Abstract
Cases of human oro-mucosal leishmaniasis are mainly reported in areas where Leishmania (Viannia) braziliensis perpetuates and the damages are mainly located at the cartilaginous nasal septum and frontal portions of the nasal fossa. In Iran, an area free of any L.(V) braziliensis, three Leishmania species are known to perpetuate through distinct (i) blood-feeding sand flies and (ii) rodents or (iii) canidae. Thus while establishing the diagnosis of any human oro-mucosal lesions, three Leishmania species – L. infantum, L. major, and L. tropica – must be considered as potential etiological agents of these damages.
With these objectives in mind, features such as localization, extent, severity of oro-mucosal lesions, and duration of symptoms at the time of diagnosis were recorded from 11 patients with respect to the presence or absence of cutaneous lesions in other body parts. The biopsy samples were collected from the oro-mucosal and cutaneous lesions and were processed for further identification of the Leishmania species.
The lesions ranged from mucosal nodules without ulceration, nodules with erosion, and shallow to deep ulcerations. Leishmania major was isolated from six (55%) cases showing lesions or scars. The scars were restricted to upper and lower extremities. For the other five patients who did not display any signs of former or active cutaneous leishmaniasis, L. major, L. tropica, and L. infantum were isolated from their lesions.
In conclusion L. major, L. infantum, and L. tropica, regardless of common tropism, can be seen in mucosal tissues. However, L. major was the predominant species detected from the lesions in the nasal, gingival, and hard and soft palates, and L. tropica was isolated from the gingival and lower lip lesions. Leishmania infantum was isolated from two severe cases of deep mucosal damage displayed by the epiglottis, cricoarytenoid muscle, and laryngeal mucosa. One important finding was the association of L. major with active or scarred skin lesions.
Keywords: Oro-mucosal leishmaniasis, L. major, L. tropica, L. infantum, Tropism, Clinical features
Introduction
Leishmaniasis has been reported from many countries, and it is particularly localized in areas of tropics, subtropics, and southern Europe , in settings ranging from deserts in western Asia to rain forests in the Americas, and from rural to peri-urban areas.1 An overall prevalence of 12 million cases and occurrence of 1–1.5 million new annual cases of the disease have previously been reported.2 Leishmania species are the causative agents of a spectrum of human diseases. The disease may be clinically presented as cutaneous leishmaniasis (CL), localized leishmania lymphadenitis, diffuse multiorgan involvement (kala azar), and rarely as mucosal leishmaniasis (ML).3 Leishmaniasis is endemic in Iran and it can involve almost any organ. Mucosal leishmaniasis is a rare disease in the world, even in endemic areas such as Iran.3 It is a well-known clinical manifestation of infection that is usually manifested after infection with species belonging to the Leishmania (Viannia) subgenus in Central and South America and has occasionally been associated with infections caused by Leishmania species endemic in the Old World.4,5 Clinically or histologically, ML can be mistaken for benign and malignant lesions. Since cytology is inexpensive and easy to perform, it is the preferred primary approach to identification. The PCR-based method not only appears to be a useful and more precise diagnostic approach to the identification of suspected cases of ML with negative cytology but is also efficient in determining the species of the parasite.5 A few ML cases have previously been described in the Old World in infections caused by Leishmania tropica, Leishmania major, and Leishmania infantum,6–8 but no etiological agents of the ML form have been isolated from the patients in Iran. The objective of the present study was to report diagnosis, prognosis, tropism, clinical features and prevalence of three Leishmania species causing the primary and secondary ML in two endemic areas of Iran.
