Abstract
Background:
Anterior cruciate ligament (ACL) injuries are common among young athletes. Biomechanical studies have led to the development of training programs to improve neuromuscular control and reduce ACL injury rates as well as screening tools to identify athletes at higher risk for ACL injury. The purpose of this study was to evaluate the cost-effectiveness of these training methods and screening strategies for preventing ACL injuries.
Methods:
A decision-analysis model was created to evaluate three strategies for a population of young athletes participating in organized sports: (1) no training or screening, (2) universal neuromuscular training, and (3) universal screening, with neuromuscular training for identified high-risk athletes only. Risk of injury, risk reduction from training, and sensitivity and specificity of screening were based on published data from clinical trials. Costs of training and screening programs were estimated on the basis of the literature. Sensitivity analyses were performed on key model parameters to evaluate their effect on base case conclusions.
Results:
Universal neuromuscular training of all athletes was the dominant strategy, with better outcomes and lower costs compared with screening. On average, the implementation of a universal training program would save $100 per player per season, and would reduce the incidence of ACL injury from 3% to 1.1% per season. Screening was not cost-effective within the range of reported sensitivity and specificity values.
Conclusions and Clinical Relevance:
Given its low cost and ease of implementation, neuromuscular training of all young athletes represents a cost-effective strategy for reducing costs and morbidity from ACL injuries. While continued innovations on inexpensive and accurate screening methods to identify high-risk athletes remain of interest, improving existing training protocols and implementing neuromuscular training into routine training for all young athletes is warranted.
Youth participation in organized sports plays a major role in the physical, social, and financial well-being of athletes and their families. Participation in sports among youth in both high school and college has been steadily increasing; three-quarters of U.S. households have children who play sports1, and female participation in particular is on the rise2,3.
This increased athletic participation has inevitably resulted in more injuries, with >3.5 million sports injuries reported annually for children under fourteen years old1,4, which includes an increased number of anterior cruciate ligament (ACL) ruptures5. ACL injuries can be physically and psychologically devastating to young athletes6, and at a minimum require prolonged withdrawal from sports as well as substantial expenses related to surgical costs and therapy7-10. Even with reconstruction, return to competition has been reported to be as low as 50%11-15.
Several recent studies have evaluated the pathomechanics and risk factors for ACL injury. Epidemiological studies have shown that the prevalence of ACL injuries is four to six times higher in females than males16-18, while biomechanical studies have shown that women tend to have both anatomic and neuromuscular differences compared with men16,19-21. These theories have been corroborated by prospective kinematic studies that have shown that athletes who land with increased knee abduction moment are at a higher risk of subsequent ACL injury22.
On the basis of this theory of altered neuromuscular control, several prevention programs that focus on retraining athletes to jump, land, and cut in biomechanical positions that reduce the strain on the ACL have been developed23-27. These programs have been prospectively tested in multiple studies25-35, and recent meta-analyses have shown they are effective in lowering the rate of ACL injuries36,37.
Widespread implementation of new training programs, however, would require additional costs that have not been well described. Some authors have also proposed various potential screening tests to identify individual athletes at higher risk for ACL injury as candidates for intervention22,38-42.
A better understanding of the trade-offs between these two approaches could aid physicians, athletic trainers, researchers, and policy makers in determining if (a) the additional cost of implementing a universal prevention program would reduce the prevalence of injuries in a cost-effective manner or (b) if a screening tool would allow us to more efficiently focus resources on athletes with a higher risk of injury.
The primary objective of this study was to establish a model to evaluate the cost-effectiveness of neuromuscular training methods and screening strategies aimed at reducing the morbidity associated with ACL injury. Additionally, we aimed to describe the costs and performance of these training and screening techniques that would be required to become optimally cost-effective.
