Abstract
Mycobacterium marinum is an atypical mycobacterium that usually causes a solitary nodule on the hand (“fish tank granuloma”) or less commonly, secondary erythematous channels and nodules spread along lymphatic drainage of the extremity, mimicking sporothricoid skin lesions of nodular lymphangitis. This report presents a case of this rare entity, a nodular lymphangitis caused by Mycobacterium marinum. Multidetector computed tomography (MDCT) imaging was very useful in determining the morphology (cellulitis with a few small subcutaneous nodules and channels) and the extension of the lesion.
Keywords: Soft-tissue infections, Mycobacterium marinum, computed tomography, nodular lymphangitis
Introduction
The disseminated form of sporotrichoid disease, due to erythematous spread and soft-tissue swelling without adenopathic masses, can mimic a deep soft-tissue infection. Computed tomography (CT) findings such as contrast-enhancing subcutaneous nodes and tubes (lymphatic channels) in a patient with hand nodes strongly suggested this rare entity. Furthermore, CT can help in ruling out air bubbles of necrotizing fasciitis; this technique is more sensitive to detect air bubbles and faster in emergencies than magnetic resonance imaging (MRI). Under these circumstances, CT imaging could be of help in diagnosis.
Case history
A 49-year-old man came to the hospital for evaluation of nodular skin lesions on the upper right extremity. The patient had been diagnosed with ankylosing spondylitis 3 years previously and was receiving treatment with adalimumab. He had a history of narcolepsy, owned a fish aquarium and several dogs, and his hobby was gardening. Physical examination revealed an ulcerated nodular lesion on the dorsal surface of the proximal phalanx of the right fourth finger. There were also erythematous nodules and swelling on the back of the right hand. On the right arm and forearm inflammatory subcutaneous nodules of different sizes followed the lymphatic drainage (Fig. 1) where erythematous and irregular linear streaks extend from primary infection site in the hand with tenderness and heat. There was no regional lymphadenopathy. C-reactive protein was slightly elevated (4.79 mg/dL). The remaining biochemical parameters and complete blood cell count were within normal limits. Chest radiography was normal. Multidetector CT (MDCT) scanning of the right hand and forearm was done to rule out extension of the infection into deeper anatomic structures. MDCT images showed subcutaneous fat stranding on the dorsum of the hand consistent with cellulitis which extended up into the dorsal and cubital aspects of the forearm, elbow, and distal arm. It also revealed a few small nodules in the subcutaneous fat of these regions with enhancement after intravenous contrast administration (Fig. 2). Given these findings, a diagnosis of nodular lymphangitis was suspected. CT excluded the presence of gas, fluid collection, or bone involvement. Skin biopsies and sputum bacilloscopy/smears were taken; a culture was positive as Mycobacterium marinum. Once a diagnosis of M. marinum infection was made, the patient started specific treatment with ethambutol, clarithromycin, and amoxicillin/clavulanic acid. Clinical improvement allowed the patient to be discharged to continue treatment at home.
Fig. 1.
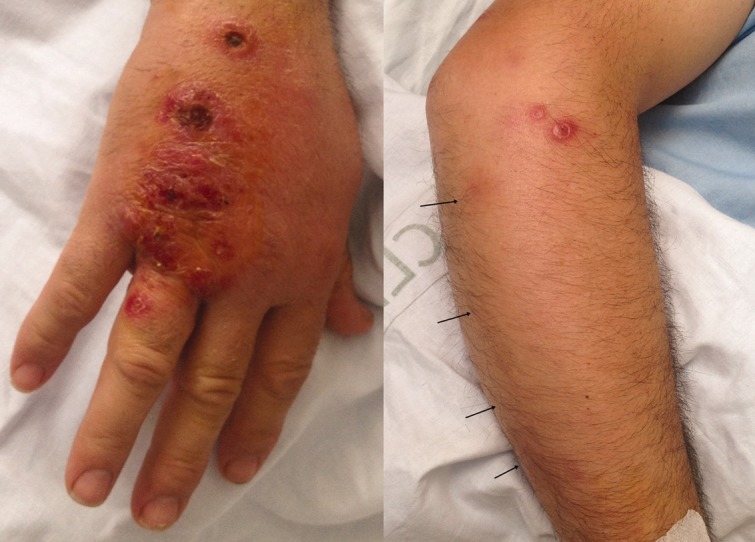
Ulcerated nodules, erythema, and swelling on the dorsal surface of the right hand mimicking a sporotrichoid aspect (left side). Linear erythematic lymphatic channel spread (arrows) and inflammatory subcutaneous nodules on the cubital side of the forearm and elbow (right side).