Materials and Methods
Eleven patients with mucosal lesions and without any history of immunosuppressed disease presented from different urban areas of Fars and Kerman Provinces in southern and south-eastern Iran were involved in this study. Of these patients, six had oral lesions; one showed oral and upper lip lesions; one demonstrated lower lip lesion; and the other three showed nasal involvement. Severity of the lesions was classified and scored based on the clinical appearance of the lesion.9 In five cases with oral and one case with lower lip involvement, scraping from the lesions was performed by a scalpel. A cytobrush was used in one case of nasal mucosa and both cases of laryngeal ML. Exfoliative cytology was performed by washing the nasal cavity of two patients with nasal lesions. Multiple smears were made on slides and were air dried, fixed, stained and screened for parasites. The entire smears were then scraped off the slide, with a sterile scalpel. For detection of the Leishmania-specific DNA, the DNA was extracted from the scrapping using the phenol–chloroform method as described by Noyes et al.10 The DNA samples were dissolved in 50 μl deionized distilled water and stored at 4°C. In the present study we used a PCR approach based on the method of Noyes et al. (1998). In this PCR method we amplified all constant and variable regions of L. major, L. infantum, and L. tropica kDNA with 560, 680, and 720 bp size, respectively, using CSB2 primers.9 The external primers CSB2XR (ATT TTT CGC GAT TTT CGC AGA ACG) and CSB2XF (CGA GTA GCA GAA ACT CCC GTT CA) were used in the first-round PCR and the internal primers LiR (TCG CAG AAC GCC CCT) and 13Z (ACT GGG GGT TGG TGT AAA ATAG) were applied in the second-round of PCR.10 The products of the second-round of the PCR were loaded onto a 1.5% agarose gel. The positive controls, the DNA extracted from the promastigote cultures of the reference strains of L. major (MHOM/IR/54/LV39), L. infantum (MCAN/IR/97/LON490), and L. tropica (MHOM/IR/89/ARA2), were run on each gel. The ultrapure distilled water was also run as negative control.
Results
The clinical data of the patients, nine men and two women, in the age range of 10 to 45 years are presented in Table 1. The lesions ranged from mucosal nodules without ulceration, nodules with erosion, shallow ulceration, to deep ulceration. The serological tests for human immunodeficiency virus were negative for all patients.
Table 1. Three Leishmania species are detected in lesions sampled from patients displaying nasal and oro-mucosal lesions.
| Patient No. | Age (year) | Sex | Duration of disease (year) | Location | Species | Skin lesion |
| 1* | 24 | M | 1.2 | Epiglottis, arytenoids, | L. infantum | Absent |
| laryngeal mucosa | ||||||
| 2* | 32 | M | 1.9 | Hard and soft palates, | L. major | Present |
| 3 | 45 | M | 0.3 | Upper lip, gingiva | L. tropica | Absent |
| 4–6+ (9) | 43 | M (2), F (1) | 0.4–10 | Nasal | L. major | Present |
| 7* | 26 | M | 7 | Palate | L. major | Absent** |
| 8 | 10 | F | 0.1 | Lower lip | L. tropica | Absent |
| 9 | 27 | M | 0.4 | Palate | L. major | Present |
| 10* | 34 | M | 1.5 | Laryngeal | L. infantum | Absent |
| 11 | 29 | F | 0.15 | Hard and soft palates | L. major | Present |
*Clinically malignant.
**He had a history of cutaneous lesion three years ago.
+Two of them diagnosed using exfoliative cytology.
M: male, F: female, ML: mucosal leishmaniasis.
The patients with laryngeal lesions presented with several months history of dysphonia, dyspnea, hoarseness, and odynophagia. Vocal cord paralysis (absence of abduction on phonation), with swelling and erythema of the cricoarytenoid muscle and epiglottis, was seen in direct laryngoscopy.
