Abstract
Aedes albopictus is a vector of dengue and chikungunya viruses in the field, along with around 24 additional arboviruses under laboratory conditions. As an invasive mosquito species, Ae. albopictus has been expanding in geographical range over the past 20 years, although the poleward extent of mosquito populations is limited by winter temperatures. Nonetheless, population densities depend on environmental conditions and since global climate change projections indicate increasing temperatures and altered patterns of rainfall, geographic distributions of previously tropical mosquito species may change. Although mathematical models can provide explanatory insight into observed patterns of disease prevalence in terms of epidemiological and entomological processes, understanding how environmental variables affect transmission is possible only with reliable model parameterisation, which, in turn, is obtained only through a thorough understanding of the relationship between mosquito biology and environmental variables. Thus, in order to assess the impact of climate change on mosquito population distribution and regions threatened by vector-borne disease, a detailed understanding (through a synthesis of current knowledge) of the relationship between climate, mosquito biology, and disease transmission is required, but this process has not yet been undertaken for Ae. albopictus. In this review, the impact of temperature, rainfall, and relative humidity on Ae. albopictus development and survival are considered. Existing Ae. albopictus populations across Europe are mapped with current climatic conditions, considering whether estimates of climatic cutoffs for Ae. albopictus are accurate, and suggesting that environmental thresholds must be calibrated according to the scale and resolution of climate model outputs and mosquito presence data.
Keywords: Aedes albopictus, Chikungunya, Environmental variables, Climatic thresholds, Mathematical modelling, Mosquito mappings
Introduction
The alphaviruses are a genus of 30 enveloped RNA viruses that cause disease in humans and domestic animals. They are transmitted by mosquitoes or other haematophagous insects.1 The alphavirus genome consists of +ssRNA, encoding four non-structural proteins essential for genome replication, the capsid, and two envelope glycoproteins.1 Chikungunya virus (CHIKV) is a member of the Semliki Forest (SF) antigenic complex of the alphavirus genus. Other viruses in the SF group are Ross River virus (RRV), Semliki Forest virus (SFV), and O'nyong-nyong virus (ONNV).
Chikungunya virus was first isolated in the 1950s in Tanzania, East Africa.2 The word 'chikungunya' translates as 'that which bends up', referring to the contorted posture of patients caused by severe joint pain.3 Symptoms of infection are similar to dengue fever, including fever, headache, painful joints, vomiting, and maculopapular rash.4 Sporadic outbreaks were reported during the 1960s–1980s throughout much of Asia and countries in Southern and Central Africa.5 These outbreaks have been unpredictable, with gaps of 7 to 20 years between epidemics.4 The primary vector was thought to be Aedes aegypti, while Aedes albopictus (the Asian tiger mosquito) acted as a secondary vector.6
A huge epidemic of CHIKV occurred in the Indian Ocean region from 2005 to 2006, with over three million cases reported in India and the surrounding islands.7 In 2006, around 38% of the population of the French island La Reunion was infected.5 Unusually, during this epidemic, few asymptomatic infections and a relatively high mortality rate were observed, most likely due to the lack of prior population immunity.8 Fears have grown for outbreaks of CHIKV and other arboviral diseases in North America and Europe following identification of the Asian tiger mosquito as the major vector.5 The Asian tiger mosquito has been spreading rapidly in geographical range. First identified in America in 1983, it invaded 36 states and several South American countries within 25 years, and is currently spreading across parts of Africa and Europe.9 A year after the CHIKV epidemic in Asia, around 200 cases were reported in two small Italian towns on the Adriatic coast, demonstrating the first instance of locally transmitted CHIKV by Ae. albopictus in Europe.10,11 This mosquito species entered Italy in 1990, probably through the importation of used tyres and has subsequently become endemic and widespread.12 An important feature of the 2005–2006 epidemic was a single amino acid substitution in the envelope protein of CHIKV that allows efficient transmission by both Ae. aegypti and Ae. albopictus.8 It was this variant that was transmitted in Italy in 2007 and it is believed that this strain could cause further outbreaks in Europe and the Americas where Ae. albopictus is becoming widespread.8
In addition to being an efficient vector of CHIKV, Ae. albopictus transmits DENV in the field, and a further 24 arboviruses in the laboratory13 and thus has the potential to transmit a variety of viruses in the field, although its precise role in disease transmission remains uncertain for these diseases. Aedes albopictus exhibits enormous ecological and physiological plasticity13 and combined with the potential effects of climate change, it is likely that its future geographical range will alter. Rising global temperatures and changing weather patterns suggested by climate model projections may further promote the spread of Ae. albopictus into temperate regions, although cold winters and rainfall intensity and frequency are likely to be limiting factors.
The global distribution of Ae. albopictus
Geographical spread of Ae. albopictus has mostly occurred within the last three decades.13 Aedes albopictus originated in tropical and temperate Asia13 where it is likely to have been a tree-dwelling, zoophilic mosquito. Over time, it has adapted to increasing anthropogenic influences on its environment (such as the introduction of cattle, change of human demographics, or the establishment of new man-made breeding sites). This process of 'domestication' has been widely described for Ae. aegypti.13 Nevertheless, in its native habitat, Ae. albopictus is not truly domesticated and in densely-populated areas with little or no vegetation it is rare or absent (although cities with relatively large amounts of vegetation can sustain Ae. albopictus).14 In sub-urban and rural areas where humans are present, Ae. albopictus breeds in a wide variety of man-made containers and this contributes to its rapid adaptation to new environments. In addition to human activities and migration patterns, major routes of expansion have also been through used tyre and lucky bamboo importation from Asia.
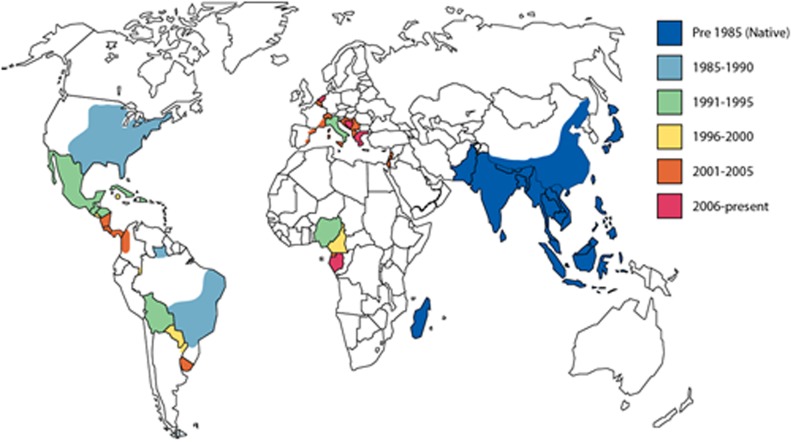
The two main factors contributing to the success of Ae. albopictus spread in the last 30 years are thought to be its physiological and ecological plasticity.13 Physiological plasticity is observed in both ancestral-type strains in Asia and recently expanded strains across the world. This plasticity is mostly conferred by the ability to survive in both tropical and temperate conditions by laying overwintering (diapausing) cold and desiccation resistant eggs.13 Ecological plasticity refers to the vast array of breeding habitats that Ae. albopictus can utilise, ranging from tree-holes and cut bamboo to a wide variety of man-made containers. In addition, despite a preference for humans and mammals, it can be zoophilic, feeding from a wide range of hosts if required and making Ae. albopictus a potentially important vector for zoonotic viruses. These factors make Ae. albopictus a highly invasive species. Figure 1 shows the expansion of Ae. albopictus from its ancestral Asian habitat to North America, South America, Africa, and Europe over the past 30 years.
Figure 1.
Native habitat of Ae. albopictus is depicted in dark blue and the subsequent invasions into North America, South America, Europe, and Africa in light blue (1985–1990), green (1991–1995), yellow (1996–2000), orange (2001–2005), and pink (2006–present).95, 111–117
Recent invasion and current global distribution of Ae. albopictus.
The global distribution of CHIKV
The historical transmission, movements, and biology of CHIKV have been reviewed recently.3,15,16 In brief, possible historic outbreaks of CHIKV occurred in Indonesia, Africa, the Caribbean, the West Indies, and India in the 16th–19th Centuries. The first confirmed outbreak was recorded in 1952 in East Africa, followed by outbreaks in Asia in 1958 and India in 1963. There are presently three genotypes of CHIKV; the East/Central African, West African, and Asian genotypes. Between the 1960s and 2000, sporadic cases were reported across Africa and localised outbreaks occurred in South Asia.15
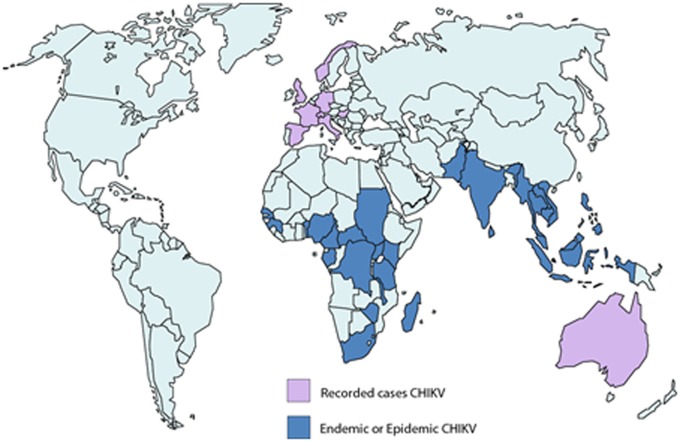
The recent outbreak of CHIKV started in Africa in 2004 and was caused by the East/Central African genotype. The disease spread throughout the Indian Ocean and Indian sub-continent in 2005–2007, infecting millions of people and facilitated by a mutation that allowed CHIKV to be transmitted by both Ae. aegypti and Ae. albopictus. Interestingly, this mutation allowing efficient transmission by Ae. albopictus appears to have evolved independently in La Reunion, India, and Cameroon/Gabon, an example of convergent selection at different locations rarely observed in nature.17 An additional mutation has subsequently been identified in India that further enhances transmission by Ae. albopictus.18 The current distribution of CHIKV is depicted in Fig. 2.
Figure 2.
The distribution of endemic or epidemic cases of CHIKV (in blue) and imported recorded cases of CHIKV (in pink) are shown as of 2010 (adapted from Ref. 3).
Distribution of CHIKV in 2010.
The mutations that have led to efficient transmission in Ae. albopictus have significantly expanded the geographic regions susceptible to CHIKV transmission, in particular to Europe and the Americas where populations are not immune to CHIKV and could potentially sustain explosive outbreaks of the disease. Recent local outbreaks of CHIKV and DENV in Europe (reviewed in Ref. 19) include more than 200 cases of CHIKV in Italy in 2007 and two local cases of CHIKV in Southern France in 2010. The first local cases of DENV since 1928 were reported in Nice in 2010, where two cases of autochthonous Dengue occurred, and later in 2010 in Croatia where local transmission of DENV was reported. In 2012, an outbreak of DENV occurred in Madeira, with more than 2000 cases reported,19 demonstrating the vulnerability of European populations to previously ‘tropical’ arboviral disease. Thus, methods for establishing the risk of disease transmission and early warning systems could help to prevent an epidemic similar in scale to that in 2006–2007 in the Indian sub-continent.