Fig. 2.
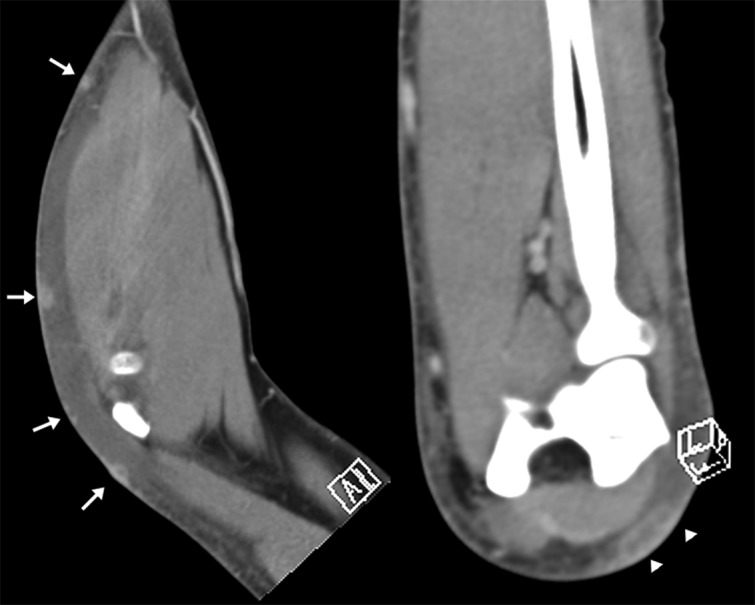
Soft-tissue algorithm 2D reformatted sagittal and coronal postcontrast MDCT images presented significant round-like contrast enhancement of the subcutaneous nodules (arrows) along the forearm and elbow. One of these nodules confirms a “tubular-like” structure (arrowheads) suggesting a lymphatic vessel.
Discussion
Lymphangitis is caused by the inflammation of lymphatic channels through tissue infection. Nodular lymphangitis is characterized by the development of inflammatory subcutaneous nodules in a linear fashion following the lymphatic drainage. These nodules spread along subcutaneous lymphatic channels from a distal primary lesion (patients generally have a history of trauma, often minor, or skin infection), which represents the inoculation site of the micro-organisms. Potential pathogens involved in nodular lymphangitis are limited. Sporothrix schenckii is the commonest, thus the classical pattern of cutaneous lesions in nodular lymphangitis is also described as “sporothricoid”. This lymphocutaneous variant of Sporothrix infection is the most common; fixed cutaneous infection without involvement of the lymphatic system or hematogenous dissemination occurring in fewer cases (1). Disseminated sporotrichosis can exceptionally mimic advanced sarcoidosis (2). Pyoderma gangrenosum and ulcerative sarcoidosis should be kept in mind when skin nodules are present without involvement of the lymphatic system (3) Cat-scratch disease, the typical clinical manifestation of Bartonella infections, generally causes swelling of the lymph nodes. Patients usually have a history of being scratched or bitten by cats. CT and MRI can depict nodules or masses of lymphadenopathy with subcutaneous edema and fat infiltration in the lymphatic drainage area. CT provides acceptable image quality with shorter acquisition time and better availability than MRI (4). Clinical signs of lymphangitis such erythematous and irregular linear streaks extend from primary infection site toward draining regional nodes, blistering of skin, and soft-tissue swelling can all be depicted by CT showing findings consistent with this, such as thickening of subcutaneous tissues, diffuse linear or ill-defined septa, lymphatic channels, and lymph nodes. Although MRI presents many advantages over CT in the evaluation of soft tissues, in this type of infection one of the initial priorities is to rule out fasciitis, where MDCT can depict contrast-enhancing subcutaneous lymphatic channels and nodes which are the hallmark of nodular lymphangitis. Moreover, CT can show the presence of air collections which are characteristic of necrotizing fasciitis or epitrochlear or axillary adenopathic masses indicative of cat-scratch disease. Ultrasound may underestimate the extent of the lesion (4). A history of cat exposure and epitrochlear or axillary adenopathic masses was not present in our case (5). Less common causal agents involved in nodular lymphangitis include some non-tuberculous Mycobacterium species, Leishmania, or Nocardia. Parasitic worms such as Filaria were discarded due to absence of suspicious clinical findings. M. marinum has rarely been reported as a cause of nodular lymphangitis. It is an atypical mycobacterium that is often found in aquatic environments. It usually causes skin infections and, less commonly, deeper infections involving the joints and tendons. It is acquired as a result of direct inoculation through a skin injury in aquatic environments such as swimming pools or fish aquariums (6–8). The incubation