Abstract
Background:
We previously demonstrated, in a rat anterior cruciate ligament (ACL) graft reconstruction model, that the delayed application of low-magnitude-strain loading resulted in improved tendon-to-bone healing compared with that observed after immediate loading and after prolonged immobilization. The purpose of this study was to determine the effect of higher levels of strain loading on tendon-to-bone healing.
Methods:
ACL reconstruction was carried out in a rat model in three randomly assigned groups: high-strain daily loading beginning on either (1) postoperative day one (immediate-loading group; n = 7) or (2) postoperative day four (delayed-loading group; n = 11) or (3) after prolonged immobilization (immobilized group; n = 8). Animals were killed two weeks after surgery and micro-computed tomography (micro-CT) and biomechanical testing of the bone-tendon-bone complex were carried out.
Results:
The delayed-loading group had greater tissue mineral density than either the immediate-loading or immobilized group (mean [and standard deviation], 813.0 ± 24.9 mg/mL compared with 778.4 ± 32.6 mg/mL and 784.9 ± 26.4 mg/mL, respectively; p < 0.05). There was a trend toward greater bone volume per total volume fraction in both the immobilized and the delayed-loading group compared with the immediate-loading group (0.24 ± 0.03 and 0.23 ± 0.06 compared with 0.20 ± 0.05; p = 0.06). Trabecular thickness was greater in the immobilized group compared with the immediate-loading group (106.5 ± 23.0 μm compared with 72.6 ± 10.6 μm; p < 0.01). There were no significant differences in failure load or stiffness between the immobilized group and either high-strain cyclic-loading group.
Conclusions:
Immediate application of high-strain loading appears to have a detrimental effect on healing in this rat model. Any beneficial effects of delayed loading on the healing tendon-bone interface (after a brief period of immobilization) may be offset by the detrimental effects of excessive strain levels or by the detrimental effects of stress deprivation on the graft.
Clinical Relevance:
The timing and magnitude of mechanical load on a healing rat ACL reconstruction graft may have important implications for postoperative rehabilitation. Avoidance of exercises that cause high graft strain in the early postoperative period may lead to improved tendon-to-bone healing in humans.
The healing of tendon to bone does not recapitulate the native architecture of the enthesis. Rather, an interposed layer of fibrovascular scar tissue is formed that is biomechanically inferior to the native enthesis and that may predispose tendon-to-bone repairs to an increased rate of failure1-7. While anterior cruciate ligament (ACL) reconstruction generally has favorable results8, incomplete graft-bone healing can lead to graft slippage, resulting in laxity and knee instability. Recent studies have identified unsatisfactory results in up to 25% of patients secondary to residual laxity and the persistence of a postoperative pivot shift9,10. Although many factors contribute to the long-term outcome of ACL reconstruction, secure tendon-to-bone healing is required for a functional graft4,6.
There is growing evidence that modulation of the mechanical environment has a critical effect on the healing graft attachment site and its ultimate mechanical integrity11-14. In previous animal studies, our group demonstrated that the delayed onset of low levels of controlled mechanical stimulation (∼2% cyclic axial strain) resulted in improved mechanical and biological parameters of tendon-to-bone healing compared with those found after immediate loading or strict immobilization14. However, the magnitude of mechanical stimulation required for optimal healing of the tendon-bone insertion site is currently undefined. A recent study revealed that the delayed application of postoperative exercise (with presumed higher levels of mechanical load) resulted in a decreased range of motion and worse mechanical properties, compared with those seen after normal cage activity, in a rat rotator cuff repair model15. However, the mechanical load was not directly controlled or quantified. Both the timing and the intensity of postoperative rehabilitation after ACL reconstruction may have important consequences with respect to the healing graft as well as the long-term outcomes of the operation.
The purpose of the present study was to determine in a rat model the effect of high levels of controlled axial loading after ACL reconstruction on tendon-to-bone healing. Our hypothesis was that the delayed onset of loading would lead to improved healing (new-bone formation at the tendon-bone interface) and increased graft load to failure compared with those found after either immediate loading or prolonged immobilization.
Materials and Methods
Approval was obtained from our Institutional Animal Care and Use Committee before this study was undertaken. Seventy male Sprague-Dawley rats (weight range, 300 to 380 g; age, less than three months) underwent ACL reconstruction with a flexor digitorum longus autograft14,16. The knees were immobilized with an external fixator. The animals were randomly assigned to (1) prolonged immobilization (immobilized group), (2) immediate daily high-strain loading beginning on postoperative day one (immediate-loading group), or (3) delayed onset of high-strain loading beginning on postoperative day four (delayed-loading group). Thirty-five animals needed to be killed prematurely due to fracture (n = 24), anesthesia-related death (n = 4), or wound complications (n = 7). The remaining thirty-five animals were killed on postoperative day fourteen and were imaged with micro-computed tomography (micro-CT). The femur-graft-tibia dissections prior to biomechanical testing revealed that nine grafts had already failed, with five of these in the immediate-loading group. Biomechanical testing was performed on the remaining twenty-six animals. The final groups consisted of (1) eight animals subjected to prolonged immobilization; (2) seven, to immediate daily high-strain loading; and (3) eleven, to delayed high-strain loading. A power analysis demonstrated that ten specimens per group would be required to detect a clinically relevant difference in load to failure.