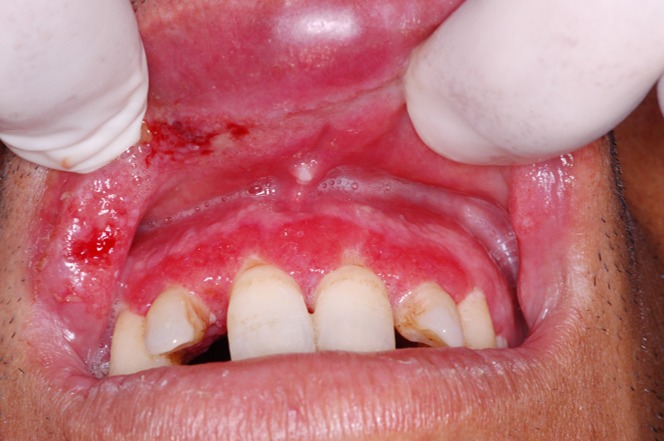
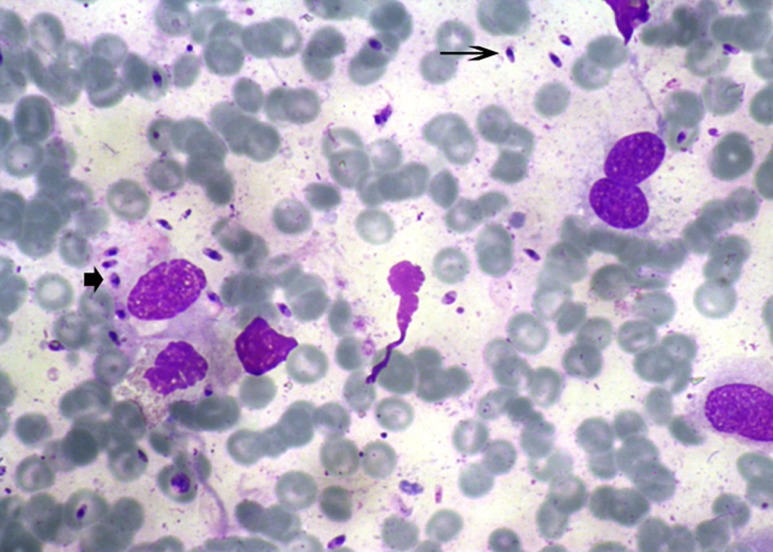
Some patients showed swelling and redness of the mucosa associated with ulceration and presence of white pseudodiphtheritic membranes on the involved mucosa (Fig. 1). The nasal lesions were located in the intranasal portion in the mucous membrane over the turbinates far from the cutaneous lesions. The amastigote forms of Leishmania were seen in the cytology smears of eight patients (Fig. 2).
Figure 1.
Swelling and redness of the mucosa associated with ulceration and presence of white pseudodiphtheritic membranes in the mucous membrane of the upper lip and gingiva caused by Leishmania major.
Figure 2.
Once air dried, smears – prepared from patient’s lesions – were stained with Wright solution and monitored for the presence of Leishmania amastigotes. Extra- (arrow) and intra-cytoplasmic (arrow head) amastigotes, ×100, Wright’s stain.
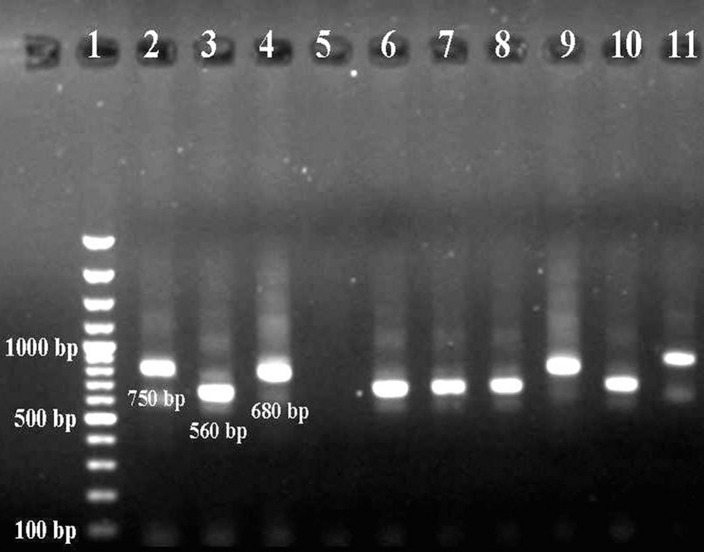
The species in seven (63.64%) of the 11 positive samples gave the 560-bp product indicative of L. major, that in two samples (18.18%) gave the 680-bp product indicative of L. infantum, and the species in two samples (18.18%) was identified as L. tropica, and gave the 750-bp second-round amplicon. No amplicon was detected in the negative-control samples (Fig. 3).
Figure 3.
Once extracted from stained smears, DNA was further processed through a nested PCR step, an agarose-based gel electrophoresis allowed the Leishmania species-specific amplicon to be detected. While in lane 5 is displayed a negative control that lacks the DNA template, from lanes 2 to 4 are shown the amplicons specific for the three Leishmania/L. species that perpetuate in Iran: L. tropica (lane 2), L. major (lane 3), and L. infantum (lane 4). From lanes 6 to 13 are shown the test sample amplicons, with L. major being identified in lanes 6, 7, 8, 10, 12, 13; L. tropica in lanes 9 and 11. In lane 1 are displayed the molecular-weight markers.