period is normally 8–30 days after exposure. The most typical presentation is a solitary papule or nodule, usually on the fingers or hands, which may occasionally ulcerate and have purulent drainage. It has been called “swimming pool granuloma” or “fish tank granuloma”. Another type resembles cutaneous sporotrichosis, in which the primary lesion appears at the site of inoculation as a verrucous papule or nodule, usually on the upper extremity. Secondary erythematous nodules spread along proximal subcutaneous lymphatic channels. Lesions are usually painless. Systemic symptoms and regional adenopathy are uncommon. Although less common, disseminated skin lesions may be seen in immunocompromised patients (9). A characteristic clinical presentation and a proper exposure history may suggest the diagnosis of M. marinum infection. Furthermore, ultrasound findings such as tenosynovitis with the presence of “rice bodies” have recently been reported in a patient (10); this finding can be characteristic though not specific, being so in other mycobacterial infections or rheumatoid arthritis. However, there are no pathognomonic clinical or imaging features and definitive diagnosis depends on histopathological and microbiological tests from skin biopsies. M. marinum is photochromogenic and requires 28–30℃ incubation temperature for optimal growth. PCR assay followed by sequencing of the PCR-amplified product is commonly used for identification of mycobacterial isolates. To our knowledge, this is the first report of CT imaging findings of nodular lymphangitis due to M. marinum infection. The use of CT was in this instance helpful in early recognition of this rare entity.
Acknowledgements
The authors thank Carlos Reguera and David Flores, TER of the CT, Radiology Department; and Jennifer Brickman, for her help with English proofreading.
References
- 1.De Lima Prearo CA, Daniguchi DP, Martinez MA, et al. Bilateral sporotrichosis. Mycoses 2002; 45: 415–417. [DOI] [PubMed] [Google Scholar]
- 2.Yang DJ, Krishnan RS, Guillen DR, et al. Disseminated sporotrichosis mimicking sarcoidosis. Int J Dermatol 2006; 45: 450–453. [DOI] [PubMed] [Google Scholar]
- 3.Liao WQ, Zang YL, Shao JZ. Sporotrichosis presenting as pyoderma gangrenosum. Mycopathologia 1991; 116: 165–168. [DOI] [PubMed] [Google Scholar]
- 4.Turecki MB, Taljanovic MS, Stubbs AY, et al. Imaging of musculoskeletal soft tissue infections. Skeletal Radiol 2010; 39: 957–971. [DOI] [PubMed] [Google Scholar]
- 5.Wang CW, Chang WC, Chao TK, et al. Computed tomography and magnetic resonance imaging of cat-scratch disease: a report of two cases. Clin Imaging 2009; 33: 318–321. [DOI] [PubMed] [Google Scholar]
- 6.Roddy K, Kao G, Dawn M, et al. The arthritic fisherman. Am J Med 2008; 121: 287–289. [DOI] [PubMed] [Google Scholar]
- 7.Aslam F, Ng B. ‘You never asked doc., I do fish’. Clin Rheumatol 2010; 29: 691–693. [DOI] [PubMed] [Google Scholar]
- 8.Adhikesavan LG, Harrington TM. Local and disseminated infections caused by Mycobacterium marinum: an unusual cause of subcutaneous nodules. J Clin Rheumatol 2008; 14: 156–160. [DOI] [PubMed] [Google Scholar]
- 9.Aubry A, Chosidow O, Caumes E, et al. Sixty-three cases of Mycobacterium marinum infection: clinical features, treatment, and antibiotic susceptibility of causative isolates. Arch Intern Med 2002; 162: 1746–1752. [DOI] [PubMed] [Google Scholar]
- 10.Furer V, Franks A, Magro C, et al. Musculoskeletal ultrasound prompts a rare diagnosis of Mycobacterium marinum infection. Scand J Rheumatol 2012; 41: 316–318. [DOI] [PubMed] [Google Scholar]