Daily Loading Protocol
The animals were anesthetized with 2% isoflurane inhalation, and loading was accomplished by attaching the external fixator (both tibial and femoral components) to a computer-controlled joint fixation/distraction system17. The fixator bar was removed, allowing for distraction or compression of the knee joint along the long axis of the graft (see Appendix) by a stepper motor, thus resulting in application of axial strain to the graft. Custom-designed software allowed input of a target displacement, load limit, frequency, and number of cycles. The software also graphed and recorded the load-displacement response of the knee joint for each loading session (see Appendix). A cadaver study was performed with use of an optical kinematic tracking system (ProReflex MCU; Qualisys, Gothenburg, Sweden) to calculate the compliance of the external fixator-loading system complex and to calibrate the distraction device17.
In the treatment groups, the daily loading protocol was initiated on either postoperative day one (immediate-loading group) or postoperative day four (delayed-loading group). The joint was then distracted at 0.24 mm/sec until the target displacement (10% of the average graft length) or the load limit (2400 g) was reached and then was returned to the starting position. The load limit had been determined in the cadaver study to be the maximal safe load to minimize the risk of iatrogenic tibial or femoral fracture or graft rupture17. Loading was repeated for fifty cycles daily. After the loading protocol was completed, the fixation bar was reattached to the external fixator and the animal resumed normal cage activity. The immobilized group of animals was allowed normal cage activity with the operatively treated limb immobilized by the external fixator throughout the study period. A clinical examination of all animals was performed daily. Behavioral change or local symptoms such as swelling, wound dehiscence, and drainage were used to diagnose a postoperative infection. Animals with postoperative infection were killed and excluded from the study.
All animals were imaged (Faxitron, model number MX-20 DC4; Faxitron X-ray Corporation, Wheeling, Illinois) on postoperative days one, four, seven, ten, and fourteen to monitor for fracture and physeal separation. Animals with a fracture or with physeal separation were killed. On postoperative day fourteen, all remaining animals were killed with carbon dioxide inhalation and were stored at −80°C.
Micro-CT Analysis
Trabecular architecture and new-bone formation along the tendon-bone interface in the tibial tunnel were assessed with use of micro-CT (MS-8 Small Specimen Scanner; Enhanced Vision Systems, London, Ontario, Canada). Twelve hours prior to imaging, the specimens were thawed to 0°C. A gross dissection was then performed to excise the foot, the fibula, and all soft tissue except for the knee joint. The remainder of the limb was then placed in a saline solution bath and the tibial tunnels were scanned (Fig. 1).
Fig. 1.
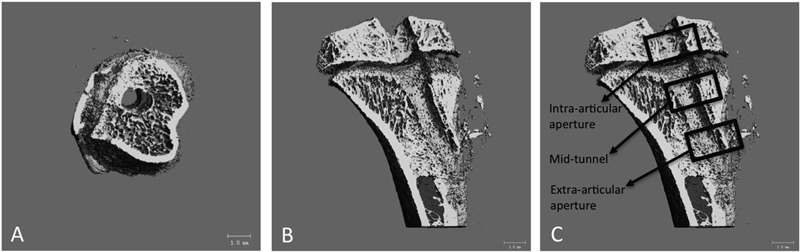
Axial (Fig. 1-A) and sagittal (Fig. 1-B) micro-CT images of the tibial tunnel as well as a sagittal image (Fig. 1-C) demonstrating the intra-articular aperture, mid-tunnel, and extra-articular aperture regions. The micro-CT scans were performed at 80 V and 80 mA. The scans included a phantom containing air, saline solution, and a bone reference material for calibration of Hounsfield units to tissue mineral density. A global threshold was determined for each specimen on the basis of the histogram of CT attenuation values based on the Otsu discriminant and used to distinguish bone voxels in the images. The Otsu method is a mathematical transformation of a gray-scale image to a binary (either black or white) image33. The tibial tunnels were scanned and reconstructed at 22.5-μm isotropic resolution. The mineral distribution along the graft was determined from the CT value for each voxel in the micro-CT scan. An SB3 standard (1100 g of hydroxyapatite/cc) was used to calibrate bone mineral density in each scan. Bone voxel threshold limits were used to differentiate new bone (300 to 400) from mature bone (401 to 1000).
Six outcome measures were evaluated: bone volume (mm3), new and total tissue mineral density (mg/mL), average trabecular thickness (μm); average trabecular number, and average trabecular spacing (μm). The volume of interest was a cylinder with a radius of 1.7 mm, which included the 0.7-mm tunnel and 1 mm of surrounding trabecular bone. The length of each tunnel was individually measured on the micro-CT images. The tunnel was then divided into three equal length subregions: intra-articular, mid-tunnel, and extra-articular. The bone volume is the total number of thresholded bone voxels within the total volume of the cylindrical volume of interest. The total bone mineral content and bone volume fraction (bone volume/total volume) were calculated for a volume of interest centered along the graft tunnel for the entire length of each graft. The outcome measures were calculated for the entire tunnel as well as for the three regions of interest. After the imaging was complete, the specimens were wrapped in saline-solution-soaked gauze and stored at −80°C.