Materials and Methods
Study Design
We conducted an economic evaluation using a decision analytic model, following guidelines by the Panel on Cost-Effectiveness in Health and Medicine43. Our reference case was a hypothetical cohort of athletes involved in organized sports, as that is a group in which broad-based interventions are feasible and high-quality data exist. The cohort included athletes between the ages of fourteen and twenty-two years (high school and college). Both males and females were included, as the literature has not clearly demonstrated a difference in training effectiveness between sexes. The literature included athletes participating in soccer, handball, volleyball, and basketball, which can broadly be categorized as “cutting sports.”
Costs were calculated from a societal perspective. We used a twenty-year time horizon, based on the longest follow-up data available on patients with ACL injuries44,45. Outcomes were expressed in quality-adjusted life years (QALYs), which takes into account decrements in quality of life due to disease and/or injury as well as the time over which those changes occurred43.
A decision tree model was created and analyzed using decision analysis software (TreeAge Pro; TreeAge Software, Williamstown, Massachusetts). The model compared multiple intervention strategies; for each strategy, the model predicted resulting outcomes (ACL reconstructed or no ACL injury) with defined probabilities (e.g., positive test, negative test, or ACL injury; see Appendix). The probabilities, costs, and outcomes were assigned values, which provided average expected-value outcomes for each strategy.
Model Design
A decision tree was constructed for evaluation of the total costs and gains in QALYs on the basis of three strategies: (a) no training or screening, (b) enrolling all athletes in neuromuscular training programs, or (c) screening all athletes for risk of ACL injury, and enrolling only so-called high-risk athletes in neuromuscular training programs (see Appendix). We assumed that all ACL ruptures would be surgically reconstructed, which is more cost-effective than conservative treatment8,46-49 and is a widely accepted treatment with the intent of restoring joint kinematics, returning athletes to sport, and limiting disability50-52.
The neuromuscular training program was based on published trials, which typically involve a specific training drill during practice and an altered warm-up routine36,53. In general, these protocols involve specific warm-up activities, core and lower extremity stretching, strengthening, plyometrics, and sport-specific agility drills, usually performed around twenty minutes before practice, three times weekly. They emphasize muscle balance, proprioception, and core strength to reinforce proper mechanics during unanticipated landing or cutting. The screening program was modeled after studies that used anthropomorphic data and kinematic measurements obtained from a video camera during a simple drop-jump test to predict the risk of subsequent ACL injury38-40.
Model Inputs (Table I)
TABLE I.
Input Values Used to Calculate the Cost-Effectiveness of Prevention Strategies for ACL Ruptures*
| Value |
||||
| Description | Base Case | Low | High | Studies |
| Surgical costs | ||||
| ACL reconstruction ($) | 8000 | 5000 | 17,000 | Gottlob et al.7, Lubowitz and Appleby8, Paxton et al.9, Genuario et al.10, and Farshad et al.46 |
| Risk of ACL injury | ||||
| Baseline incidence of ACL rupture (per season) | 0.03 | 0.02 | 0.04 | Arendt and Dick17, Myklebust et al.25, Mandelbaum et al.26, Steffen et al.27, Caraffa et al.28, Gilchrist et al.29, Heidt et al.30, Hewett et al.31, Petersen et al.32, and Pfeiffer et al.34 |
| Prevention program | ||||
| Cost of prevention program ($/player/yr) | 1.25 | 1.25 | 25 | Mandelbaum et al.26, Caraffa et al.28, Gilchrist et al.29, Heidt et al.30, Hewett et al.31, Petersen et al.32, and Pfeiffer et al.34 |
| Risk ratio of prevention program | 0.38 | 0.2 | 0.72 | Sadoghi et al.36 |
| Screening test | ||||
| Cost of screening test ($/player) | 1.5 | 0 | 15 | Uhorchak et al.20, Hewett et al.22, Padua et al.38, and Myer et al.42 |
| Sensitivity of screening test | 65 | 61 | 78 | Hewett et al.22 and Myer et al.39-41 |
| Specificity of screening test | 60 | 56 | 73 | Hewett et al.22 and Myer et al.39-41 |
| Quality of life (utility value) | ||||
| After ACL reconstruction | 0.78 | 0.72 | 0.98 | Gottlob et al.7, Lubowitz and Appleby8, Paxton et al.9, Genuario et al.10, Farshad et al.46, Biau et al.56, and Tibor et al.57 |
ACL = anterior cruciate ligament.