Modelling Climatic and Environmental Influences On CHIKV Transmission and Ae. albopictus Population Dynamics
Modelling studies seeking to better understand (and quantify) the link between environmental variables, human CHIKV infection, and vector/parasite biology may take one of two forms. Statistical models develop empirical relationships between historical environmental data and, for example, CHIKV incidence (or prevalence), enabling predictions of future disease scenarios to be made in the given climate forecasts and the assumption that these relationships remain stationary over time. Statistical models do not explicitly represent CHIKV natural history or the ecological or biological processes underpinning vector population dynamics. Nonetheless, several such models have been developed for CHIKV – Geographic Information System (GIS) models based on temperature, annual rainfall, and other factors have been used to predict the distribution of Ae. albopictus and the resultant risk of CHIKV outbreaks; examples include assessment of whether Ae. albopictus could settle in the UK20 and the Netherlands21 (due to unintentional vector imports). Geographic Information System has been similarly used to establish the speed and route of CHIKV and DENV spread in Thailand using rainfall and temperature.22 Generalised logistic models have also been developed to investigate the distribution and characteristics of suitable breeding sites for vector development and two such studies have focussed on Ae. albopictus in La Réunion23 and Ae. aegypti, Ae. albopictus, and Aedes lilii in Mayotte,24 while the distribution of Ae. albopictus in Northern and North Eastern Italy using Land Surface Temperature (LST) maps has also been recently considered.25,26
In contrast to statistical models, mathematical models adopt a process-based approach by incorporating relevant ecological, epidemiological, entomological, and environmental processes (consistent with a set of assumptions) into a mechanistic mathematical framework. By incorporating the known effects of environmental variables on CHIKV infection and Ae. albopictus populations, such models provide a valuable quantitative framework for a better understanding of observed patterns of human CHIKV prevalence in terms of underlying processes, thus offering explanatory power that statistical models cannot provide. Despite this, CHIKV modelling has received relatively little attention to date; although the first known model was a computer simulation developed in 1970,27 existing knowledge on (and future directions for) CHIKV mathematical modelling was not synthesised until 2008,28 with the need for more modelling studies, better entomological knowledge of the disease vector and wider assessment (using models) of the impact of interventions representing key recommendations. Since then modelling has markedly increased, with several studies developing methods and models to use outbreak data to estimate the CHIKV basic reproduction number R0 (the number of secondary cases generated per infectious individual in a wholly susceptible population),28,29 the effect of seasonal fluctuations in Ae. albopictus populations,30,31 and the sensitivity of CHIKV vector populations to climatic conditions32 (with similar studies elsewhere on the response of Aedes africanus and Aedes furcifer populations, vectors of yellow fever, to climatic and environmental conditions33). Other studies have considered the role of temperature and rainfall on CHIKV transmission,34–36 as well as the risk of CHIKV infection in DENV-endemic regions.37 Using mathematical models to assess the impact of interventions against CHIKV has also attracted increasing attention, with the need to explicitly account for the role of climatic variables highlighted38 and important policy-decisions, such as whether the early combination of chemical imagicide spraying and active reduction of the number of breeding sites may have controlled the 2006 La Réunion epidemic,39 considered. These studies have been further extended to consider the use of the sterile insect technique (SIT) to reduce transmission,40 demonstrating that pulsed SIT with small and frequent releases may be used as an alternative to chemical control, and this is also consistent with other modelling findings elsewhere for Ae. aegypti.41
However, in order to more rigorously understand and assess the impact of climatic variables (and climate change) on CHIKV transmission dynamics, developing realistic process-based models of how Ae. albopictus population dynamics (and abundance) in different CHIKV-affected regions depend on local environmental factors is essential; indeed, this prerequisite has already been recognised elsewhere in the context of malaria eradication.42 In turn, however, key to developing more reliable Ae. albopictus population models is the need for reliable parameterisation that can only be obtained through a thorough understanding of the relationship between mosquito biology (as characterised by key life history parameters) and environmental variables; these relationships may then be incorporated into mathematical models of the vector population dynamics and this process has been recently illustrated for An. gambiae s.s. mosquitoes that transmit malaria,43 while reviewing current knowledge on these dependencies also aids identification of where future experimental (and field) work is needed to further improve model parameterisation. To the best of our knowledge, this process has not yet been rigorously undertaken for Ae. albopictus, despite its importance in understanding CHIKV (and DENV) transmission; to this end, this paper therefore aims to undertake a literature review to synthesise current knowledge on the role of climatic and environmental variables on Ae. albopictus life history parameters in order to provide the parameterisation basis of analogous modelling studies to that undertaken for An. gambiae s.s.43 and Ae. albopictus (and, subsequently, CHIKV and DENV transmission dynamics) and these will follow in the forthcoming articles. In the approach of Ref. 43, a parsimonious An. gambiae s.s. model was developed that required knowledge of how different climatic and environmental variables (including density-dependence) affect (a) the daily survival probability of the three immature (aquatic) stages (eggs, larvae, and pupae) and adult mosquitoes, (b) the development rate of vectors between life cycle states, and (c) adult fecundity (influenced, in turn, by the dependence of the gonotrophic cycle on temperature), together with threshold values of climatic variables above or below which processes reach limiting values, as well as knowledge of preferred breeding habitats. This article undertakes an analogous literature review for Ae. albopictus that aims to provide the basis for future Ae. albopictus population models and vector-borne diseases mediated by this mosquito.
Aedes Albopictus Biology and its Response to Environmental Variables
Mosquitoes, like many invertebrates, are directly affected by changes in weather to a greater extent than warm-blooded animals and external temperatures are therefore required to be above critical thresholds for adult activity or immature stage development. Since immature vector stages are entirely aquatic, many species also rely on regular rainfall to provide suitable breeding sites, while relative humidity (RH) (affecting water loss and adult survival) and, to a lesser extent, wind patterns (affecting mosquito dispersal and hydrological processes) are also likely to affect vector population dynamics. In this review, current European Ae. albopictus populations are overlayed with maps of meteorological conditions. The corresponding climate data used to produce the relevant maps have been generated by the EMAC general global circulation model, a global numerical chemistry and climate simulation system comprising of sub-models that describe tropospheric, middle atmosphere processes and their interactions with land, oceans, and human influences.44 The EMAC system consists of two major components: (a) the base/core atmospheric model (ECHAM5)45 and (b) the interface, Modular Earth Sub-Model System (MESSy), which includes sub-models, mainly concerned with atmospheric chemistry processes, coupled to the base system. For these particular maps, we have utilised EMAC version 1.9 based on ECHAM version 5.3 and MESSy version 1.9. The simulations are performed on the T85L19 resolution, namely with a spherical spectral truncation of T85, corresponding to a quadratic Gaussian grid of approximately 1.4 by 1.4 degrees in longitude and latitude, with 19 vertical hybrid pressure levels up to 10 hPa. The AMIP II simulations46 for sea-surface temperature and sea-ice distribution between the years 2000–2009 are imposed as boundary conditions to the model.
Aedes albopictus presence is defined at the NUTS2 level (www.vbornet.org) as of December 2011, giving relatively low resolution data, but allowing for large regions to be studied. The resolution of this data must be taken into consideration when drawing conclusions about environmental thresholds, and as will be discussed, it must be stressed that these results are appropriate only when using relatively coarse-scale modelling and are unlikely to reflect the true conditions within microclimates on the ground. As modelling of microclimates across large regions is not reasonably possible at present, appropriate environmental conditions at the scale or resolution of models used to generate maps are still needed.
Temperature
Several studies have reported temperature thresholds for Ae. albopictus survival and activity, and these include winter minimum and summer maximum temperatures. In this review, we will consider the impact of temperature on each stage of the mosquito life cycle from egg to adult.
Eggs and overwintering
Of key importance when considering suitable habitats for invasive mosquito species such as Ae. albopictus are winter minimum temperatures, as these define whether populations in the form of diapausing eggs can overwinter. Winter minimum temperatures in the northern hemisphere are often characterised by the average January temperature (JanTm). A range of JanTm values have been reported as cutoffs for the overwintering of Ae. albopictus: >−3°C for populations in China and South Korea;47 >−5°C for North Western China (with seasonal expansions reaching areas with JanTm around −10°C);48 0°C to −2°C in Japan;49 and >0°C to −5°C for temperate populations in North America.47 A recent study using a statistical model and Ae. albopictus presence data in North America predicted almost zero probability of Ae. albopictus presence at −2°C, and the highest probability of presence at 0–1°C.50
Studies of egg mortality in response to cold temperatures demonstrate remarkable cold-resistance. Experiments have shown that 78–99% of eggs from US and Asian temperate strains may survive −10°C for 24 hours.14 A comparison of European temperate strains of Ae. albopictus with tropical strains of Ae. albopictus and Ae. aegypti demonstrated that minimum survival temperatures for temperate diapausing eggs were −10°C for long-term exposure (12–24 hours) and −12°C for short-term exposure (1 h), while tropical Ae. albopictus and Ae. aegypti both survived long-term temperatures of −2°C and short-term temperatures of −7°C.51 This study confirms that temperate, diapausing Ae. albopictus eggs are cold-adapted and able to survive winter nights down to −10°C (although only around 10% of eggs hatched after exposure to this temperature). The major limitation of this study is the short timescale of exposure, as eggs would presumably be repeatedly exposed to such low temperatures throughout the winter. In order to really answer the question of whether Ae. albopictus eggs can overwinter in these conditions, field studies would need to be carried out to observe survival in field conditions over a whole winter.
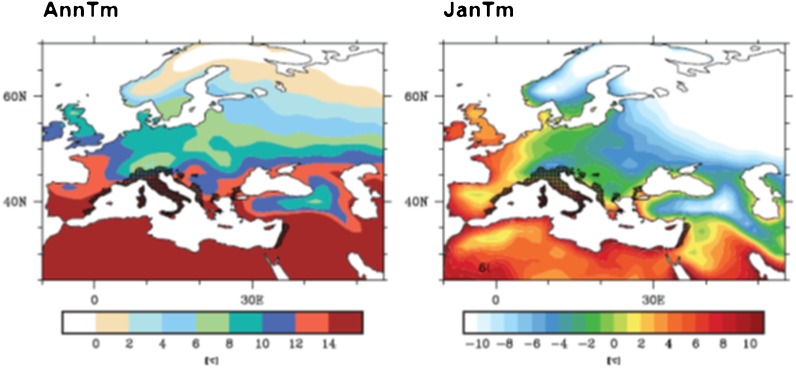
A JanTm of >0°C has been generally accepted as delineating overwintering populations for modelling climatic suitability for Ae. albopictus.49,52,53 Whilst this may be biologically true at the ground level, using climate models at the regional or global scale, with resolutions of hundreds of kilometres, a threshold of 0°C can exclude large regions where Ae. albopictus are present. For example, overlaying the current distribution of Ae. albopictus (as of December 2011) in Europe with JanTm from the EMAC GMC with 1.4 degree (approx 150 km) resolution suggests that a cut-off of −4°C may be more accurate (Fig. 3A). There are two factors that can explain this disparity. Firstly, any given 150 km square region will contain a large number of microclimate zones. This is especially true, for example, at the northern limits of Ae. albopictus in Italy, in the Alpes, where valleys and lakes can provide dramatically different microclimates to close by mountain peaks. In this grid square, JanTms might range from −10°C to 0°C, and Ae. albopictus might likely only overwinter where warmer temperatures prevail. This grid square, however, might be modelled as having a JanTm of −4°C and being present for Ae. albopictus. Although it is incorrect to say Ae. albopictus should not be present in this grid square because (according to the model) JanTm<0°C, it may also be incorrect to say that Ae. albopictus overwinter at JanTms of −4°C. The reality is likely to be somewhere in-between. A second factor that explains this disparity could be re-invasion of neighbouring regions on a yearly basis. In this instance, populations do not actually overwinter in the form of eggs, but are recorded during the summer months as present for Ae. albopictus when nearby populations from warmer regions spread during the spring/summer months. In reality, a combination of microclimate zones and annual re-invasions will exist in the field. What is important from the perspective of using models to predict Ae. albopictus populations is the scale or resolution of both the climate data and the recorded Ae. albopictus data, as these will influence the appropriate thresholds of environmental conditions. Using a GCM at the 150 km resolution, we suggest that a JanTm of −4°C currently reflects Ae. albopictus distribution at the NUTS2 level. This illustrates the necessity to calibrate any biological thresholds used in conjunction with climate models with Ae. albopictus presence data, as GCMs and RCMs cannot yet accurately model microclimate (i.e. true biological) conditions.