Biomechanical Testing
The femur-graft-tibia construct was thawed to room temperature, and all soft tissue was dissected under a microscope, with only the graft crossing the knee preserved. The tibia and femur were potted in cement (Bondo; 3M Bondo, Atlanta, Georgia). Specimens were mounted on a custom-designed tensile testing apparatus that allowed for distraction parallel to the long axis of the graft. A preload of 0.2 N was applied, and the specimens were preconditioned for five cycles between 0 and 0.2 N. The specimens were loaded in uniaxial tension at a rate of 167 μm/sec until graft failure. The ultimate load was obtained from the highest load recorded on the load-deformation curve. Stiffness was calculated from the linear portion of this curve, with use of Microsoft Office Excel 2002 (Microsoft, Redmond, Washington).
Statistical Analysis
Statistical analysis comparing the micro-CT and biomechanical data among groups was performed with use of a two-way analysis of variance (ANOVA) followed by the post hoc Tukey test. Significance was set at p < 0.05.
Source of Funding
This study was financially supported by National Institutes of Health Grant R01 AR053689-01A1.
Results
In the immediate-loading group, which underwent thirteen days of loading, the target displacement was achieved on a mean of 5.1 days and the load limit was reached on a mean of 7.9 days (see Appendix). In the delayed-loading group (ten days of loading), the target displacement was achieved on a mean of 4.9 days and the load limit was reached for a mean 5.1 days.
Micro-CT
Regional tunnel analysis revealed that the delayed-loading group had greater tissue mineral density compared with either the immediate-loading or the immobilized group at the extra-articular region (mean [and standard deviation], 813.0 ± 24.9 mg/mL versus 778.4 ± 32.6 mg/mL and 784.9 ± 26.4 mg/mL, respectively; p < 0.05) (Table I). There was a trend toward greater bone volume/total volume (p = 0.06) in both the immobilized (0.24 ± 0.03) and delayed-loading (0.23 ± 0.06) groups compared with the immediate-loading group (0.20 ± 0.05) (Fig. 2). Trabecular thickness in the mid-tunnel region was greater in the immobilized group (106.5 ± 23.0 μm) than in the immediate-loading group (72.6 ± 10.6 μm; p < 0.01). Tissue mineral density in the tibial tunnels was greater in both loading groups (immediate and delayed) than it was in the immobilized animals (589.7 ± 14.8 mg/mL and 586.5 ± 12.7 mg/mL, respectively, versus 571.0 ± 3.6 mg/mL; p < 0.05). Analyses did not show any significant differences for bone volume (p = 0.241), new-bone volume (p = 0. 331), trabecular spacing (extra-articular: p = 0.723, mid-tunnel: p = 0.331, intra-articular: p = 0.542), or trabecular number (extra-articular: p = 0.538, mid-tunnel: p = 0.272, intra-articular: p = 0.317).
TABLE I.
Summary of Micro-CT Data for Tibial Tunnels of the Three Groups*
| Immobilized | Immediate Loading | Delayed Loading | |
| Tissue mineral density† (mg/mL) | |||
| Entire tunnel (new bone) | 571.0 ± 3.6 | 589.7 ± 14.8‡ | 586.5 ± 12.7‡ |
| Entire tunnel (total bone) | 819.4 ± 26.8 | 827.0 ± 17.2 | 837.9 ± 29.2 |
| Extra-articular aperture | 784.9 ± 26.4 | 778.4 ± 32.6 | 813.0 ± 24.9‡§ |
| Mid-tunnel | 794.7 ± 32.7 | 776.3 ± 35.0 | 792.0 ± 45.8 |
| Intra-articular aperture | 837.3 ± 65.6 | 845.4 ± 45.9 | 852.6 ± 52.8 |
| Bone volume/total volume (total bone) | |||
| Entire tunnel | 0.24 ± 0.03 | 0.20 ± 0.05 | 0.23 ± 0.06 |
| Extra-articular aperture | 0.18 ± 0.06 | 0.22 ± 0.26 | 0.15 ± 0.08 |
| Mid-tunnel | 0.15 ± 0.07 | 0.13 ± 0.07 | 0.10 ± 0.06 |
| Intra-articular aperture | 0.27 ± 0.12 | 0.24 ± 0.07 | 0.21 ± 0.10 |
| Bone volume/total volume (new bone only) | |||
| Entire tunnel | 0.13 ± 0.02 | 0.11 ± 0.02 | 0.12 ± 0.03 |
| Extra-articular aperture | 0.069 ± 0.020 | 0.057 ± 0.022 | 0.069 ± 0.031 |
| Mid-tunnel | 0.069 ± 0.030 | 0.057 ± 0.023 | 0.066 ± 0.027 |
| Intra-articular aperture | 0.090 ± 0.030 | 0.071 ± 0.025 | 0.079 ± 0.021 |
| Trabecular number (avg./mm) | |||
| Extra-articular aperture | 2.67 ± 0.47 | 2.49 ± 0.48 | 2.95 ± 1.20 |
| Mid-tunnel | 3.07 ± 0.65 | 2.47 ± 0.60 | 2.88 ± 0.80 |
| Intra-articular aperture | 3.62 ± 0.78 | 3.16 ± 1.15 | 3.19 ± 1.11 |
| Trabecular thickness (μm) | |||
| Extra-articular aperture | 99.0 ± 21.8 | 83.8 ± 16.0 | 99.5 ± 24.0 |
| Mid-tunnel | 106.5 ± 23.0 | 72.6 ± 10.6# | 89.7 ± 21.6 |
| Intra-articular aperture | 143.8 ± 27.9 | 120.6 ± 25.4 | 127.8 ± 27.9 |
The values are given as the mean and standard deviation.