Costs
The main cost inputs in this model were those associated with ACL reconstruction, the neuromuscular training and injury prevention program, and the risk assessment or screening program. Costs for ACL reconstruction, which were based on previous literature, have been reported to range from $5000 to $17,0007-10,46, although some recent studies have estimated the long-term societal costs to be as high as $38,000 for operatively treated injuries49.
The costs of the training programs were based on the programs that have been described in prospective comparative trials26,28-32,34. We estimated the resources required on the basis of both personnel and equipment needs. The costs in our reference case were based on the routine described by Mandelbaum et al.26, which included additional training for the coach (watching instructional videos online) and additional training for the team (learning how to execute the new routine). Assuming the coach takes thirty minutes to watch the instructional materials at the beginning of each season, and an additional thirty minutes to teach the routine to the players, the cost would be $1.25 per player per season (assuming the coach is salaried at $50,000 yearly54, and the average team size is twenty players). A higher cost estimate, which we evaluated in the sensitivity analysis, was based on the study by Hewett et al.31, which involved preseason sessions with a dedicated athletic trainer for sixty to ninety minutes for a total of eighteen sessions, increasing the cost estimate to $25 per player per season.
The cost of a potential screening program was estimated on the basis of the protocols reported in the literature. Protocols requiring extensive setup and sophisticated biomechanical monitoring equipment22 were excluded, as the expense and time required to conduct these would not be conducive to large-scale screening.
The more feasible protocols follow a common set of steps, which involve (a) gathering anthropomorphic data on the subject, then (b) videotaping a drop jump to analyze the kinematics, and (c) performing simple calculations to estimate the injury risk39,40. Under the most optimistic estimates, a dedicated screening center with minimal equipment (two cameras and one computer) and full-time staff salaried at $50,000 yearly would take at least five minutes per subject (including registration, data gathering, and analysis), which would cost approximately $2 to $3 per player screened. If, instead, the screening was performed by coaches or athletic trainers using similar equipment, the equipment cost per player increases and the cost per screening would rise to $15 per player. If coaches or athletic trainers were able to perform a screening test without cameras or equipment, the cost would drop to $1.50 per player screened.
Clinical Outcome Probabilities
The baseline ACL injury rate, which was based on multiple prospective studies17,25-32,34, ranged from 0.003 to 0.08 per player per season. Although there were a few outliers, the data were relatively consistent, with >70% of studies noting injury rates between 0.02 and 0.03. An incidence of 0.03 was used in the reference case, with a range of 0.02 to 0.04 used in the sensitivity analysis.
The reduction in injury from prevention programs was based on a recent meta-analysis that reported a mean risk ratio of 0.38 (i.e., a 62% reduction in risk; 95% confidence interval, 0.2 to 0.72)36.
Sensitivity and Specificity of Screening
There are no published reports of the diagnostic performance of simple jump tests for predicting ACL injury. In order to determine overall sensitivity and specificity, data from previous studies examining two types of tests were combined. Hewett et al. created a gold-standard screening test for predicting ACL injury, using measured knee abduction moments in 205 patients who were prospectively followed, and found a sensitivity of 78% and specificity of 73% for subsequent ACL rupture22. However, these data required use of a sophisticated motion capture laboratory, which is not feasible for large-scale implementation. Alternatively, there are lower-cost studies that have described the use of simple anthropometric measurements, a camcorder, and free software that uses a single drop jump to predict a high knee abduction moment, with reported sensitivities ranging from 73% to 84% and specificities, from 67% to 71%39-41. The data from these two tests were combined; one set used simple equipment to predict knee abduction moment, and another used more elaborate protocols that measured knee abduction moment and predicted ACL injury. The data were synthesized to calculate an effective sensitivity and specificity of simple jump tests for ACL injury (sensitivity ranged from 61% to 70% and specificity, from 56% to 61%).