While L. major was isolated from seven cases of mucosal lesions having non-ulcerating nodules with erosion and shallow to deep ulceration in the mucous membranes of nasal, gingival, and hard and soft palates, Leishmania infantum was isolated from two cases of deep mucosal ulcers present in the epiglottis, cricoarytenoid muscle, and laryngeal mucosa. However, L. tropica was detected from the lesions present in the gingiva and lower lip of two cases having mucosal nodules and erosion.
A history of CL lesions with evidence of active cutaneous lesions or presence of scarring in skin was observed in six patients. All the cutaneous lesions of these patients were located in the lower extremities, upper extremities, and trunk whereas head and face were free of any relevant cutaneous lesions. Leishmania major was isolated from both the cutaneous and mucosal lesions of these patients. The other five patients had only mucosal lesions, but L. major, L. tropica, and L. infantum were isolated from their lesions. The duration of the disease in the present patients was variable, ranging from 30 days to 7 years. The minimum and maximum duration of disease was in the case of L. major and L. tropica, respectively (Table 1). The patients were treated by intravenous infusion of amphotericin B at 1 mg/kg/day for 14 days, and resolution of the lesions started 1 week after the treatment started.
Discussion
Mucosal leishmaniasis is a destructive disease that mainly affects the mucous membranes of the mouth and nose and does not heal spontaneously.11 In general, all clinically manifest cases of visceral leishmaniasis (VL) and ML should be treated, whereas not all cases of CL require treatment. While spontaneous healing happens in CL, ML leads to scarring and disfigurement and VL is life threatening.12 Mucosal leishmaniasis usually appears as mucosal ulcerations in the cheek, lips, soft palate, hard palate, tongue, tonsils, and larynx; however, it can affect other sites too.13 There was diversity in the clinical features of all patients. The clinical features of the disease depend on complex interactions resulting from the pathogenicity and tropism of the Leishmania species, the parasite’s invasiveness, and the host’s genetically determined immune response.14 The clinical data from this study are in support of the previous studies and confirm the possible relationship between tropism of the Leishmania species and clinical features of patients.13,14
Mucosal leishmaniasis is a disease mainly associated with L. braziliensis infection, although it has occasionally been documented in infections caused by other Leishmania species.6–8,15–21
Leishmania major or L. tropica is the causative agent of almost all the human CL in Iran, but L. infantum is the etiological agent causing VL. In the present study three species of Leishmania including L. tropica, L. major, and L. infantum causing ML in Iran have been reported. Review of the literature shows a few reports on Leishmania species causing Old and New Word ML (Table 2).6,8,16,22–34,35
Table 2. Mucosal leishmaniasis caused by different Leishmania species throughout the world.
| Reference | Location | Species | Country |
| Guerra et al.22 | Nasal, oropharynx, nasopharynx, larynx, pharynx | L. braziliensis, L. guyanensis | Brazil |
| Richter et al.23 | Nasal (1), hard palate (1), buccal (1) | L. infantum | Spain |
| Faucher et al.24 | Larynx (5), vocal cordnose (3), palate (1) | L. infantum | France |
| Kaltoft et al.25 | Larynx | L. donovani | Scandinavia |
| Sethuraman et al.26 | Lower lip, lower gum, mouth, palate | L. donovani | India |
| Motta et al.27 | Nasal, oropharynx, tongue, palate, lip | L. amazonensis | Brazil |
| Mahdi et al.8 | Oral, nasal, cheek | L. donovani | Sudan |
| Casolari et al.16 | Larynx | L. infantum | Italy |
| Lawn et al.28 | Pharyngeal, nasal mucosa (1), turbinate (2), | L. viannia | America |
| Nasal septum (3), inferior turbinate (1), post | |||
| nasal space to the piriform fossa (1), arythenoid | |||
| fold (1), right vocal cord (1) | |||
| Ahluwalia et al.29 | Nasal (2), septum (1), oropharynx (1) | L. viannia | South America |
| Weinstock et al.30 | Pharyngeal mucosa | L. tropica | Afghanistan |
| Morsy et al.6 | Not available (2 cases) | L. tropica | Saudi Arabia |
| Ghalib et al.31 | Oral and nasal | L. donovani, L. major | Sudan |
| Alvar32 et al. | Nasal | L. infantum | Spain |
| Osorio33 et al. | Nasal, oral, lip and larynx | L. panamensis | Colombia |
| Naiff34 et al. | Nasal | L. guyanensis | Brazil |
| Shirian35 et al. | Nasal and oral | Mixed (L. major, L. tropica) | Iran |
| Present study | Table 1 | L. major, L. tropica, L. infantum | Iran |
Mucosal L. tropica infection has been reported in Saudi Arabia,6 whereas L. major and occasionally L. infantum have been isolated from the patients with ML in Sudan31 and Europe,36 respectively.