New bone was defined as the bone voxel threshold limit of 300 to 400. Total bone was defined as the bone voxel threshold limit of 300 to 1000.
Significantly different from immobilized group (p < 0.05).
Significantly different from immediate-loading group (p < 0.05).
Significantly different from immobilized group (p < 0.01).
Fig. 2.
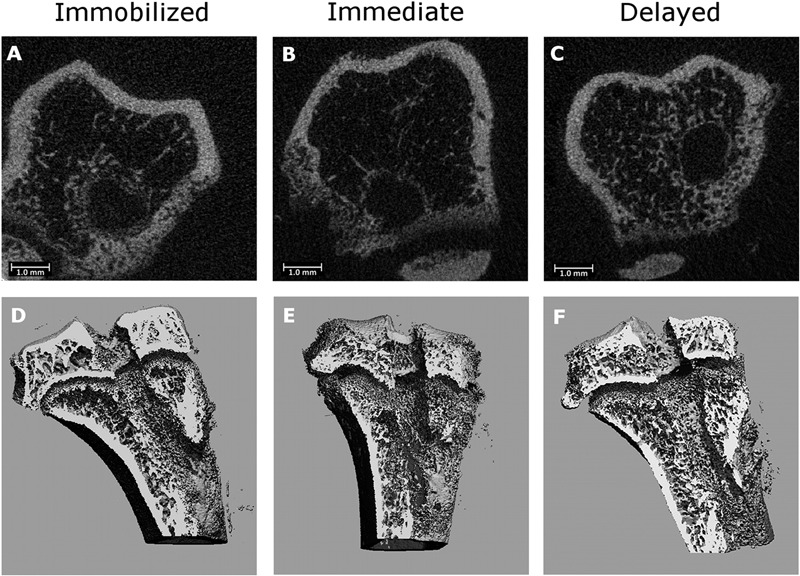
Representative axial micro-CT images of the tibial tunnel after immobilization (Fig. 2-A), immediate high-strain loading (Fig. 2-B), and delayed high-strain loading (Fig. 2-C). Three-dimensional reconstructions of the tibial tunnel after immobilization (Fig. 2-D), immediate high-strain loading (Fig. 2-E), and delayed high-strain loading (Fig. 2-F). There is greater bone formation in the immobilized and delayed-loading groups than in the immediate-loading group.
Biomechanical Testing
There were no significant differences in failure load (p = 0.366) or stiffness (p = 0.247) between the immobilized group and either loading group (Fig. 3). The mean load to failure in the immobilized, immediate-loading, and delayed-loading groups was 7.8 ± 2.3 N, 6.5 ± 1.6 N, and 6.4 ± 2.4 N, respectively. The mean stiffness of the tibia-ACL graft-femur construct in the immobilized group, immediate-loading, and delayed-loading groups was 3.0 ± 0.3 N/mm, 3.5 ± 1.3 N/mm, and 4.2 ± 1.6 N/mm, respectively.
Fig. 3.
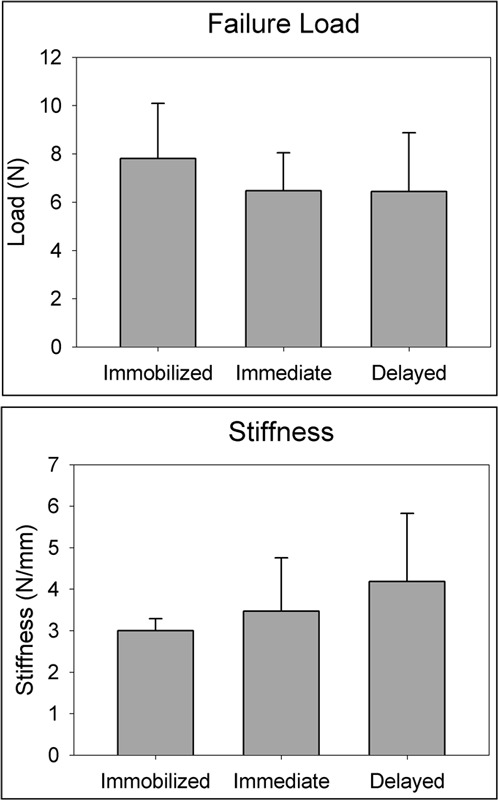
Biomechanical testing results. No significant differences were detected among the immobilized (n = 8), immediate-loading (n = 7), and delayed-loading (n = 11) groups with regard to either load to failure (top) or stiffness (bottom).
