new plant at a wound site, without production of callus. Now, Liu et al. (1081–1093) have devised a system in which to study such de novo organogenesis using Arabidopsis thaliana.
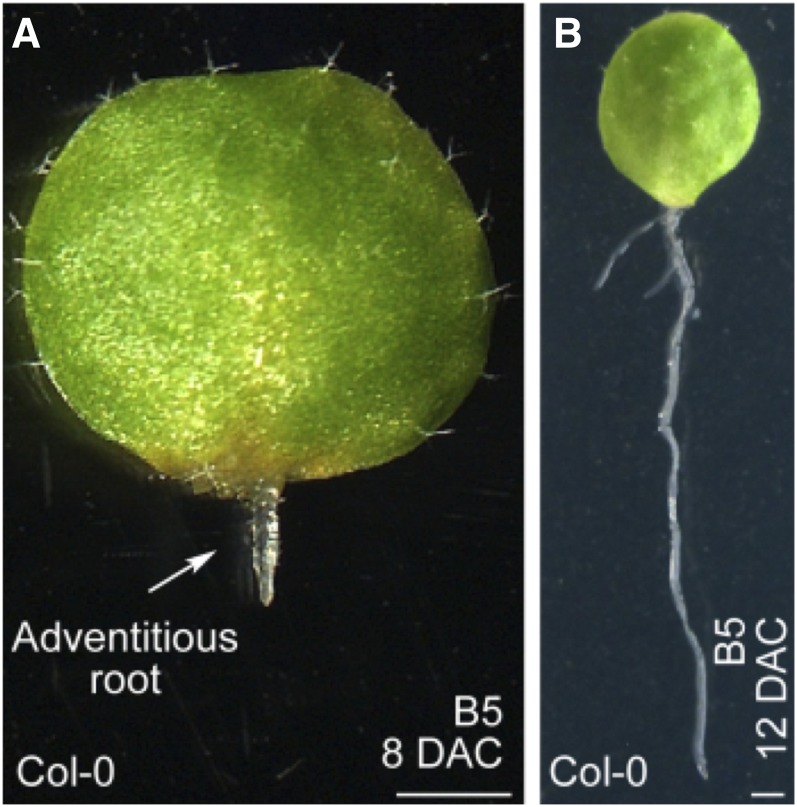
When Liu et al. cultured leaf explants on medium without added hormones, they formed adventitious roots (see figure). Chemical treatments and analysis of auxin reporter lines revealed that initiation of these adventitious root primordia requires polar auxin transport and a local auxin maximum in procambium cells. Liu et al. further found that mutants unable to form callus also failed to form adventitious roots from leaf explants in their system.
Arabidopsis leaf explants form adventitious roots on medium without added hormones. Leaf explants grown for 8 (A) or 12 (B) d on B5 medium. Bars = 1 mm. (Reprinted from Liu et al. [2014], Figure 1.)
The authors reasoned that genes responsible for the first-step cell fate transition leading to adventitious root formation should be directly regulated by auxin and likely were among those differentially regulated during the leaf-to-callus transition. This led them to WUSCHEL RELATED HOMEOBOX11 (WOX11), whose rice (Oryza sativa) homolog is involved in development of crown roots (a type of adventitious root) (Zhao et al., 2009). The authors found that WOX11 is induced by auxin in the procambium where the root founder cell will form. They went on to show that WOX11 and its homolog WOX12 likely function in the initial cell fate transition step of both adventitious rooting and callus formation, acting through at least some of the same downstream regulatory factors in both processes.
In addition to these insights into the molecular mechanisms of regeneration, this work provides an elegant system for studying de novo organogenesis—a process often held up as proof of the amazing plasticity of plants.
References
- Liu J., Sheng L., Xu Y., Li J., Yang Z., Huang H., Xu L. (2014). WOX11 and 12 are involved in the first-step cell fate transition during de novo root organogenesis in Arabidopsis. Plant Cell 26: 1081–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto K., Jiao Y., Meyerowitz E.M. (2010). Arabidopsis regeneration from multiple tissues occurs via a root development pathway. Dev. Cell 18: 463–471. [DOI] [PubMed] [Google Scholar]
- Sugimoto K., Gordon S.P., Meyerowitz E.M. (2011). Regeneration in plants and animals: dedifferentiation, transdifferentiation, or just differentiation? Trends Cell Biol. 21: 212–218. [DOI] [PubMed] [Google Scholar]
- Xu L., Huang H. (2014). Genetic and epigenetic controls of plant regeneration. Curr. Top. Dev. Biol. 108: 1–33. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Hu Y., Dai M., Huang L., Zhou D.X. (2009). The WUSCHEL-related homeobox gene WOX11 is required to activate shoot-borne crown root development in rice. Plant Cell 21: 736–748. [DOI] [PMC free article] [PubMed] [Google Scholar]