The nasal mucosa is the preferential site for the lesions caused by L. braziliensis in ML, and the lesions may spread beyond the nasal mucosa to the upper airways and the digestive tract such as larynx and pharynx of humans.37 Oral involvement in leishmaniasis is rare and unusual and in most cases it becomes evident after several years of resolution of the original cutaneous lesions. Classically, the oral lesions appear as ulcerations, mainly in the hard or soft palates.27 The results of the present investigation show that the mucosa of the nasal, and hard and soft palates are the metastatic locations for L. major, whereas, the laryngeal mucosa and lips are the primary locations for L. infantum and L. tropica. Therefore, L. major is possibly the causative agent of secondary ML, but L. infantum and L. tropica may result in primary ML.
In four patients, a malignant tumor was clinically suspected. It has been stated that the lesions of ML may also present as nodular, exophytic, indurated and generally red or purple in colour that can mimic a malignant lesion and manifest as a tumor, polypoid lesion or granulomatous inflammation.13 Leishmania major and L. infantum were isolated from the four patients in whom a malignant tumor was clinically suspected. These four patients had suffered for more than one year from leishmaniasis. The severity of the lesions in the patients with ML caused by L. major and L. infantum was more than that caused by L. tropica. Duration of the disease was another key factor in determining the severity of the lesions and conspicuous tissue destruction was seen in the patients who presented with a long history of mucosal lesions.
Five patients with ML in the present study did not relate a preceding skin lesion and other six were associated with CL. The association between ML and previous skin lesions is widely accepted, as both forms can be caused by a single species.22 It has been shown that localization of the parasites in the nasal, oral, and pharyngeal mucosa results from migration of the Leishmania via lymphatic or by hematogenous dissemination of the amastigotes from the skin of 5% of the patients affected with CL or it occurs in those persons without a former history of cutaneous lesions.26 For those cases associated with CL it is important to know the time of evolution of these skin lesions, especially those healed, because there is a direct relationship between the time of appearance of skin diseases and mucosal lesions. In most cases, ML becomes apparent some years or even decades after resolution of the preceding cutaneous lesions, but it can also develop while the skin lesions are still present.38 It has also been stated that the ML usually evolves during the course of several years before the patient seeks medical attention.26 This may explain why in some patients of the present study the skin lesions were not observed. Although the reason why ML subsequently develops only in a few patients with CL is unknown, Guddo et al. (2005) indicated that the primary ML may occur in both immunocompetent and immunosuppressed patients.39
Based on the present findings, L. major, L. infantum, and L. tropica, regardless of common tropism, can be seen in mucosal tissues. However, L. major was the predominant species detected from the lesions in the nasal, gingival, and hard and soft palates, and L. tropica was isolated from the gingival and lower lip lesions. Leishmania infantum was isolated from two severe cases of deep mucosal damaging processes displayed by the epiglottis, cricoarytenoid muscle, and laryngeal mucosa. One important finding was the association of L. major with active or scarred skin lesions. On the other hand, the metastatic ML has occurred as the result of L. major infection, but the primary ML has happened due to the L. tropica and L. infantum infection. In the endemic areas of the Old World leishmaniasis, early diagnosis of ML should be the main concern of the physicians to prevent severe tissue destruction in the patients with long duration of mucosal lesions. In such areas any nodules, erosion, ulceration and granulomatous lesion in the mucous membrane of nasal and oro-mucosal areas should firstly be considered as ML and relevant diagnostic tests such as PCR or immunodiagnosis be applied for its detection.