Discussion
The aim of the current study was to investigate the effect of high levels of mechanical strain on tendon-to-bone healing after ACL reconstruction. We used an established animal model of ACL reconstruction and a novel in vivo loading system to apply a daily, controlled axial strain to the healing graft. We chose to examine graft strain because it has strong clinical relevance, as the different ACL graft tunnel positions that are being used have varying degrees of non-isometry, which results in varying levels of strain on the healing ACL graft. Identification of the optimal strain regimen for a healing graft will ultimately have strong relevance for postoperative rehabilitation design. Our hypothesis was partially supported by our data, suggesting that delayed mechanical stimulation improved trabecular bone remodeling compared with that seen after immediate loading. However, neither group had significantly improved biomechanical or microstructural properties compared with the prolonged-immobilization group. While previous studies in our laboratory showed that the biomechanical properties of tendon-to-bone healing were better after delayed application of 2% axial strain than they were in the immediate-loading and immobilized groups14, the increase to 10% axial strain in the present study did not yield similar results. This suggests that the beneficial effects of delayed loading after a brief period of immobilization may have been offset by the detrimental effects of excessive graft-strain levels.
The micro-CT and biomechanical testing data following high-strain loading in the current study can be compared with our group’s data from animals that received low-strain loading (2%) in an otherwise identical model14. Although there are limitations regarding the conclusions that can be drawn with the use of historical data, there were no differences in the micro-CT data between these groups. There were also no significant differences between the high and low-strain delayed-loading groups with regard to stiffness or load to failure.
There is substantial evidence that normal bone-tendon attachment-site morphology is not recapitulated during healing1-7. The native insertion site contains four distinct types of tissue: tendon, unmineralized fibrocartilage, mineralized fibrocartilage, and bone. However, following ligament reconstruction or tendon repair, the structure and composition of the graft attachment site are replaced by disorganized scar. The global hypothesis of our laboratory’s ongoing work is that, while initial healing depends on biological signals such as cytokines12,18-21, mechanical load is of paramount importance for subsequent remodeling of the healing attachment site.
It has been well established that mechanical stimuli are essential for the normal maintenance of ligament, tendon, and bone integrity22-29. Stress deprivation decreases collagen organization and ultimate tissue strength27. However, the relationship between mechanical stress and healing of a tendon-bone insertion site has not been as well established. Recent studies have shown that application of load in the immediate postoperative period impairs tendon-to-bone healing11,13,14,30. Thomopoulos et al. demonstrated that, after rotator cuff surgery, immobilized animals had superior tendon-to-bone healing than an early-exercise group13. Sakai et al. found that immediate postoperative immobilization of rabbits after ACL reconstruction resulted in improved healing and graft attachment strength compared with a group with normal postoperative cage activity30. In previous work from our group, delayed application of cyclic axial load after ACL reconstruction resulted in improved mechanical and biological parameters of tendon-to-bone healing compared with those seen after immediate loading or prolonged immobilization of the knee14. The results of the current study are partially consistent with these findings, as we found that the mean bone volume of the entire tibial tunnel was higher in both the immobilized group and the delayed-loading group when compared with the immediate-loading group (p = 0.06). However, these differences did not result in an improvement in the biomechanical parameters.
Despite this growing evidence to support delayed initiation of mechanical stimulation of a healing tendon-to-bone site, little is known about the effect of the magnitude of this load on healing. Peltz et al. studied enthesis healing after rotator cuff surgery in a rat model15. After two weeks of immobilization, the animals began normal cage activity, began exercise, or remained immobilized. The exercise group had significantly decreased motion and mechanical properties at twelve weeks compared with the cage-activity group. The authors concluded that, after a short period of immobilization, increased activity was detrimental to both tendon mechanical properties and shoulder joint mechanics because of an increase in scar production. However, the mechanical stimulation was neither controlled nor quantified.
In the current study, we controlled and quantified the amount of axial loading applied to the healing tendon-bone interface. We used micro-CT to quantify new-bone formation at the tendon-bone interface as the primary outcome for tendon-to-bone healing, since numerous animal studies have shown that tendon-to-bone healing occurs by new-bone formation along the bone tunnel with bone ingrowth into the interface tissue and outer part of the tendon4-6.
Our study design did not demonstrate that high strain impairs new trabecular bone formation along the tunnel, at least compared with our prior data derived with low-strain loading in this model14. However, indirect support for this hypothesis comes from the finding that the delayed high-strain loading group was equivalent to the immobilized group, which suggests that the potential benefit of delayed mechanical loading (as found in our prior work) may have been mitigated by a negative effect of high strain. A possible explanation for these findings is that the greater loads disrupted healing by increasing shear and micromotion at the tendon-bone interface. This could have stimulated an inflammatory response, resulting in increased production of disorganized scar tissue. An inhibition of ossification could have prevented bone ingrowth, as demonstrated by our micro-CT data. However, these data should be considered preliminary, as we studied only one early time point. Additional studies, including histologic analyses, are needed to determine if these results are maintained at later time points with progressive remodeling.