Figure 3.
The horizontal bar represents degrees Celsius. Aedes albopictus data were taken from the ECDC Vbornet (www.vbornet.org). Dark hatched areas show regions where Ae. albopictus have been recorded as present as of December 2011.
Current Aedes albopictus distribution and average annual temperature (AnnTm) and January temperature (JanTm) across Europe for the reference period 2000–2009.
In Ref. 51, the air surface temperature in Europe is plotted on one of the coldest winter nights in the unusually cold winter of 2011. Regions reaching below −10°C overlap very closely with regions with JanTm<−4°C from the EMAC GCM. These maps indicate that the majority of Europe and large parts of North America would support overwintering populations of Ae. albopictus.
Temperature and the immature stages
Although overwintering through diapausing eggs may occur at JanTm<0°C, the larval and pupal stages cannot develop, and adults will not survive, at these temperatures. Figure 4 illustrates the survival rate of Ae. albopictus eggs (Fig. 4A), larvae (Fig. 4B), pupae (Fig. 4C), and adults (Fig. 4D) with temperature from several studies. In general, mortality rates of immature stages greatly increase at temperature extremes. Survival of larvae and pupae at different temperatures is fairly consistent across studies, with optimal survival at 25–30°C and high mortality at <15°C and >36°C. Temperatures above 40°C are generally accepted as the limit for immature survival and laboratory studies have shown that eggs fail to hatch above this.23,54 Large variability in egg survival is observed between studies, possibly reflecting differences in physiology between diapausing and non-diapausing eggs. Fitting quadratic curves to egg (Fig. 4E), larval (Fig. 4F), and pupal (Fig. 4G) survival allows us to estimate upper and lower mortality thresholds. For larval stages, survival is estimated to cease at 10 and 40°C, while for pupae, survival is estimated to cease at 10 and 37°C. Curve fitting for egg survival is complicated by the fact that at low temperatures, diapausing eggs are laid. Data from the two studies presented here also differ significantly, making clear conclusions hard to infer. The data presented by Delatte et al.,55 however, support 40°C as an upper threshold for egg survival.
Figure 4.
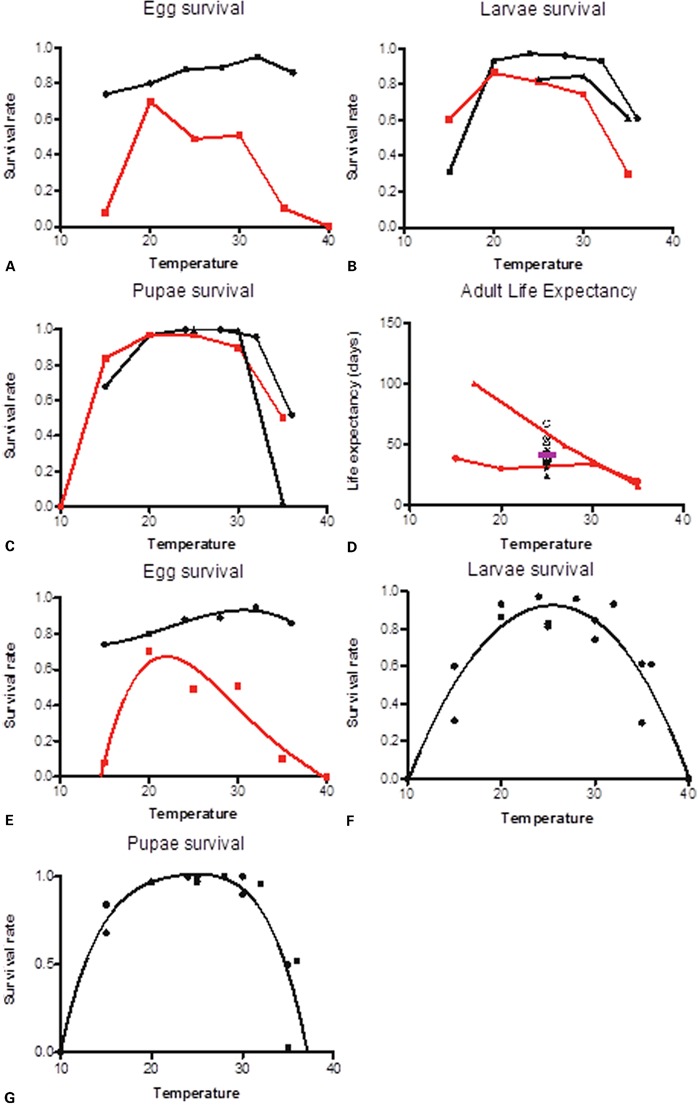
Three independent laboratory studies investigated the effect of temperature on survival of immature Ae. albopictus. Temperature effects on (a) egg survival, (b) larval survival, and (c) pupal survival were established for laboratory colonies in Refs. 55 (squares), 54 (circles), and 65 (triangles). (d) Laboratory adult life expectancy for females was additionally estimated in Refs. 55 and 65 over a range of temperatures. Median female survival in days from multiple studies (conducted at 25°C) have been added to the graph (black symbols with the mean plotted as pink bar). Red lines indicate temperate strains and black lines indicate tropical strains. Second or third-order polynomials were fitted to (e) egg survival, (f) larval survival, and (g) pupal survival.
The effect of temperature on Aedes albopictus survival.
In addition to survival, temperature also significantly affects mosquito development rates, with the general trend of increased temperatures causing decreased development times. Several studies have gathered data from field and laboratory studies investigating this relationship in Ae. albopictus and these are shown in Fig. 5 (data from Refs. 14, 54–65). Large differences in embryonic development time (here, the time between laying and hatching) at lower temperatures are observed between tropical and temperate strains (Fig. 5A). Very long embryonation times are observed at lower temperatures for tropical strains, whereas those with shorter embryonation times at lower temperatures are a mixture of temperate and tropical strains. This is likely due to cold-adaptation of temperate strains, where eggs can withstand low temperatures and desiccation. Tropical strain embryonation ranges from 42 to 2 days between 15 and 36°C. Temperate strain embryonation ranges from 7.5 to 2 days between 15 and 36°C.
Figure 5.
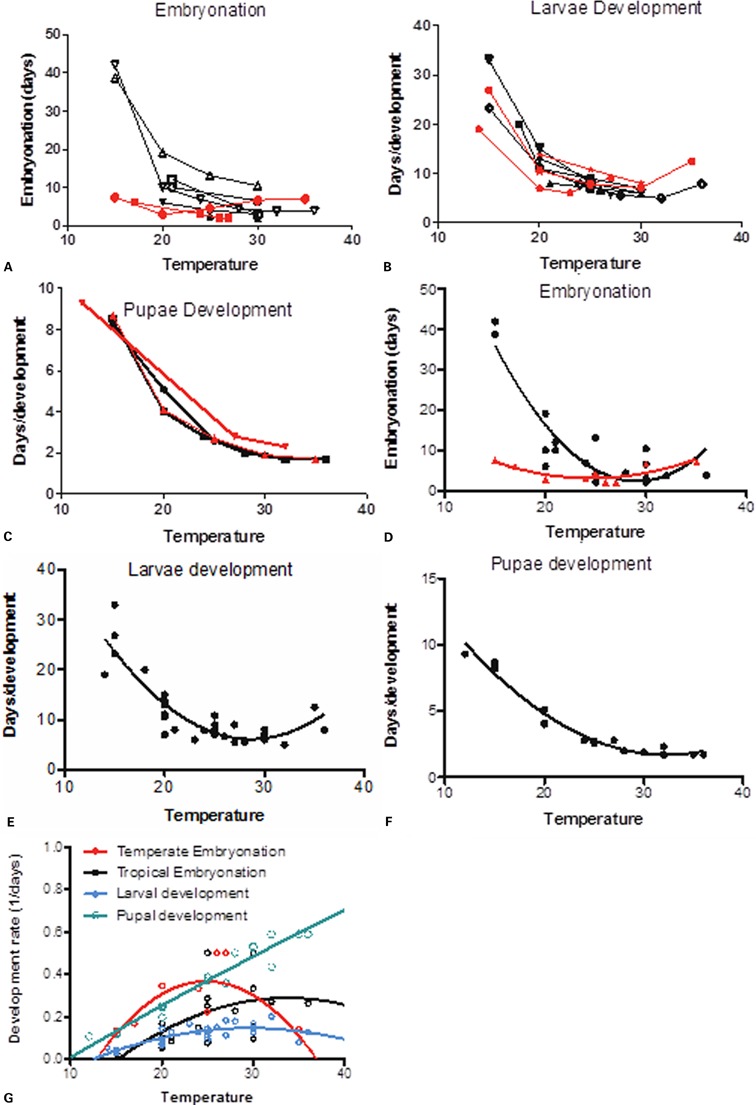
The development in days of (a) eggs, (b) larvae, and (c) pupae in response to temperature from multiple studies in the literature. Best-fit curves (second-order or third-order polynomials) were plotted for (d) embryonation, (e) larvae development, and (f) pupae development for calculating average development time of immature stages in response to temperature (data from Ref. 14,54–65). Red lines indicate temperate strains of Ae. albopictus and black lines indicate tropical strains of Ae. albopictus. The rate of development (g) was calculated for temperate strain eggs (red), tropical strain eggs (black), larvae (blue), and pupae (green). Data were fitted to polynomial curves for estimating threshold temperatures for development.
The effect of temperature on Aedes albopictus immature development.
Larval growth is also dependent on temperature (Fig. 5B), with a commonly accepted lower threshold (below which development ceases) of around 11°C.14,52 Temperatures of 35°C and above have been shown to adversely affect larval development.54,55 No difference in tropical and temperate strains have been reported. Larval development ranges from 27 to 5.5 days between 15 and 36°C. Pupae are a distinct and remarkable stage of mosquito development. They are relatively short-lived, but have a distinct morphology, their own mechanism of movement and undergo the transition from aquatic swimming immatures to flying adults.66 The duration of this transition also depends on temperature (Fig. 5C), with pupal development times ranging from 8.5 to 1.7 days between 15 and 36°C. No difference between tropical and temperate strains has been observed.
Best-fit curves are fitted to embryonation (Fig. 5D), larval (Fig. 5E), and pupal (Fig. 5F) development data and the resulting curves can be used to estimate temperature-dependent development times for each immature life stage. The sensitivity of immature development times to temperature is illustrated by an approximate doubling in time from egg to hatching between 15 and 20°C (from 5.4 to 3.1 weeks). Quadratic curves fitted to development rates as a function of temperature (Fig. 5G) provide an estimate of developmental thresholds from the collated data. Lower thresholds are estimated as 15°C (embryonation in tropical strain eggs), 12.8°C (embryonation in temperate strain eggs), 12.5°C (larval development), 9.6°C (pupal development), and these values compare well with values given in other studies.26,54,55 For upper thresholds, the only stage where development rates are estimated to fall to zero below 40°C are temperate strain eggs, with an estimated upper threshold of 37°C. Values above 40°C can be considered irrelevant as all immature and adult stages die above 40°C.