Funding
None
Conflict of interest
None declared.
Ethical approval
The Ethics Committee of the Medical School, Shiraz University, and the authors' institutional review board of Dr Daneshbod Laboratory approved the study and the author group collected written informed consent from all study participants.
Acknowledgments
The authors would like to thank the Veterinary School of Shiraz University, the Medical School of Shiraz University of Medical Sciences, and Institute of Experimental Pathology, University of Münster, Germany, for their support. We also would like to thank Dr M. Davarmanesh, Dr M. M. Davarpanah, Dr M. Khanlari, Dr A. Saeedzadeh, and Dr G. Randau, Dr T. Rozhdestvensky, and Prof. J. Brosius from Institute of Experimental Pathology, University of Münster, Germany, for their help and advice.
References
- 1.Herwaldt BL. Leishmaniasis. Lancet. 1999;354:1191–9. doi: 10.1016/S0140-6736(98)10178-2. [DOI] [PubMed] [Google Scholar]
- 2.Desjeux P. Leishmaniasis: current situation and new perspectives. Comp Immunol Microbiol Infect Dis. 2004;27:305–18. doi: 10.1016/j.cimid.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Daneshbod Y, Oryan A, Davarmanesh M, Shirian S, Negahban S, Aledavood A, et al. Clinical, histopathologic, and cytologic diagnosis of mucosal leishmaniasis and literature review. Arch Pathol Lab Med. 2011;135:478–82. doi: 10.5858/2010-0069-OA.1. [DOI] [PubMed] [Google Scholar]
- 4.Dedet JP, Pratlong F.Leishmaniasis. In: Cook GC, Zumla AI (eds.), Manson’s tropical diseases. 22nd ed. London: Saunders; 2009:1341–1365. [Google Scholar]
- 5.Shirian S, Oryan A, Hatam GR, Daneshbod K, Daneshbod Y. Molecular diagnosis and species identification of mucosal leishmaniasis in Iran and correlation with cytological findings. Acta Cytol. 2012;56:304–9. doi: 10.1159/000337450. [DOI] [PubMed] [Google Scholar]
- 6.Morsy TA, Khalil NM, Salama MM, Hamdi KN, al Shamrany YA, Abdalla KF. Mucosal leishmaniasis caused by Leishmania tropica in Saudi Arabia. J Egypt Soc Parasitol. 1995;25:73–9. [PubMed] [Google Scholar]
- 7.Kharfi M, Fazaa B, Chaker E, Kamoun MR. Mucosal localization of leishmaniasis in Tunisia: 5 cases. Ann Dermatol Vénéréol. 2003;130:27–30. [PubMed] [Google Scholar]
- 8.Mahdi M, Elamin EM, Melville SE, Musa AM, Blackwell JM, Mukhtar MM, et al. Sudanese mucosal leishmaniasis: isolation of a parasite within the Leishmania donovani complex that differs genotypically from L. donovani causing classical visceral leishmaniasis. Infect Genet Evol. 2005;5:29–33. doi: 10.1016/j.meegid.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 9.Lessa HA, Lessa MM, Guimarães LH, Lima CM, Arruda S, Machado PR, et al. A proposed new clinical staging system for patients with mucosal leishmaniasis. Trans R Soc Trop Med Hyg. 2012;106:376–81. doi: 10.1016/j.trstmh.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noyes HA, Reyburn H, Bailey JW, Smith D. A nested PCR-based schizodeme method for identifying Leishmania kinetoplast minicircle classes directly from clinical samples and its application to the study of the epidemiology of Leishmania tropica in Pakistan. J Clin Microbiol. 1998;36:2877–81. doi: 10.1128/jcm.36.10.2877-2881.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaudhry Z, Barrett AW, Corbett E, French PD, Zakrzewska JM. Oral mucosal leishmaniasis as a presenting feature of HIV infection and its management. J Oral Pathol Med. 1999;28:43–6. doi: 10.1111/j.1600-0714.1999.tb01993.x. [DOI] [PubMed] [Google Scholar]