We recognize that a large percentage (>60%) of the animals were lost prematurely. This is a challenging model, as axial distraction of the knee is accomplished by pulling on the pins in the femur and tibia. In order to distract the knee adequately to produce high graft strains, high loads are placed on the pin-bone interface, which can lead to pin loosening with subsequent infection and fractures. The compliance of the external fixator had a coefficient of variance of 20%17. Factors that influenced compliance included the distance between Kirschner wires and the distance between the femur or tibia and the external fixator. Therefore, there was likely some variation in the actual displacement of the knee joint among animals.
It is important to recognize the limitations of our study. This model and loading protocol applied cyclic axial load along the axis of the bone tunnels, resulting in interface shear strain. However, the mechanical loads on an in vivo human ACL graft are complex. A purely axial load was selected for this model to produce a controlled and quantified strain to the bone-graft-bone construct. We also acknowledge that strain on the graft changes over time as the graft heals to bone. Although all of the collateral and cruciate ligaments were sectioned during the operation, the soft tissue surrounding the joint progressively healed via scar formation in the postoperative period. Therefore, as time elapsed, the joint required progressively increasing loads to achieve the target joint distraction. For this reason, we chose a target displacement as the loading goal so that the graft experienced a target strain independent of the force. However, as the surrounding soft tissues healed, many of the loading curves reached the load limit. Therefore, the displacement goal was not reached and less strain was applied to the graft. Ultimately, it was difficult to apply a reproducible strain every day in different animals. Despite this variability, however, the strains were still substantially greater than those applied in our prior work with use of this model and loading protocol14,17.
This study provides early-term data but does not provide insight into the healing and maturation of the ACL attachment site beyond two weeks. We chose this time point because of the resilient and accelerated healing capacity found in rodent species1. We chose postoperative day four as the starting day for delayed-onset loading because, in our previous work with low strain loading14, starting loading on postoperative day four resulted in significantly improved tendon-to-bone healing compared with that seen with prolonged immobilization or with the onset of loading on postoperative day one or ten. While the present study showed differences in trabecular bone remodeling through micro-CT analysis, it is important to note that these results did not correlate with ultimate load to failure. Each specimen underwent biomechanical testing after micro-CT imaging. It is possible (although unlikely) that the imaging process and the additional freeze-thaw cycle had an effect on the collagen and tendon-bone interface. Prior investigators have reported that freezing and freeze-thaw-freeze cycles had no effect on tissue viscoelastic and tensile properties31,32.
This study does not provide recommendations regarding specific rehabilitation parameters following human ACL reconstruction. Although bone formation is important for tendon-to-bone healing, there were no differences in load to failure or stiffness. The magnitude of trabecular bone changes at the healing tendon-bone interface that is clinically relevant is currently unknown. Although we found significant differences in several micro-CT parameters, further study is necessary to determine if these findings are clinically important.
In conclusion, this study confirms our prior finding that the timing of initiation of mechanical loading of the healing tendon-bone interface has an important effect on healing. There is likely an interaction between timing and magnitude of strain, as the potential benefits of delayed loading may have been mitigated by high strain levels. The findings in the current study may have important implications for rehabilitation after ligament reconstruction. Knee motion is commonly prescribed following ACL reconstruction; however, the effect of this activity on healing of the tendon to the bone tunnel is poorly understood. Furthermore, the optimal duration of immobilization prior to the initiation of rehabilitation is not known. While there is controversy over the ideal intensity of early rehabilitation, our findings may provide reasonable support for humans avoiding exercises that will lead to a high strain on the ACL graft in the early postoperative period. This may be an especially important consideration with contemporary “anatomic” ACL reconstruction techniques in which non-isometric graft placement may lead to higher graft strains. The results of this animal study provide some support for a rehabilitation program that begins with a brief period of strict immobilization followed by low to moderate-intensity exercises to optimize tendon-to-bone healing.
Appendix
A table showing loading results in each animal on each postoperative day and figures demonstrating the loading device and the load-displacement graph are available with the online version of this article as a data supplement at jbjs.org.
Supplementary Material
Disclosure of Potential Conflicts of Interest
A table showing loading results in each animal on each postoperative day and a figure demonstrating the loading device and the load-displacement graph
Acknowledgments
Note: The authors acknowledge Dr. Peter Torzilli for his guidance throughout the design and completion of these experiments. They also acknowledge Lyuda Lukashova for contributions with micro-CT data collection.
Footnotes
Disclosure: One or more of the authors received payments or services, either directly or indirectly (i.e., via his or her institution), from a third party in support of an aspect of this work. In addition, one or more of the authors, or his or her institution, has had a financial relationship, in the thirty-six months prior to submission of this work, with an entity in the biomedical arena that could be perceived to influence or have the potential to influence what is written in this work. No author has had any other relationships, or has engaged in any other activities, that could be perceived to influence or have the potential to influence what is written in this work. The complete Disclosures of Potential Conflicts of Interest submitted by authors are always provided with the online version of the article.