Temperature and adults
Adult mortality has been reported as approximately age-independent in laboratory survival assays.67 Females are generally reported to survive longer than males,14,55,61,68 although similar life expectancies have also been observed and it has been suggested that longevity for males and females is more similar below 20°C. Figure 4D shows the relationship between adult survival and temperature from two independent studies. Both studies used temperate strains of Ae. albopictus from La Reunion55 and Louisiana.65 Despite a large difference in the life expectancy at lower temperatures (with the North American strain demonstrating much longer life expectancy than the La Reunion strain), both data sets highlight the same trend of longevity increasing at lower temperatures. Although a further study reported no effect of temperature on adult survival between 22 and 26°C,69 this is consistent with the La Reunion strain,55 where a very small difference in life expectancy is observed between 20 and 25°C. Figure 4D also shows laboratory female Ae. albopictus median survival at 25°C from a number of other studies. These data demonstrate the variability in life expectancy from strain to strain, ranging from 24 to 67 days. No significant difference in median survival is observed between temperate and tropical strains (data not shown).
The increase in longevity at lower temperatures may be explained by the rate at which mosquitoes acquire, store, and use energy supplies. The cold-blooded nature of mosquitoes has a direct effect on their metabolism, which, in turn, affects their ability to acquire and store energy for hatching, metamorphosis, flying, and oviposition. Studies investigating the effect of temperature on protein, lipid, and glycogen content of Ae. albopictus show that at lower temperatures, the larval stages have a longer phagoperiod since development is slower, during which the immature stages are able to consume and store increased nutrients compared to faster-growing counterparts.65 At lower temperatures (17°C), mosquitoes may have larger body sizes, consume more blood, have higher levels of protein, lipids, and glycogen in their ovaries, and develop more eggs with higher lipid levels than counterparts at higher temperatures (27–32°C).65 This contributes to the longer life expectancy of adults at lower temperatures (up to a threshold) and increased desiccation resistance.70 Aedes albopictus thus appears to adapt to cold conditions through increasing lipid synthesis, resulting in increased desiccation tolerance,70 female longevity, and egg production.65 Increased longevity at lower temperatures is important when considering the seasonality of adult activity (and thus potential disease transmission season), as longer-lived adults have a greater chance of transmitting disease.
The threshold for the seasonal emergence of host-seeking temperate females in the field (in Italy) has been estimated as 13°C, with the lower threshold for adult activity ceasing similarly estimated as 9°C.71 Summer maximum temperature thresholds for Ae. albopictus are less well-documented, although temperatures above 40°C are generally accepted as the limit for adult survival and laboratory studies have shown that eggs fail to hatch at this temperature.23,54 Although, in the field, it is possible that mosquitoes seek shade or cooler conditions, whether mosquitoes display temperature-regulated behaviour, actively seeking out cooler or warmer microhabitats, is controversial.
While average annual temperatures (AnnTm) of 11°C have been cited as a requirement for breeding Ae. albopictus populations, overlaying maps of existing populations in Europe with the EMAC GCM indicate that AnnTm>8°C may better reflect the current distribution, again illustrating the need to consider the resolution of the climate models used to generate maps in the assessment of environmental thresholds (Fig. 3).
Egg laying and biting behaviour
The number of eggs per gonotrophic cycle has been found to be independent of temperature55 and follows no consistent trend between studies that have investigated eggs/gonotrophic cycle over a range of temperatures.57,65 The number of eggs oviposited per gonotrophic cycle commonly ranges from 30 to 8055,72–77 and declines with age.14,72,77 While large numbers of eggs are laid in the laboratory, these are typically from larger females derived from well-fed immature stages. In the field, the number of eggs laid is likely to be closer to the lower end of this range due to more adverse conditions compared to the laboratory.14
The trigger for Ae. albopictus laying diapausing eggs is partly photoperiodic, with exposure of pupae and adults to short-day lengths causing females to oviposit.78 Critical photoperiods (CPPs) for onset of diapause have been given as (light:dark hours) 14:10 (Italy),79 13:11 (China, Japan and North America),80 and 12:12 (Japan).49 Photoperiodic diapause is common in insects and allows species to ‘anticipate' the onset of unfavourable conditions. Hatching stimuli for diapausing eggs include age, desiccation, temperature changes, the oxygen tension of water, flooding, and photoperiod.14
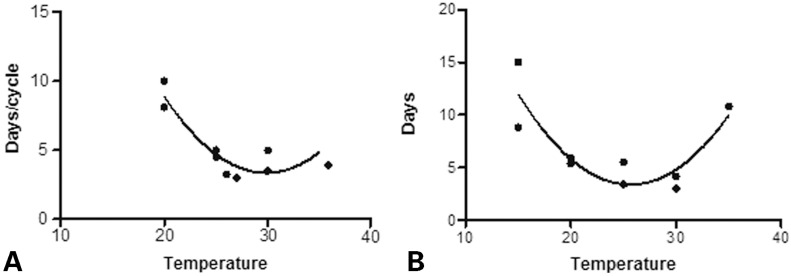
Experimental data suggest that both the duration of a single gonotrophic cycle (the time between taking blood meals) and the time between adult emergence and taking a first blood meal are dependent on temperature. Reports on gonotrophic cycle duration for Ae. albopictus range from 3.5 to 10 days depending on the temperature (Fig. 6A),55,60,62,64,81 while the time between adult emergence and taking the first blood meal is also important and depends on the temperature (Fig. 6B), typically around 4.2–15 days.55 Other studies of the latter have given lower values around 2–3 days at 25°C.60 Aedes albopictus have a strong preferences for human feeding in host-choice experiments, although they are able to take blood meals from a wide variety of hosts and may even have blood meals from multiple hosts within short durations.82 Field experiments studying host-seeking and resting behaviour have found that the majority of females are exophilic and engage in exophagy.82 Biting occurs with a bimodal activity peak, typically around 2 hours before sunset (largest peak of activity) and 8.30am (smaller peak), while night activity is minimal or absent.14,82,83 Although several biting rates have been reported for Ae. aegypti,84 little data are available for Ae. albopictus, although the biting rate of Ae. albopictus in Southern China has been estimated as 0.31 blood meals per day under laboratory conditions.83 Although temperature effects on feeding behaviour have not been published to date for Ae. albopictus (to the best of our knowledge), the biting rate is likely to correlate with temperature, as a minimum of one blood meal is taken per gonotrophic cycle and gonotrophic cycles are shorter at higher temperatures.
Figure 6.
Data from Refs. 55, 60, 62, 64, 81, and 118. A secondorder polynomial (quadratic) curve was fitted to each data set.
The effect of temperature on Aedes albopictus (a) gonotrophic cycle duration and (b) time between hatching and taking a first blood meal.
Precipitation
Precipitation is probably the most complex environmental variable affecting Ae. albopictus populations, and one of the most difficult to model for several reasons. The first is due to the ecology of the species as container breeders; whilst the ancestral tree-hole dwelling Ae. albopictus would have been dependent on rainfall to provide breeding water sources, modern, more urbanised, Ae. albopictus often breed in water sources that are independent of rainfall, for example through watering of plants in urban gardens, or the provision of water in vases in cemeteries. A second issue is the reliability of projected rainfall through climate modelling. Future precipitation patterns are generally recognised as containing greater uncertainty in the output of global/regional climate models (compared to temperature) as a result of complex atmospheric chemistry and poor measurement records in many regions of the world.
Studies investigating the link between rainfall and Ae. albopictus have looked at population density responses to rainfall in the field, and have investigated the effects of rainfall flushing on immature stages. Changes in population density in response to rainfall are variable, reflecting the presence of rainfall-dependent and rainfall-independent breeding sites. Precipitation is often correlated with increases in egg, larval, and/or adult density in the field, with peaks in weekly mean rainfall correlating with subsequent peaks in adult Ae. albopictus abundance.85 Three studies in India found a strong correlation between rainfall and seasonal increases in larval density86–88 and a study of egg populations in Malaysia also reported a strong correlation between rainfall and the number of collected eggs.89 Conversely, no correlation between seasonal variation in rainfall and Ae. albopictus collections was found in the Dominican Republic,90 and larval densities in permanent breeding sites in India were found to be independent of rainfall.86
These studies suggest that the response of Ae. albopictus populations to rainfall is likely due to the creation of new breeding sites and that breeding in permanent water sources is independent of rainfall. Populations can therefore be considered to be comprised of two components: those breeding in ‘permanent’ sites unaffected by rainfall (for example in urban garden environments) and populations breeding in sites created by, and dependent on, rainfall.
For rainfall-dependent populations, it is the larval and pupal stages that are vulnerable to desiccation. The volume of water in breeding containers does not appear to affect the development time of the immature stages,91 however as larval and pupal stages have little to no desiccation resistance,92 when rainfall is insufficient to maintain water levels, immature survival (and thus population sizes) will decrease. Eggs, however, can survive once embryonation is complete for extended periods of time after desiccation; up to 243 days has been observed in the laboratory.14 Studies indicate that desiccation does not significantly decrease egg survival.92 Thus, egg survival will be independent of rainfall regardless of water source.
One negative factor associated with rainfall is the flushing of immature stages from aquatic environments under heavy precipitation.93 It is difficult to extrapolate losses from a single simulated rainfall event in an experiment to monthly or annual rainfall levels, since the number of rain falls per shower and frequency of such events are generally unclear from data on the latter. Nonetheless, simulated light rainfall conditions corresponding to fairly typical rainfall conditions in temperate countries demonstrate the loss of 2–10% of Ae. albopictus immature stages depending on container size.93 Flushing of larvae during rainfall has also been observed for An. gambiae mosquitoes.94 For rainfall-independent breeding sites, low precipitation may be advantageous through reduced flushing of immature stages, further adding to the complexity of rainfall effects on population density.
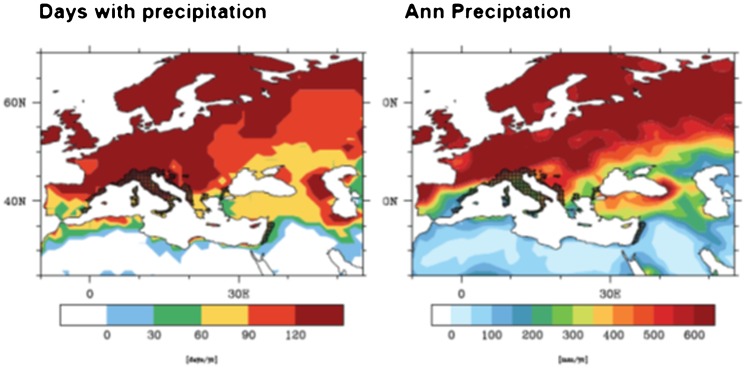
Despite the uncertainty in how strongly precipitation drives Ae. albopictus populations, a minimum requirement of 500 mm of annual rainfall has been reported previously,52 although the presence of Ae. albopictus has been confirmed in regions of Spain where annual rainfall is 292 mm.95 If lower thresholds are to be used for predicting Ae. albopictus presence using global or regional meteorological models, then plotting the current European distribution of Ae. albopictus against annual rainfall may give a better indication of precipitation levels relevant to Ae. albopictus ecology within the context of a climate model. Figure 7, overlaying European distribution of Ae. albopictus with precipitation, suggests that when using the EMAC GCM with a resolution of 1.4 degrees (approx. 150 km), a threshold of 200–250 mm per annum is more accurate. Thus, within the limits of modelling at coarse resolutions (as already discussed), thresholds should be lowered or adjusted to reflect the resolution of the climate model used. In addition, the complexity of breeding site origins should be taken into consideration for modelling Ae. albopictus populations. One possible way to reflect the two distinct breeding populations (rainfall-dependent and rainfall-independent) would be to use a measure of land use or urbanisation to predict the likelihood of rainfall-independent water sources existing for breeding. This could be used to define areas where low rainfall would have a significant or insignificant impact on Ae. albopictus population density.