Health-Related Quality of Life (Health Utility)
Health-related quality of life was calculated on the basis of the literature on subjective outcomes after ACL reconstruction7-10. Most literature currently has described outcomes after ACL reconstruction using International Knee Documentation Committee (IKDC) scores ranging from A (perfect function during stressful sports) to D (symptomatic pain and/or instability during activities of daily living). Utilities were derived from those measured in a survey of 285 university students by Gottlob and Baker48, which was later adapted to the IKDC scale by Paxton et al.9. After ACL reconstruction, patients were assigned health utilities on the basis of the IKDC score, which was distributed according to those reported in recent meta-analyses (Table II). We used a weighted average of the IKDC score breakdowns to generate the utility in the reference case.
TABLE II.
Outcomes by International Knee Documentation Committee (IKDC) Group and Corresponding Utility
| Percent in Each IKDC Group (Utility*) |
||||||
| Analysis | No. of Subjects | A (1.0) | B (0.697) | C (0.328) | D (0.233) | Overall Average Utility |
| Gottlob et al.7 | 373 | 66.5 | 23.9 | 6.3 | 3.3 | 0.86 |
| Paxton et al.9 | 989 | 59.3 | 34.4 | 5.8 | 0.5 | 0.85 |
| Biau et al.56 | 1125 | 37.1 | 41.5 | 21.4† | 0.73 | |
| Tibor et al.57 | 1970 | 39 | 43.8 | 13.3 | 3.9 | 0.75 |
| Model | 45.3 | 39.5 | 13.1 | 2.1 | 0.78 | |
Sensitivity Analysis
Sensitivity analysis was performed to evaluate the uncertainties in key parameter values. The ACL rupture rate, reconstruction cost, utility after reconstruction, and time horizon were varied in a series of one-way sensitivity analyses. A multi-way sensitivity analysis was also performed to evaluate the combined uncertainties of the training program cost, risk reduction with training, screening program cost, and screening program sensitivity and specificity.
Finally, we evaluated a hypothetical scenario in which a novel, more intensive neuromuscular training program was developed. All athletes would be trained under the current neuromuscular program, but those identified as at risk would be given additional training in the novel program. We then conducted a threshold analysis to answer the question: If this hypothetical training program were used, what are the cost and risk reduction parameters for which universal screening would be cost-effective?
Source of Funding
There was no external funding for this investigation.
Results
Reference Case
Universal training was estimated to reduce the incidence of ACL injury by an average of 63% (from 3% per season to 1.1% per season), while the screening program reduced the incidence by an average of 40% (from 3% to 1.8%). In other words, the model predicted that, of 10,000 athletes, 300 would have ACL injuries in the no-screening arm; 110, in the treat-all arm; and 180, in the screen-and-treat arm. On a per-case basis, the average cost of the universal training strategy was $100 lower than no training and $25 lower than screening. The universal training strategy also results in a net gain of 0.05 QALY, on average, compared with no training and an average gain of 0.03 QALY compared with screening. Universal training was therefore the dominant strategy, resulting in lower costs overall as well as improved health outcomes.
Sensitivity Analyses
In all of the one-way sensitivity analyses, in which parameters were varied according to the ranges specified in Table I, universal training was the dominant strategy. The results remained unchanged for ACL reconstruction costs as low as $1000 and ACL injury rates as low as 0.001 per player per season. The cost of the training program and its risk reduction were more influential parameters, although universal training still dominated other alternatives for almost all parameter values tested (Fig. 1). Two-way sensitivity analysis evaluating both sensitivity and specificity of the screening test showed that universal training remained dominant for all values.
Fig. 1.