- 12.Nylén S, Eidsmo L. Tissue damage and immunity in cutaneous leishmaniasis. Parasite Immunol. 2012;34:551–61. doi: 10.1111/pim.12007. [DOI] [PubMed] [Google Scholar]
- 13.Aliaga L, Cobo F, Mediavilla JD, Bravo J, Osuna A, Amador JM, et al. Localized mucosal Leishmaniasis due to Leishmania (Leishmania) infantum: clinical and microbiologic findings in 31 patients. Medicine. 2003;82:147–58. doi: 10.1097/01.md.0000076009.64510.b8. [DOI] [PubMed] [Google Scholar]
- 14.Pearson RD, Sousa AQ, Jeronimo SMB.Cutaneous and mucosal leishmaniasis. In: Gerald L, Mandell JE, Bennett RD (eds.), Mandell, Douglas and Bennett’s Principles and Practice of Infectious Diseases. Vol 5. 5th ed. Philadelphia: Churchill Livingstone; 2000. p. 2831–41 [Google Scholar]
- 15.Van Damme PA, Keuter M, Van Assen S, DeWilde PCM, Beckers JA. A rare case of oral leishmaniasis. Lancet Infect Dis. 2004;4:53. doi: 10.1016/s1473-3099(03)00861-2. [DOI] [PubMed] [Google Scholar]
- 16.Casolari C, Guaraldi G, Pecorari M, Tamassia G, Cappi C, Fabio G, et al. A rare case of localized mucosal leishmaniasis due to Leishmania infantum in an immunocompetent Italian host. Eur J Epidemiol. 2005;20:559–61. doi: 10.1007/s10654-005-1249-7. [DOI] [PubMed] [Google Scholar]
- 17.Cobo F, Aliaga L, Talavera P, Concha A. The histological spectrum of non-granulomatous localized mucosal leishmaniasis caused by Leishmania infantum. Ann Trop Med Parasitol. 2007;101:689–94. doi: 10.1179/136485907X229095. [DOI] [PubMed] [Google Scholar]
- 18.Garcia de Marcos JA, Dean Ferrer A, Granados FA, Ruiz Masera JJ, Cortés Rodríguez B, Vidal Jiménez A, et al. Localized leishmaniasis of the oral mucosa. A report of three cases. Med Oral Patol Oral Cir Bucal. 2007;12:281–6. [PubMed] [Google Scholar]
- 19.Benmously-Mlika R, Fenniche S, Kerkeni N, Aoun K, Khedim A, Mokhtar I. Primary Leishmania infantum MON-80 endonasal leishmaniasis in Tunisia. Ann Dermatol Vénéréol. 2008;135:389–92. doi: 10.1016/j.annder.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 20.El Fékih N, Sliti N, Kharfi M, Trabelsi S, Khaled S, Fazaa B, et al. Mucosal leishmaniasis by contiguity with a skin lesion: another case report from Tunisia. Med Trop. 2008;68:634–6. [PubMed] [Google Scholar]
- 21.Pau M, Azzori L, Aste N, Aste N. Two cases of primary endonasal leishmaniasis in Sardinia (Italy). Dermatol Online J. 2009;15:5. [PubMed] [Google Scholar]
- 22.Guerra JA, Prestes SR, Silveira H, Coelho LI, Gama P, Moura A, et al. Mucosal leishmaniasis caused by Leishmania (Viannia) braziliensis and Leishmania (Viannia) guyanensis in the Brazilian Amazon. PLoS Negl Trop Dis. 2011;5(3):E980. doi: 10.1371/journal.pntd.0000980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richter J, Hanus I, Häussinger D, Löscher T, Harms G. Mucosal Leishmania infantum infection. Parasitol Res. 2011;109:559–62. doi: 10.1007/s00436-011-2356-x. [DOI] [PubMed] [Google Scholar]
- 24.Faucher B, Pomares C, Fourcade S, Benyamine A, Marty P, Pratlong L, et al. Mucosal Leishmania infantum leishmaniasis: Specific pattern in a multicentre survey and historical cases. J Infect. 2011;63:76–82. doi: 10.1016/j.jinf.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 25.Kaltoft M, Munch-Petersen HR, Møller H. Leishmaniasis isolated to the larynx as cause of chronic laryngitis. Ugeskr Laeger. 2010;17:2898–9. [PubMed] [Google Scholar]