Figure 7.
The horizontal bar represents (a) days and (b) annual precipitation in millimetres. Aedes albopictus data were taken from the ECDC Vbornet (www.vbornet.org). Dark hatched areas show regions where Ae. albopictus have been recorded as present as of December 2011.
Aedes albopictus distribution and annual rainfall across Europe for the reference period 2000–2009.
Relative humidity
While rainfall has impacts mostly on the aquatic immature stages, RH affects mostly the egg and adult stages of Ae. albopictus. The Asian tiger mosquito has been reported to survive well at a range of RH values,14 although little data studying the relationship between RH and Ae. albopictus survival can be found in the literature. A complicating factor of studies on RH effects on survival is that temperature and RH are not independent.
Aedes albopictus egg hatch rates have been shown to be higher at higher RH.96 Additionally, RH has been shown to affect egg survival in desiccation experiments. A linear relationship between RH and survival at 26°C (where mortality is highest at 25% RH and lowest at 95% RH) was observed for a North American strain.97 At 24°C, mortality also increased nonlinearly with decreasing RH. At 22°C, mortality was highest at 25% RH, but lowest at 55% RH, demonstrating the complex relationship between temperature, RH, and survival.
Adult survival data in response to RH are very limited. Within an RH range of 60–90%, little difference in adult survival is typically observed.55,61,65,67,69 Two studies report a decrease in adult longevity at lower RH: adult survival decreased from 8 days (at 85% RH) to 6 days (at 35% RH),98 while adult survival decreased from 2.1–4 days at 90% RH (for different Ae. albopictus strains) to 1.4–1.8 days at 70% RH.99 A later study linked increased desiccation resistance with higher glycogen and fatty acid content.70
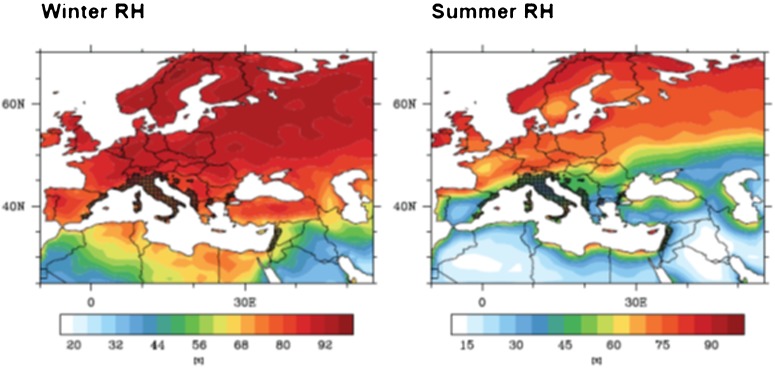
Since evidence suggests that strains adapt to environmental conditions, it is perhaps more appropriate to use current observations of Ae. albopictus populations and map RH conditions to give a reference range of RH (Fig. 8). European populations of Ae. albopictus are found in regions with RH as low as 35% in the summer, demonstrating the broad range of RHs at which this species can survive.
Figure 8.
The horizontal bar represents percentage relative humidity. Aedes albopictus data were taken from the ECDC Vbornet (www.vbornet.org). Dark hatched areas show the regions where Ae. albopictus have been recorded as present as of December 2011.
Aedes albopictus distribution and RH across Europe for the reference period of 2000–2009.
Field Estimates of Survival Probabilities
Field estimates of survival probabilities are generally considerably lower than estimates from laboratory populations, predominantly due to predation and other density-dependent processes. The immature stages are a source of food for other mosquito and copepod species, as well as being parasitised by a number of fungi, yet quantitative assessments of the impact of predation on survival have not been undertaken.14 The density of immature stages also affects development and survival, with higher densities resulting in reduced development times14,65,100 and production of smaller adults,14,98,100 although density has not been observed to affect either adult longevity98 or the susceptibility of adult Ae. albopictus to SINV infection.100 Food availability also affects survival, with limited food sources causing increased mortality and immature development time. Table 1 illustrates mortality rates from field estimates for Ae. albopictus life stages from either parity dissections or mark-release-recapture experiments in various regions. Egg hatching rates for Ae. albopictus in semi-field conditions have been reported as 0.5/day in tree holes and 0.7/day in tyres.101 Larval mortality and adult survival estimates from field studies are collated by Hawley,14 with estimates around 80% and a range of 0.71–0.89 for female adults respectively.14,102 A daily survival probability of 0.95 has been ascertained from capture-mark-release experiments in La Reunion,91 a value much higher than expected (and previous observations), potentially suggesting experimental error, although very high daily survival probabilities for Ae. albopictus have also been reported based on parity observations of field-caught mosquitoes in Tennessee (0.96 at 24.9°C and 0.99 at 15.7°C).103 Increased daily survival at lower temperatures have also been observed in laboratory experiments.55
Table 1. Field survival estimates of immature and adult Ae. albopictus.
| Eggs | Larvae | Adults (parity dissections) | Adults (Mark-release-recapture) | ||||
| Mortality (%) | Reference | Mortality (%) | Reference | DSR | Reference | DSR | Reference |
| 50 (tree holes) | Vitek and Livdahl (2006)101 (semi-field) | 80 | Hawley (1988)14 | 0.72 | Hawley (1988)14 | 0.77 | Hawley (1988)14 (unpublished data) |
| 30 (tyres) | Vitek and Livdahl (2006)101 (semi-field) | 88 | Hawley (1988)14 | 0.75 | Hawley (1988)14 | 0.89 | Niebylski and Craig (1994)102 |
| 73 | Hawley (1988)14 | 0.71 | Hawley (1988)14 | ||||
| 80 | Hawley (1988)14 | 0.78 | Hawley (1988)14 | ||||
| 70–80 | Hawley (1988)14 | 0.78 | Hawley (1988)14 | ||||
| 0.8 | Hawley (1988)14 | ||||||
| 0.85 | Hawley (1988)14 | ||||||
| 0.88 | Hawley (1988)14 | ||||||
| 0.82 | Hawley (1988)14 | ||||||
| 0.85 | Hawley (1988)14 | ||||||
| 0.95 | Lacroix et al. (2009)91 | ||||||
| 0.96–0.99 | Gottfried et al. (2002)103 | ||||||
| 0.948–0.966 | Almeida et al (2005)83 | ||||||
DSR: daily survival rate.
Response of Viral Transmission Dynamics to Environmental Variables
A number of studies have demonstrated a relationship between temperature and viral replication in several mosquito genera, with higher temperatures generally leading to shorter extrinsic incubation periods (EIPs), increased infection rates and faster dissemination rates, although these vary considerably in different mosquito/virus combinations. Within the Flavivirus genus, yellow fever (YF) infection of Ae. aegypti shows a decreased EIP at higher temperatures, YF infection of Haemagogus mosquitoes demonstrates lower infection rates at lower temperatures,104 and large daily temperature fluctuations decrease midgut DENV infection rates without affecting the EIP.105 DENV transmission is also affected by temperature – transmission from infected Ae. aegypti to monkeys has been shown to occur only above temperatures of 30°C, with the EIP decreasing from 12 days at 30°C to 7 days at 32/35°C.106 Alphaviruses also demonstrate reduce EIPs at higher temperatures, but display variation in the effects on infection and transmission rates – EEEV and WNV infection rates in Aedes triseriatus and Culex univittatus are independent of temperature, while WEEV infection rates in Culex tarsalis decrease at higher temperatures (32°C) compared to moderate temperatures (25°C).104 EEEV, WEEV, and WNV all have reduced EIPs at higher temperatures.104,107 The variation in temperature effects on infection and dissemination rates suggests that further experimental evidence of temperature effects on CHIKV infection in Ae. albopictus is needed for accurate modelling of transmission response to climatic changes, but to the best of our knowledge, such experiments have not been published to date.
Diurnal Temperature Cycles and Microclimate
Diurnal temperature range (DTR) has been shown to affect Ae. aegypti adult survival and dengue virus (DENV) transmission potential, with large fluctuations in temperature (20°C with a mean of 26°C) causing significantly higher Ae. aegypti mortality than under moderate temperature fluctuations (10°C) or constant temperatures.105 At lower temperatures (14°C), higher DTR increases disease transmission probability (presumably through enhanced virus ability to replicate and disseminate at higher temperatures during the daily fluctuation), while at higher temperatures (26°C), increased DTR reduces transmission probability (possibly through the deleterious effects of very high temperatures on viral infection and replication).105 The effect of fluctuating temperatures on Anopheles stephensi (through survival and development) and malaria transmission (through parasite development) has been similarly studied.108 DTR increases the rate of parasite development at lower temperatures (and vice versa), indicating that increases in temperature may not necessarily result in increased disease transmission since the relationship between them is nonlinear. This is critical when modelling the effects of changing climate on vector populations and disease transmission. The effect of DTR on immature and adult life stages confirms the need for appropriate empirical data to reliably capture vector–pathogen interactions when modelling disease transmission. Nonetheless, the complex relationship between temperature and mosquito life cycle has been highlighted,105,108 although no studies of fluctuating temperatures on Ae. albopictus dynamics have been carried out to date.
Despite the complex ecology and biology of mosquitoes, ambient air temperature is often used in studies investigating the effect of temperature on mosquito population dynamics and/or disease transmission. Yet given the aquatic nature of the immature stages, water temperatures in breeding sites should ideally be considered (which tend to be consistently higher than air temperatures by up to 6°C depending on the size and location of the water pool109), while adult mosquitoes frequently rest indoors (at different temperatures to those outdoors) after blood meals. Studies investigating such ‘microclimate’ effects on malaria risk have been reported elsewhere.109,110 The relationship between air and water temperature is generally nonlinear (1°C increases in the former typically cause smaller increases in the latter) and An. gambiae immature development times may be shortened by 4–11 days when using water, rather than air, temperatures. Thus, models considering air temperatures alone may incorrectly estimate An. gambiae response to increasing temperatures. However, the importance of differences in air and water temperatures varies from species to species, as well as depending on breeding site preferences – Ae. albopictus often selects from a vast array of containers in which to breed, ranging from used tyres and tin cans to 55 gallon drums, complicating consideration of air and water temperature differences. Weekly water temperatures at Ae. albopictus breeding sites may be around 0–5°C higher than ambient air temperatures or closely follow it depending on site type.92 Host-seeking and post blood-feeding behaviour also affect the temperatures to which adult mosquitoes are exposed and the effect of indoor resting by An. gambiae mosquitoes on malaria risk has been considered,110 although Ae. albopictus blood-feeding and resting behaviour is exophagic and, as such, microclimate is unlikely to affect pathogen development or blood meal digestion.