References
- 1.Carpenter JE, Thomopoulos S, Flanagan CL, DeBano CM, Soslowsky LJ. Rotator cuff defect healing: a biomechanical and histologic analysis in an animal model. J Shoulder Elbow Surg. 1998 Nov-Dec;7(6):599-605 [DOI] [PubMed] [Google Scholar]
- 2.Cooper RR, Misol S. Tendon and ligament insertion. A light and electron microscopic study. J Bone Joint Surg Am. 1970 Jan;52(1):1-20 [PubMed] [Google Scholar]
- 3.Galatz LM, Sandell LJ, Rothermich SY, Das R, Mastny A, Havlioglu N, Silva MJ, Thomopoulos S. Characteristics of the rat supraspinatus tendon during tendon-to-bone healing after acute injury. J Orthop Res. 2006 Mar;24(3):541-50 [DOI] [PubMed] [Google Scholar]
- 4.Rodeo SA, Arnoczky SP, Torzilli PA, Hidaka C, Warren RF. Tendon-healing in a bone tunnel. A biomechanical and histological study in the dog. J Bone Joint Surg Am. 1993 Dec;75(12):1795-803 [DOI] [PubMed] [Google Scholar]
- 5.Whiston TB, Walmsley R. Some observations on the reactions of bone and tendon after tunnelling of bone and insertion of tendon. J Bone Joint Surg Br. 1960 May;42-B:377-86 [DOI] [PubMed] [Google Scholar]
- 6.Grana WA, Egle DM, Mahnken R, Goodhart CW. An analysis of autograft fixation after anterior cruciate ligament reconstruction in a rabbit model. Am J Sports Med. 1994 May-Jun;22(3):344-51 [DOI] [PubMed] [Google Scholar]
- 7.Panni AS, Milano G, Lucania L, Fabbriciani C. Graft healing after anterior cruciate ligament reconstruction in rabbits. Clin Orthop Relat Res. 1997 Oct;(343):203-12 [PubMed] [Google Scholar]
- 8.Oiestad BE, Holm I, Aune AK, Gunderson R, Myklebust G, Engebretsen L, Fosdahl MA, Risberg MA. Knee function and prevalence of knee osteoarthritis after anterior cruciate ligament reconstruction: a prospective study with 10 to 15 years of follow-up. Am J Sports Med. 2010 Nov;38(11):2201-10 Epub 2010 Aug 16 [DOI] [PubMed] [Google Scholar]
- 9.Bach BR, Jr, Tradonsky S, Bojchuk J, Levy ME, Bush-Joseph CA, Khan NH. Arthroscopically assisted anterior cruciate ligament reconstruction using patellar tendon autograft. Five- to nine-year follow-up evaluation. Am J Sports Med. 1998 Jan-Feb;26(1):20-9 [DOI] [PubMed] [Google Scholar]
- 10.Beynnon BD, Johnson RJ, Fleming BC, Kannus P, Kaplan M, Samani J, Renström P. Anterior cruciate ligament replacement: comparison of bone-patellar tendon-bone grafts with two-strand hamstring grafts. A prospective, randomized study. J Bone Joint Surg Am. 2002 Sep;84(9):1503-13 [DOI] [PubMed] [Google Scholar]
- 11.Gimbel JA, Van Kleunen JP, Williams GR, Thomopoulos S, Soslowsky LJ. Long durations of immobilization in the rat result in enhanced mechanical properties of the healing supraspinatus tendon insertion site. J Biomech Eng. 2007 Jun;129(3):400-4 [DOI] [PubMed] [Google Scholar]
- 12.Bedi A, Fox AJ, Kovacevic D, Deng XH, Warren RF, Rodeo SA. Doxycycline-mediated inhibition of matrix metalloproteinases improves healing after rotator cuff repair. Am J Sports Med. 2010 Feb;38(2):308-17 Epub 2009 Oct 13 [DOI] [PubMed] [Google Scholar]
- 13.Thomopoulos S, Williams GR, Soslowsky LJ. Tendon to bone healing: differences in biomechanical, structural, and compositional properties due to a range of activity levels. J Biomech Eng. 2003 Feb;125(1):106-13 [DOI] [PubMed] [Google Scholar]
- 14.Bedi A, Kovacevic D, Fox AJ, Imhauser CW, Stasiak M, Packer J, Brophy RH, Deng XH, Rodeo SA. Effect of early and delayed mechanical loading on tendon-to-bone healing after anterior cruciate ligament reconstruction. J Bone Joint Surg Am. 2010 Oct 20;92(14):2387-401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peltz CD, Sarver JJ, Dourte LM, Würgler-Hauri CC, Williams GR, Soslowsky LJ. Exercise following a short immobilization period is detrimental to tendon properties and joint mechanics in a rat rotator cuff injury model. J Orthop Res. 2010 Jul;28(7):841-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brophy RH, Kovacevic D, Imhauser CW, Stasiak M, Bedi A, Fox AJ, Deng XH, Rodeo SA. Effect of short-duration low-magnitude cyclic loading versus immobilization on tendon-bone healing after ACL reconstruction in a rat model. J Bone Joint Surg Am. 2011 Feb 16;93(4):381-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stasiak M, Imhauser C, Packer J, Bedi A, Brophy R, Kovacevic D, Jackson K, Deng XH, Rodeo S, Torzilli P. A novel in vivo joint loading system to investigate the effect of daily mechanical load on a healing anterior cruciate ligament reconstruction. J Med Device. 