- 26.Sethuraman G, Sharma VK, Salotra P. Indian mucosal leishmaniasis due to Leishmania donovani infection. N Engl J Med. 2008;358:313–5. doi: 10.1056/NEJMc072391. [DOI] [PubMed] [Google Scholar]
- 27.Motta ACF, Lopes MA, Ito FA, Carlos-Bregni R, de Almeida OP, Roselino AM. Oral leishmaniasis: a clinicopathological study of 11 cases. Oral Dis. 2007;13:335–40. doi: 10.1111/j.1601-0825.2006.01296.x. [DOI] [PubMed] [Google Scholar]
- 28.Lawn SD, Whetham J, Chiodini PL, Kanagalingam J, Watson J, Behrens RH, et al. New world mucosal and cutaneous leishmaniasis: an emerging health problem among British travellers. QJM. 2004;97:781–8. doi: 10.1093/qjmed/hch127. [DOI] [PubMed] [Google Scholar]
- 29.Ahluwalia S, Lawn SD, Kanagalingam J, Grant H, Lockwood DNJ. Mucocutaneous leishmaniasis: an imported infection among travellers to central and South America. BMJ. 2004;329:842–4. doi: 10.1136/bmj.329.7470.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weinstock C, Knobloch J, Schultheis W, Northoff H. Impaired production of cytokines in a case of human leishmaniasis. Clin Infect Dis. 1997;25:1334–9. doi: 10.1086/516123. [DOI] [PubMed] [Google Scholar]
- 31.Ghalib HW, Eltoum EA, Kroon CCM, El Hassan AM. Identification of Leishmania from mucosal leishmaniasis by recombinant DNA probes. Trans R Soc Trop Med Hyg. 1992;86:158–60. doi: 10.1016/0035-9203(92)90549-r. [DOI] [PubMed] [Google Scholar]
- 32.Alvar J, Ballesteros JA, Soler R, Benito A, van Eys GJ, Schoone GJ, et al. Mucocutaneous leishmaniasis due to Leishmania (Leishmania) infantum: biochemical characterization. Am J Trop Med Hyg. 1990;43:614–8. doi: 10.4269/ajtmh.1990.43.614. [DOI] [PubMed] [Google Scholar]
- 33.Osorio LE, Castillo CM, Ochoa MT. Mucosal leishmaniasis due to Leishmania (Viannia) panamensis in Colombia: clinical characteristics. Am J Trop Med Hyg. 1998;59:49–52. doi: 10.4269/ajtmh.1998.59.49. [DOI] [PubMed] [Google Scholar]
- 34.Naiff RD, Talhari S, Barrett TV. Isolation of Leishmania guyanensis from lesions of the nasal mucosa. Mem Inst Oswaldo Cruz. 1998;83:529–30. doi: 10.1590/s0074-02761988000400022. [DOI] [PubMed] [Google Scholar]
- 35.Shirian S, Oryan A, Hatam GR, Daneshbod Y. Mixed mucosal leishmaniasis caused by L. tropica and L. major and brief literature review. J Clin Microbiol. 2012;50:3805–8. doi: 10.1128/JCM.01469-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harms G, Schönian G, Feldmeier H. Leishmaniasis in Germany. Emerg Infect Dis. 2003;9:872–5. doi: 10.3201/eid0907.030023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lessa H, Carvalho EM, Marsden PD. Eustachian tube blockage with consequent middle ear infection in mucosal leishmaniasis. Rev Soc Bras Med Trop. 1994;27:103. doi: 10.1590/s0037-86821994000200008. [DOI] [PubMed] [Google Scholar]
- 38.Marsden PD. Mucosal leishmaniasis due to Leishmania (Viannia) braziliensis L(V)b in Três Braços, Bahia-Brazil. Rev Soc Bras Med Trop. 1994;27:93–101. doi: 10.1590/s0037-86821994000200007. [DOI] [PubMed] [Google Scholar]
- 39.Guddo F, Gallo E, Cillari E, La Rocca AM, Moceo P, Leslie K, et al. Detection of Leishmania infantum kinetoplast DNA in laryngeal tissue from an immunocompetent patient. Hum Pathol. 2005;36:1140–2. doi: 10.1016/j.humpath.2005.07.006. [DOI] [PubMed] [Google Scholar]