Conclusion
The global distribution of Ae. albopictus has changed significantly over the last few decades and current indications suggest that this is likely to continue under future climate scenarios. It will be crucial to our understanding of how the future burden of mosquito-borne diseases may shift under climate change to better understand how the ecology and biology of disease-carrying vectors (such as Anopheles, Aedes, and Culex mosquitoes, together with variability between sub-species) depend on a range of biotic and abiotic factors likely to influence their global distribution and local abundance. In this article, we have reviewed the dependence of the lifecycle parameters of Ae. albopictus (a key vector of CHIKV and DENV transmission) on climatic variables and other environmental factors to better understand the role of such variables on disease risk and hence the implications for future transmission (given knowledge of how viral replication dynamics additionally depend on environmental factors and the use of mathematical models). Key heterogeneities between tropical and temperate Ae. albopictus species exist. Including the complex interactions between temperature, rainfall, desiccation, RH, and density-dependent processes and main vector lifecycle processes are discussed. Both statistical and mathematical models have important roles to play in assessing the likely global distribution of CHIKV transmission over the coming decades under different scenarios, as well as country-specific disease risk (and, if realised, the likely severity, intensity and consequences of emergence or re-emergence). Given the characteristic decadal timescales of climate change, however, robust, validated process-based transmission models arguably offer a more reliable basis for developing projections of disease risk than those derived from statistical models (which, by definition, extrapolate current relationships between environmental variables and indicators of transmission and assume all other factors remain unchanged). However, the reliability and hence usefulness of process-based models relies heavily on accurate model parameterisation and it is hoped that this article provides a thorough basis for future modelling efforts within the field. Good mathematical modelling practice additionally demands that model outcomes, projections, and implications (e.g. for CHIKV control and the design of intervention programmes) are assessed for sensitivity to uncertainty in model inputs, one component of which is parameter uncertainty. Thus, we have also highlighted where key uncertainties and limitations in our current knowledge and understanding of the relationships between Ae. albopictus biology/ecology and environmental variables lie, and hence where future experimental and modelling research may be directed to further improve parameterisation in vector and CHIKV transmission models. One example is the need to better understand the role of temporal fluctuations (on a range of timescales) in climatic variables on Ae. albopictus population dynamics, as well as the role of changes in mean environmental conditions, a consideration that has received little attention to date. This process of identifying, assessing, and quantifying such uncertainties not only enables us to better exploit the explanatory power of process-based mathematical models, but also, ultimately, to produce more reliable projections of future spatiotemporal patterns of CHIKV transmission under different climate scenarios.
Acknowledgments
This work was co-funded by the European Regional Development Fund and the Republic of Cyprus through the Research Promotion Foundation (Project YΓEΙA/ΔYΓEIA/0311(BIE)/13). The research leading to these results has received funding from the European Research Council under the European Union’s Seventh Framework Programme (FP7/2007-2013)/ERC grant agreement number 226144. EM acknowledges the Eck Institute for Global Health at the University of Notre Dame, as well as NIH R01 grant number AI069387-01A1, for partial funding of this work. PEP and EM would like to thank the Grantham Institute for Climate Change at Imperial College London for funding this research. None of the funding bodies mentioned contributed to the design or content of this study, nor the writing of the manuscript or decision to submit.
References
- 1.Strauss JH, Strauss EG. The alphaviruses: gene expression, replication, and evolution. Microbiol Rev. 1994;58(3):491–562. doi: 10.1128/mr.58.3.491-562.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ross RW. The Newala epidemic. III. The virus: isolation, pathogenic properties and relationship to the epidemic. J Hyg (Lond). 1956;54(2):177–91. doi: 10.1017/s0022172400044442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwartz O, Albert ML. Biology and pathogenesis of chikungunya virus. Nat Rev Microbiol. 2010;8(7):491–500. doi: 10.1038/nrmicro2368. [DOI] [PubMed] [Google Scholar]
- 4.Solignat M, Gay B, Higgs S, Briant L, Devaux C. Replication cycle of chikungunya: a re-emerging arbovirus. Virology. 2009;393(2):183–97. doi: 10.1016/j.virol.2009.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chhabra M, Mittal V, Bhattacharya D, Rana U, Lal S. Chikungunya fever: a re-emerging viral infection. Indian J Med Microbiol. 2008;26(1):5–12. doi: 10.4103/0255-0857.38850. [DOI] [PubMed] [Google Scholar]
- 6.Chevillon C, Briant L, Renaud F, Devaux C. The Chikungunya threat: an ecological and evolutionary perspective. Trends Microbiol. 2008;16(2):80–8. doi: 10.1016/j.tim.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Pialoux G, Gauzere BA, Jaureguiberry S, Strobel M. Chikungunya, an epidemic arbovirosis. Lancet Infect Dis. 2007;7(5):319–27. doi: 10.1016/S1473-3099(07)70107-X. [DOI] [PubMed] [Google Scholar]
- 8.Tolle MA. Mosquito-borne diseases. Curr Probl Pediatr Adolesc Health Care. 2009;39(4):97–140. doi: 10.1016/j.cppeds.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Enserink M. Entomology. A mosquito goes global. Science. 2008;320(5878):864–6. doi: 10.1126/science.320.5878.864. [DOI] [PubMed] [Google Scholar]
- 10.Rezza G, Nicoletti L, Angelini R, Romi R, Finarelli AC, Panning M, et al. Infection with chikungunya virus in Italy: an outbreak in a temperate region. Lancet. 2007;370(9602):1840–6. doi: 10.1016/S0140-6736(07)61779-6. [DOI] [PubMed] [Google Scholar]
- 11.Watson R. Europe witnesses first local transmission of chikungunya fever in Italy. BMJ. 2007;335(7619):532–3. doi: 10.1136/bmj.39332.708738.DB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knudsen AB, Romi R, Majori G. Occurrence and spread in Italy of Aedes albopictus, with implications for its introduction into other parts of Europe. J Am Mosq Control Assoc. 1996;12(2 Pt 1):177–83. [PubMed] [Google Scholar]
- 13.Paupy C, Delatte H, Bagny L, Corbel V, Fontenille D. Aedes albopictus, an arbovirus vector: from the darkness to the light. Microbes Infect. 2009;11(14–15):1177–85. doi: 10.1016/j.micinf.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Hawley WA. The biology of Aedes albopictus. J Am Mosq Control Assoc Suppl. 1988;1:1–39. [PubMed] [Google Scholar]
- 15.Ng LC, Hapuarachchi HC. Tracing the path of chikungunya virus–evolution and adaptation. Infect Genet Evol. 2010;10(7):876–85. doi: 10.1016/j.meegid.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 16.Singh SK, Unni SK.Chikungunya virus: host pathogen interaction. Rev Med Virol. 201121278–88. [DOI] [PubMed] [Google Scholar]
- 17.de Lamballerie X, Leroy E, Charrel RN, Ttsetsarkin K, Higgs S, Gould EA. Chikungunya virus adapts to tiger mosquito via evolutionary convergence: a sign of things to come? Virol J. 2008;5:33. doi: 10.1186/1743-422X-5-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsetsarkin KA, Weaver SC. Sequential adaptive mutations enhance efficient vector switching by chikungunya virus and its epidemic emergence. PLoS Pathog. 2011;7(12):e1002412. doi: 10.1371/journal.ppat.1002412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schaffner F, Medlock JM, Van Bortel W.Public health significance of invasive mosquitoes in Europe. Clin Microbiol Infect 2013. [published online ahead of print]. [DOI] [PubMed] [Google Scholar]
- 20.Medlock JM, Avenell D, Barrass I, Leach S. Analysis of the potential for survival and seasonal activity of Aedes albopictus (Diptera: Culicidae) in the United Kingdom. J Vector Ecol. 2006;31(2):292–304. doi: 10.3376/1081-1710(2006)31[292:aotpfs]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 21.Takumi K, Scholte E-J, Braks M, Reusken C, Avenell D, Medlock JM. Introduction, scenarios for establishment and seasonal activity of Aedes albopictus in The Netherlands. Vector Borne Zoonotic Dis. 2009;9(2):191–6. doi: 10.1089/vbz.2008.0038. [DOI] [PubMed] [Google Scholar]
- 22.Ditsuwan T, Liabsuetrakul T, Chongsuvivatwong V, Thammapalo S, McNeil E. Assessing the spreading patterns of dengue infection and chikungunya fever outbreaks in lower southern Thailand using a geographic information system. Ann Epidemiol. 2011;21(4):253–61. doi: 10.1016/j.annepidem.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Delatte H, Dehecq JS, Thiria J, Domerg C, Paupy C, Fontenille D. Geographic distribution and developmental sites of Aedes albopictus (Diptera: Culicidae) during a chikungunya epidemic event. Vector Borne Zoonotic Dis. 2008;8(1):25–34. doi: 10.1089/vbz.2007.0649. [DOI] [PubMed] [Google Scholar]
- 24.Bagny L, Delatte H, Elissa N, Quilici S, Fontenille D. Aedes (Diptera: Culicidae) vectors of arboviruses in Mayotte (Indian Ocean): distribution area and larval habitats. J Med Entomol. 2009;46(2):198–207. doi: 10.1603/033.046.0204. [DOI] [PubMed] [Google Scholar]
- 25.Neteler M, Roiz D, Rocchini D, Castellani C, Rizzoli A. Terra and Aqua satellites track tiger mosquito invasion: modelling the potential distribution of Aedes albopictus in north-eastern Italy. Int J Health Geogr. 2011;10:49. doi: 10.1186/1476-072X-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roiz D, Neteler M, Castellani C, Arnoldi D, Rizzoli A. Climatic factors driving invasion of the tiger mosquito (Aedes albopictus) into new areas of Trentino, northern Italy. PloS ONE. 2011;6(4):e14800. doi: 10.1371/journal.pone.0014800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Moor PP, Steffens FE. A computer-simulated model of an arthropod-borne virus transmission cycle, with special reference to chikungunya virus. Trans R Soc Trop Med Hyg. 1970;64(6):927–34. doi: 10.1016/0035-9203(70)90114-8. [DOI] [PubMed] [Google Scholar]
- 28.ECDC. Expert meeting on chikungunya modelling, Stockholm, Sweden, April, 2008 2009 [Google Scholar]
- 29.Moulay D, Aziz-Alaoui MA, Cadivel M. The chikungunya disease: modeling, vector and transmission global dynamics. Math Biosci. 2011;229(1):50–63. doi: 10.1016/j.mbs.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 30.Bacaer N. Approximation of the basic reproduction number R0 for vector-borne diseases with a periodic vector population. Bull Math Biol. 2007;69(3):1067–91. doi: 10.1007/s11538-006-9166-9. [DOI] [PubMed] [Google Scholar]