2010;4(1):15003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheung EV, Silverio L, Sperling JW. Strategies in biologic augmentation of rotator cuff repair: a review. Clin Orthop Relat Res. 2010 Jun;468(6):1476-84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gulotta LV, Kovacevic D, Ehteshami JR, Dagher E, Packer JD, Rodeo SA. Application of bone marrow-derived mesenchymal stem cells in a rotator cuff repair model. Am J Sports Med. 2009 Nov;37(11):2126-33 Epub 2009 Aug 14 [DOI] [PubMed] [Google Scholar]
- 20.Gulotta LV, Kovacevic D, Montgomery S, Ehteshami JR, Packer JD, Rodeo SA. Stem cells genetically modified with the developmental gene MT1-MMP improve regeneration of the supraspinatus tendon-to-bone insertion site. Am J Sports Med. 2010 Jul;38(7):1429-37 Epub 2010 Apr 16 [DOI] [PubMed] [Google Scholar]
- 21.Rodeo SA, Potter HG, Kawamura S, Turner AS, Kim HJ, Atkinson BL. Biologic augmentation of rotator cuff tendon-healing with use of a mixture of osteoinductive growth factors. J Bone Joint Surg Am. 2007 Nov;89(11):2485-97 [DOI] [PubMed] [Google Scholar]
- 22.Gomez MA, Woo SL, Amiel D, Harwood F, Kitabayashi L, Matyas JR. The effects of increased tension on healing medical collateral ligaments. Am J Sports Med. 1991 Jul-Aug;19(4):347-54 [DOI] [PubMed] [Google Scholar]
- 23.Kamps BS, Linder LH, DeCamp CE, Haut RC. The influence of immobilization versus exercise on scar formation in the rabbit patellar tendon after excision of the central third. Am J Sports Med. 1994 Nov-Dec;22(6):803-11 [DOI] [PubMed] [Google Scholar]
- 24.Thomopoulos S, Zampiakis E, Das R, Silva MJ, Gelberman RH. The effect of muscle loading on flexor tendon-to-bone healing in a canine model. J Orthop Res. 2008 Dec;26(12):1611-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woo SL, Gomez MA, Sites TJ, Newton PO, Orlando CA, Akeson WH. The biomechanical and morphological changes in the medial collateral ligament of the rabbit after immobilization and remobilization. J Bone Joint Surg Am. 1987 Oct;69(8):1200-11 [PubMed] [Google Scholar]
- 26.Binkley JM, Peat M. The effects of immobilization on the ultrastructure and mechanical properties of the medial collateral ligament of rats. Clin Orthop Relat Res. 1986 Feb;(203):301-8 [PubMed] [Google Scholar]
- 27.Hannafin JA, Arnoczky SP, Hoonjan A, Torzilli PA. Effect of stress deprivation and cyclic tensile loading on the material and morphologic properties of canine flexor digitorum profundus tendon: an in vitro study. J Orthop Res. 1995 Nov;13(6):907-14 [DOI] [PubMed] [Google Scholar]
- 28.Noyes FR. Functional properties of knee ligaments and alterations induced by immobilization: a correlative biomechanical and histological study in primates. Clin Orthop Relat Res. 1977 Mar-Apr;(123):210-42 [PubMed] [Google Scholar]
- 29.Gelberman RH, Woo SL, Lothringer K, Akeson WH, Amiel D. Effects of early intermittent passive mobilization on healing canine flexor tendons. J Hand Surg Am. 1982 Mar;7(2):170-5 [DOI] [PubMed] [Google Scholar]
- 30.Sakai H, Fukui N, Kawakami A, Kurosawa H. Biological fixation of the graft within bone after anterior cruciate ligament reconstruction in rabbits: effects of the duration of postoperative immobilization. J Orthop Sci. 2000;5(1):43-51 [DOI] [PubMed] [Google Scholar]
- 31.Moon DK, Woo SL, Takakura Y, Gabriel MT, Abramowitch SD. The effects of refreezing on the viscoelastic and tensile properties of ligaments. J Biomech. 2006;39(6):1153-7 Epub 2005 Apr 05 [DOI] [PubMed] [Google Scholar]
- 32.Woo SL, Orlando CA, Camp JF, Akeson WH. Effects of postmortem storage by freezing on ligament tensile behavior. J Biomech. 1986;19(5):399-404 [DOI] [PubMed] [Google Scholar]
- 33.Otsu N. A threshold selection method from gray-level histograms. IEEE Trans Syst Man Cyber. 1979;9(1):62-6 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosure of Potential Conflicts of Interest
A table showing loading results in each animal on each postoperative day and a figure demonstrating the loading device and the load-displacement graph