- 31.Bacaer N, Gomes MG. On the final size of epidemics with seasonality. Bull Math Biol. 2009;71(8):1954–66. doi: 10.1007/s11538-009-9433-7. [DOI] [PubMed] [Google Scholar]
- 32.Chaves LF, Morrison AC, Kitron UD, Scott TW.Nonlinear impacts of climatic variability on the density-dependent regulation of an insect vector of disease. Global Change Biol 2012182457–468. [Google Scholar]
- 33.Schaeffer B, Mondet B, Touzeau S. Using a climate-dependent model to predict mosquito abundance: application to Aedes (Stegomyia) africanus and Aedes (Diceromyia) furcifer (Diptera: Culicidae). Infect Genet Evol. 2008;8(4):422–32. doi: 10.1016/j.meegid.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 34.Mecoli M, De Angelis V, Brailsford SC, editors. Modelling the risk of mosquito-borne diseases by system dynamics: the case of human travel between different geographic regions. IEEE International Conference on Systems, Man and Cybernetics; 2010 Oct 10–13; Istanbul, Turkey [Google Scholar]
- 35.Ramchurn S, Goorah S, Mungla D, Ramsurrun B, Pydiah V, Summun A. A study of the 2006 chikungunya epidemic outbreak in Mauritius. Internet J Med Update. 2008;3(1):11–21. [Google Scholar]
- 36.Poletti P, Messeri G, Ajelli M, Vallorani R, Rizzo C, Merler S. Transmission potential of chikungunya virus and control measures: the case of Italy. PloS ONE. 2011;6(5):e18860. doi: 10.1371/journal.pone.0018860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Massad E, Ma S, Burattini MN, Tun Y, Coutinho FAB, Ang LW. The risk of chikungunya fever in a dengue-endemic area. J Travel Med. 2008;15(3):147–55. doi: 10.1111/j.1708-8305.2008.00186.x. [DOI] [PubMed] [Google Scholar]
- 38.Dumont Y, Chiroleu F, Domerg C. On a temporal model for the Chikungunya disease: modeling, theory and numerics. Math Biosci. 2008;213(1):80–91. doi: 10.1016/j.mbs.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 39.Dumont Y, Chiroleu F. Vector control for the chikungunya disease. Math Biosci Eng. 2010;7(2):313–45. doi: 10.3934/mbe.2010.7.313. [DOI] [PubMed] [Google Scholar]
- 40.Dumont Y, Tchuenche JM.Mathematical studies on the sterile insect technique for the chikungunya disease and Aedes albopictus. J Math Biol. 2012655809–854. [DOI] [PubMed] [Google Scholar]
- 41.White SM, Rohani P, Sait SM. Modelling pulsed releases for sterile insect techniques: fitness costs of sterile and transgenic males and the effects on mosquito dynamics. J Appl Ecol. 2010;47(6):1329–39. [Google Scholar]
- 42.Ferguson HM, Dornhaus A, Beeche A, Borgemeister C, Gottlieb M, Mulla MS, et al. Ecology: a prerequisite for malaria elimination and eradication. PLoS Med. 2010;7(8):e1000303. doi: 10.1371/journal.pmed.1000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parham PE, Pople D, Christiansen-Jucht C, Lindsay S, Hinsley W, Michael E. Modeling the role of environmental variables on the population dynamics of the malaria vector Anopheles gambiae sensu stricto. Malar J. 2012;11:271. doi: 10.1186/1475-2875-11-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jöckel P, Tost H, Pozzer A, Brühl C, Buchholz J, Ganzeveld L, et al. The atmospheric chemistry general circulation model ECHAM5/MESSy1: consistent simulation of ozone from the surface to the mesosphere. Atmos Chem Phys. 2006;6:5067–104. [Google Scholar]
- 45.Roeckner E, Brokopf R, Esch M, Giorgetta M, Hagemann S, Kornblueh L, et al. Sensitivity of simulated climate to horizontal and vertical resolution in the ECHAM5 atmosphere model. J Clim. 2006;19(16):3771–91. [Google Scholar]
- 46.Taylor K, Williamson D, Zwiers F. The sea surface temperature and sea ice concentration boundary conditions for AMIP II simulations. PCMDI Report, Tech Rep Program for Climate Model Diagnosis and Inter-comparison. 2000:60. Livermore, CA, USA: Lawrence Livermore National Laboratory. [Google Scholar]
- 47.Nawrocki SJ, Hawley WA. Estimation of the northern limits of distribution of Aedes albopictus in North America. J Am Mosq Control Assoc. 1987;3(2):314–7. [PubMed] [Google Scholar]
- 48.Wu F, Liu Q, Lu L, Wang J, Song X, Ren D. Distribution of Aedes albopictus (Diptera: Culicidae) in northwestern China. Vector Borne Zoonotic Dis. 2011;11(8):1181–6. doi: 10.1089/vbz.2010.0032. [DOI] [PubMed] [Google Scholar]
- 49.Kobayashi M, Nihei N, Kurihara T. Analysis of northern distribution of Aedes albopictus (Diptera: Culicidae) in Japan by geographical information system. J Med Entomol. 2002;39(1):4–11. doi: 10.1603/0022-2585-39.1.4. [DOI] [PubMed] [Google Scholar]
- 50.Rochlin I, Ninivaggi DV, Hutchinson ML, Farajollahi A. Climate change and range expansion of the Asian tiger mosquito (Aedes albopictus) in Northeastern USA: implications for Public Health Practitioners. PLoS ONE. 2013;8(4):e60874. doi: 10.1371/journal.pone.0060874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thomas SM, Obermayr U, Fischer D, Kreyling J, Beierkuhnlein C. Low-temperature threshold for egg survival of a post-diapause and non-diapause European aedine strain, Aedes albopictus (Diptera: Culicidae). Parasites Vectors. 2012;5:100. doi: 10.1186/1756-3305-5-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Medlock JM, Avenell D, Barrass I, Leach S. Analysis of the potential for survival and seasonal activity of Aedes albopictus (Diptera: Culicidae) in the United Kingdom. J Vector Ecol. 2006;31(2):292–304. doi: 10.3376/1081-1710(2006)31[292:aotpfs]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 53.Caminade C, Medlock JM, Ducheyne E, McIntyre KM, Leach S, Baylis M, et al. Suitability of European climate for the Asian tiger mosquito Aedes albopictus: recent trends and future scenarios. J R Soc Interface. 2012;9(75):2708–17. doi: 10.1098/rsif.2012.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee SJ. Development of eggs, larvae and pupae of Aedes albopictus (Skuse) (Diptera:Culicidae). Chin J Entomol. 1994;14:13–32. (in Chinese) [Google Scholar]
- 55.Delatte H, Gimonneau G, Triboire A, Fontenille D. Influence of temperature on immature development, survival, longevity, fecundity, and gonotrophic cycles of Aedes albopictus, vector of chikungunya and dengue in the Indian Ocean. J Med Entomol. 2009;46(1):33–41. doi: 10.1603/033.046.0105. [DOI] [PubMed] [Google Scholar]
- 56.Tseng SM, Wu I. An ecological study of mosquitoes in the Wuhan area. Bull Entomol Res. 1951;42:527–33. [Google Scholar]
- 57.Udaka M. Some ecological notes on Aedes albopictus in Shikoku, Japan. Kontyu. 1959;27:202–8. [Google Scholar]
- 58.Livingtsone D, Krishnamoorthy K. Studies on the active patterns of the larvae and adults of Aedes albopictus (Skuse) and Aedes vittatus (Bigot) of the scrub jungles of Palghat-Gap, India. J Bombay Nat Hist Soc. 1985;82:30–7. [Google Scholar]
- 59.Hien DS. Biology of Aedes aegypti (L. 1762) and Aedes albopictus (Skuse, 1895) (Diptera, Culicidae). III. Effect of certain environmental conditions on the development of larvae and pupae. Acta Parasitol Pol. 1975;23:553–68. [Google Scholar]
- 60.Rosario D. Studies on the biology of Phippine mosquitoes. II. Observations of the life and behaviour of Aedes albopictus (Skuse) in the laboratory. Phil J Sci. 1963;92:89–103. [Google Scholar]
- 61.Calado DC, Silva MA. Avaliacao da influencia da temperatura sobre o desenvolvimento de Aedes albopictus [Evaluation of the temperature influence on the development of Aedes albopictus]. Rev Saude Publica. 2002;36(2):173–9. doi: 10.1590/s0034-89102002000200009. (in Portuguese) [DOI] [PubMed] [Google Scholar]
- 62.Galliard H, Golvan YJ. Influences de certains facteurs nutritionels et hormonaux a des temperatures variables, sur la croissance des larves d’Aedes (S.) aegypti, Aedes (S.) albopictus et Anopheles (M.) stephensi. [Effects of certain nutritional and hormonal factors at various temperatures on growth of the larvae of Aedes (S.) aegypti, Aedes (S.) albopictus and Anopheles (M.) stephensi]. Ann Parasitol Hum Comp. 1957;32(5–6):563–79. (in French) [PubMed] [Google Scholar]
- 63.Galliard H. Recherches sur la biologie des Culicides a Hanoi (tonkin, Nord Vietnam). I. Developpement larvaire comparatif de differentes souches d'Aedes albopictus, A. aegypti, Culex fatigans et Armigeres obturbans. [Findings on the biology of Culicidae in Hanoi (Tonkin, North Vietnam). I. Comparative larval development of various strains of Aedes albopictus, Aedes aegypti, Culex fatigans & Armigeres obturans]. Ann Parasitol Hum Comp. 1958;33(1–2):131–44. (in French) [PubMed] [Google Scholar]
- 64.Halcrow JG. Notes on a laboratory colony of Aedes (Stegomyia) albopictus (Skuse) and its distribution in Mauritius. Proc R Entomol Soc Lond. 1955;30:40–2. [Google Scholar]
- 65.Briegel H, Timmermann SE. Aedes albopictus (Diptera: Culicidae): physiological aspects of development and reproduction. J Med Entomol. 2001;38(4):566–71. doi: 10.1603/0022-2585-38.4.566. [DOI] [PubMed] [Google Scholar]
- 66.Gillett JD. Chaucer Press; 1971. Mosquitos. Worthing, West Sussex, UK: Littlehampton Book Services Ltd. [Google Scholar]
- 67.Lowenberg Neto P, Navarro-Silva MA. Development, longevity, gonotrophic cycle and oviposition of Aedes albopictus Skuse (Diptera: Culicidae) under cyclic temperatures. Neotrop Entomol. 2004;33:29–33. [Google Scholar]
- 68.Rosenstein DL, Kalogeras KT, Kalafut M, Malley J, Rubinow DR, Joshi DS. Effect of fluctuating and constant temperatures on development, adult longevity and fecundity in the mosquito Aedes krombeini. J Therm Biol. 1996;21(3):151–4. [Google Scholar]
- 69.Alto BW, Juliano SA. Temperature effects on the dynamics of Aedes albopictus (Diptera: Culicidae) populations in the laboratory. J Med Entomol. 2001;38(4):548–56. doi: 10.1603/0022-2585-38.4.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sawabe K, Mogi M. Differences in energy metabolism and adult desiccation resistance among three Aedes (Stegomyia) species (Diptera: Culicidae) from South Sulawesi, Indonesia. J Med Entomol. 1999;36(1):101–7. doi: 10.1093/jmedent/36.1.101. [DOI] [PubMed] [Google Scholar]
- 71.Roiz D, Rosa R, Arnoldi D, Rizzoli A. Effects of temperature and rainfall on the activity and dynamics of host-seeking Aedes albopictus females in northern Italy. Vector Borne Zoonotic Dis. 2010;10(8):811–6. doi: 10.1089/vbz.2009.0098. [DOI] [PubMed] [Google Scholar]
- 72.Basuki TA, Cerone A, Barbuti R, Maggiolo-Schettini A, Milazzo P, Rossi E.Modelling the dynamics of an Aedes albopictus population. In: Milazzo P, Perez Jimenez MJ, editors. Applications of membrane computing, concurrency and agent-based modelling in population biology. 2010. p. 18–36. [Google Scholar]
- 73.Erickson RA, Presley SM, Allen LJS, Long KR, Cox SB. A stage-structured, Aedes albopictus population model. Ecol Modell. 221(9):1273–82. [Google Scholar]
- 74.Aida HN, Dieng H, Ahmad AH, Satho T, Nurita AT, Salmah RC, et al. The biology and demographic parameters of Aedes albopictus in northern peninsular Malaysia. Asian Pac J Trop Biomed. 2011:472–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gubler DJ. Competitive displacement of Aedes (Stegomyia) Polynesiensis Marks by Aedes (Stegomyia) albopictus Skuse in laboratory populations. J Med Entomol. 1970;7(2):229–35. doi: 10.1093/jmedent/7.2.229. [DOI] [PubMed] [Google Scholar]
- 76.Xue RD, Barnard DR, Ali A. Influence of multiple blood meals on gonotrophic dissociation and fecundity in Aedes albopictus. J Am Mosq Control Assoc. 2009;25(4):504–7. doi: 10.2987/09-5912.1. [DOI] [PubMed] [Google Scholar]
- 77.Dieng H, Saifur RG, Hassan AA, Salmah MR, Boots M, Satho T, et al. Indoor-breeding of Aedes albopictus in northern peninsular Malaysia and its potential epidemiological implications. PLoS ONE. 2010;5(7):e11790. doi: 10.1371/journal.pone.0011790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Urbanski JM, Benoit JB, Michaud MR, Denlinger DL, Armbruster P. The molecular physiology of increased egg desiccation resistance during diapause in the invasive mosquito, Aedes albopictus. Proc Biol Sci. 2010;277(1694):2683–92. doi: 10.1098/rspb.2010.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Toma L, Severini F, Di Luca M, Bella A, Romi R. Seasonal patterns of oviposition and egg hatching rate of Aedes albopictus in Rome. J Am Mosq Control Assoc. 2003;19(1):19–22. [PubMed] [Google Scholar]
- 80.Pumpuni CB, Knepler J, Craig GB., Jr Influence of temperature and larval nutrition on the diapause inducing photoperiod of Aedes albopictus. J Am Mosq Control Assoc. 1992;8(3):223–7. [PubMed] [Google Scholar]
- 81.Mori A, Wada Y. The Gonotrophic cycle of Aedes albopictus in the field. Trop Med. 1977;19(3):141–6. [Google Scholar]
- 82.Delatte H, Desvars A, Bouetard A, Bord S, Gimonneau G, Vourc’h G, et al. Blood-feeding behavior of Aedes albopictus, a vector of chikungunya on La Reunion. Vector Borne Zoonotic Dis. 2010;10(3):249–58. doi: 10.1089/vbz.2009.0026. [DOI] [PubMed] [Google Scholar]
- 83.Almeida AP, Baptista SS, Sousa CA, Novo MT, Ramos HC, Panella NA, et al. Bioecology and vectorial capacity of Aedes albopictus (Diptera: Culicidae) in Macao, China, in relation to dengue virus transmission. J Med Entomol. 2005;42(3):419–28. doi: 10.1093/jmedent/42.3.419. [DOI] [PubMed] [Google Scholar]
- 84.Scott TW, Amerasinghe PH, Morrison AC, Lorenz LH, Clark GG, Strickman D, et al. Longitudinal studies of Aedes aegypti (Diptera: Culicidae) in Thailand and Puerto Rico: blood feeding frequency. J Med Entomol. 2000;37(1):89–101. doi: 10.1603/0022-2585-37.1.89. [DOI] [PubMed] [Google Scholar]
- 85.Ho B, Chan K, Chan Y. Aedes aegypti (L.) and Aedes albopictus (Skuse) in Singapore City: 3. Population fluctuations*. Bull WHO. 1971;44(5):635. [PMC free article] [PubMed] [Google Scholar]
- 86.Ray S, Tandon N. Breeding habitats & seasonal variation in the larval density of Aedes aegypti (L) & Ae. albopictus (Skuse) in an urban garden in Calcutta city. Indian J Med Res. 1999;109:221–4. [PubMed] [Google Scholar]
- 87.Tandon N, Ray S. Breeding habitats and larval indices of Aedes aegypti and Ae. albopictus in the residential areas of Calcutta City. J Commun Dis. 2000;32(3):180–4. [PubMed] [Google Scholar]
- 88.Khan AR. Studies on the breeding habitats and seasonal prevalence of larval population of Aedes aegypti (L.) and Aedes albopictus (Skuse) in Dacca city. Bangladesh Med Res Counc Bull. 1980;6(2):45–52. [PubMed] [Google Scholar]
- 89.Rozilawati H, Zairi J, Adanan CR. Seasonal abundance of Aedes albopictus in selected urban and suburban areas in Penang, Malaysia. Trop Biomed. 2007;24(1):83–94. [PubMed] [Google Scholar]
- 90.Pena CJ, Gonzalvez G, Chadee DD. Seasonal prevalence and container preferences of Aedes albopictus in Santo Domingo City, Dominican Republic. J Vector Ecol. 2003;28:208–12. [PubMed] [Google Scholar]
- 91.Lacroix R, Delatte H, Hue T, Reiter P. Dispersal and survival of male and female Aedes albopictus (Diptera: Culicidae) on Reunion Island. J Med Entomol. 2009;46(5):1117–24. doi: 10.1603/033.046.0519. [DOI] [PubMed] [Google Scholar]
- 92.Alto BW, Juliano SA. Precipitation and temperature effects on populations of Aedes albopictus (Diptera: Culicidae): implications for range expansion. J Med Entomol. 2001;38(5):646–56. doi: 10.1603/0022-2585-38.5.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dieng H, Rahman GM, Abu Hassan A, Che Salmah MR, Satho T, Miake F, et al. The effects of simulated rainfall on immature population dynamics of Aedes albopictus and female oviposition. Int J Biometeorol. 2012;56(1):113–120. doi: 10.1007/s00484-011-0402-0. [DOI] [PubMed] [Google Scholar]
- 94.Paaijmans KP, Wandago MO, Githeko AK, Takken W. Unexpected high losses of Anopheles gambiae larvae due to rainfall. PLoS ONE. 2007;2(11):e1146. doi: 10.1371/journal.pone.0001146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Eritja R, Escosa R, Lucientes J, Marques E, Roiz D, Ruiz S. Worldwide invasion of vector mosquitoes: present European distribution and challenges for Spain. Biol Invasions. 2005;7:87–97. [Google Scholar]
- 96.Dickerson CZ.2007. The effects of temperature and humidity on the eggs of Aedes aegypti (L.) and Aedes albopictus (Skuse) in Texas Texas: Texas A&M University. 119p [Google Scholar]
- 97.Juliano SA, O’Meara GF, Morrill JR, Cutwa MM. Desiccation and thermal tolerance of eggs and the coexistence of competing mosquitoes. Oecologia. 2002;130(3):458–69. doi: 10.1007/s004420100811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Reiskind MH, Lounibos LP. Effects of intraspecific larval competition on adult longevity in the mosquitoes Aedes aegypti and Aedes albopictus. Med Vet Entomol. 2009;23(1):62–8. doi: 10.1111/j.1365-2915.2008.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mogi M, Miyagi I, Abadi K. Syafruddin. Inter- and intraspecific variation in resistance to desiccation by adult Aedes (Stegomyia) spp. (Diptera: Culicidae) from Indonesia. J Med Entomol. 1996;33(1):53–7. doi: 10.1093/jmedent/33.1.53. [DOI] [PubMed] [Google Scholar]
- 100.Muturi EJ, Costanzo K, Kesavaraju B, Alto BW. Can pesticides and larval competition alter susceptibility of Aedes mosquitoes (Diptera: Culicidae) to arbovirus infection? J Med Entomol. 2011;48(2):429–36. doi: 10.1603/me10213. [DOI] [PubMed] [Google Scholar]
- 101.Vitek CJ, Livdahl TP. Field and laboratory comparison of hatch rates in Aedes albopictus (Skuse). J Am Mosq Control Assoc. 2006;22(4):609–14. doi: 10.2987/8756-971X(2006)22[609:FALCOH]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 102.Niebylski ML, Craig GB., Jr Dispersal and survival of Aedes albopictus at a scrap tire yard in Missouri. J Am Mosq Control Assoc. 1994;10(3):339–43. [PubMed] [Google Scholar]
- 103.Gottfried KL, Gerhardt RR, Nasci RS, Crabtree MB, Karabatsos N, Burkhalter KL, et al. Temporal abundance, parity, survival rates, and arbovirus isolation of field-collected container-inhabiting mosquitoes in eastern Tennessee. J Am Mosq Control Assoc. 2002;18(3):164–72. [PubMed] [Google Scholar]
- 104.Turell MJ, Rossi CA, Bailey CL. Effect of extrinsic incubation temperature on the ability of Aedes taeniorhynchus and Culex pipiens to transmit Rift Valley fever virus. Am J Trop Med Hyg. 1985;34(6):1211–8. doi: 10.4269/ajtmh.1985.34.1211. [DOI] [PubMed] [Google Scholar]
- 105.Lambrechts L, Paaijmans KP, Fansiri T, Carrington LB, Kramer LD, Thomas MB, et al. Impact of daily temperature fluctuations on dengue virus transmission by Aedes aegypti. Proc Natl Acad Sci USA. 2011;108(18):7460–5. doi: 10.1073/pnas.1101377108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Watts DM, Burke DS, Harrison BA, Whitmire RE, Nisalak A. Effect of temperature on the vector efficiency of Aedes aegypti for dengue 2 virus. Am J Trop Med Hyg. 1987;36(1):143–52. doi: 10.4269/ajtmh.1987.36.143. [DOI] [PubMed] [Google Scholar]
- 107.Reisen WK, Fang Y, Martinez VM. Effects of temperature on the transmission of west nile virus by Culex tarsalis (Diptera: Culicidae). J Med Entomol. 2006;43(2):309–17. doi: 10.1603/0022-2585(2006)043[0309:EOTOTT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 108.Paaijmans KP, Blanford S, Bell AS, Blanford JI, Read AF, Thomas MB. Influence of climate on malaria transmission depends on daily temperature variation. Proc Natl Acad Sci USA. 2010;107(34):15135–9. doi: 10.1073/pnas.1006422107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Paaijmans KP, Imbahale SS, Thomas MB, Takken W. Relevant microclimate for determining the development rate of malaria mosquitoes and possible implications of climate change. Malar J. 2010;9:196. doi: 10.1186/1475-2875-9-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Paaijmans KP, Thomas MB. The influence of mosquito resting behaviour and associated microclimate for malaria risk. Malar J. 2011;10:183. doi: 10.1186/1475-2875-10-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Reiter P. Aedes albopictus and the world trade in used tires, 1988–1995: the shape of things to come? J Am Mosq Control Assoc. 1998;14(1):83–94. [PubMed] [Google Scholar]
- 112.Ogata K, Lopez Samayoa A. Discovery of Aedes albopictus in Guatemala. J Am Mosq Control Assoc. 1996;12(3 Pt 1):503–6. [PubMed] [Google Scholar]
- 113.Fontenille D, Toto JC. Aedes (Stegomyia) albopictus (Skuse), a potential new dengue vector in southern Cameroon. Emerg Infect Dis. 2001;7(6):1066–7. doi: 10.3201/eid0706.010631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Benedict MQ, Levine RS, Hawley WA, Lounibos LP. Spread of the tiger: global risk of invasion by the mosquito Aedes albopictus. Vector-Borne Zoonotic Dis. 2007;7(1):76–85. doi: 10.1089/vbz.2006.0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Coffinet T, Mourou JR, Pradines B, Toto JC, Jarjaval F, Amalvict R, et al. First record of Aedes albopictus in Gabon. J Am Mosq Control Assoc. 2007;23(4):471–2. doi: 10.2987/5636.1. [DOI] [PubMed] [Google Scholar]
- 116.Haddad N, Harbach RE, Chamat S, Bouharoun-Tayoun H. Presence of Aedes albopictus in Lebanon and Syria. J Am Mosq Control Assoc. 2007;23(2):226–8. doi: 10.2987/8756-971X(2007)23[226:POAAIL]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 117.Cuellar-Jimenez ME, Velasquez-Escobar OL, Gonzalez-Obando R, Morales-Reichmann CA. Deteccion de Aedes albopictus (Skuse) (Diptera: Culicidae) en la ciudad de Cali, Valle del Cauca, Colombia. [Detection of Aedes albopictus (Skuse) (Diptera: Culicidae) in the city of Cali, Valle del Cauca, Colombia]. Biomedica. 2007;27(2):273–9. (in Spain) [PubMed] [Google Scholar]
- 118.Hien DS. Biology of Aedes aegypti (L. 1762) and Aedes albopictus (Skuse, 1895) (Diptera, Culicidae). II. Effect of certain environmental conditions on the hatching of larvae. Acta Parasitol Pol. 1975;23:537–52. [Google Scholar]