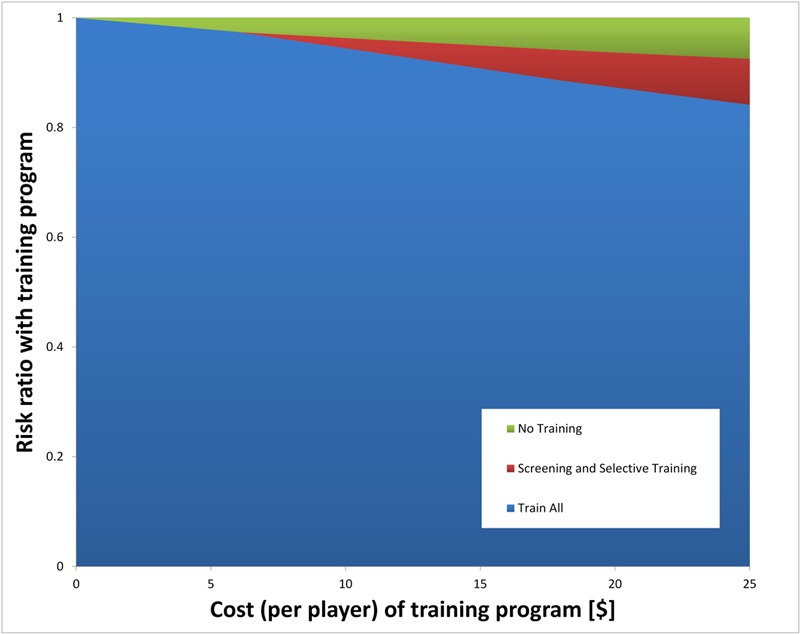
Effect of training cost and training risk reduction on the model. Two-way sensitivity analysis evaluating the cost of the training program and the risk ratio (effectiveness) of the training program. As shown, universal training dominated for all risk ratios below 0.9, with a narrow range of cost/risk ratio values where screening would be cost-effective.
Hypothetical Training Scenario
Assuming a societal willingness to pay $100,000/QALY55 in a hypothetical scenario, in which a new training program is more costly but also more effective, universal training with the novel protocol could be cost-effective if the cost was <$2500 per player per season (Fig. 2). If the cost of the training is between $3000 and $8000 and reduces injury risk by at least 80% (relative risk of <0.2), universal screening would become the cost-effective option. Protocols requiring expenses of >$8000 per player were unlikely to be more cost-effective than current protocols.
Fig. 2.
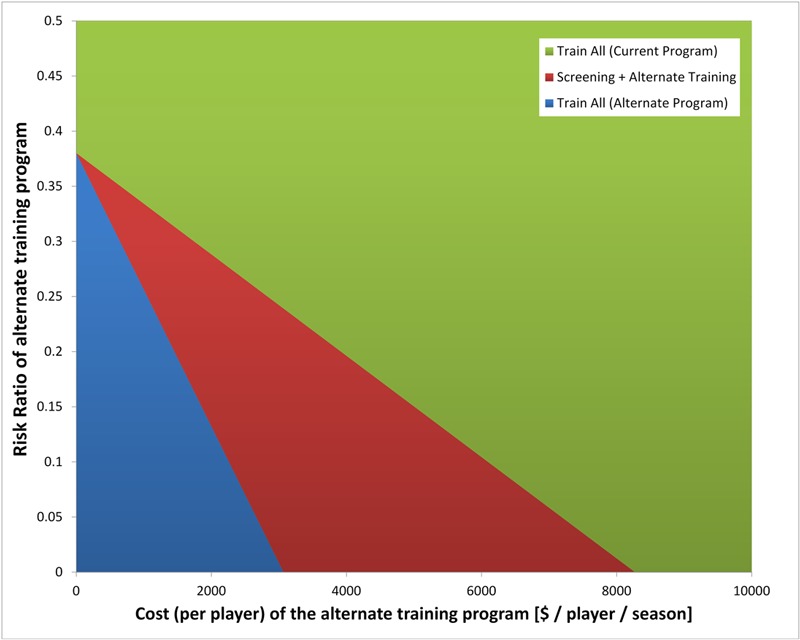
Effect of hypothetical training program cost and risk reduction. Two-way sensitivity analysis evaluating cost and risk ratio of a hypothetical new training program. For highly effective (i.e., low risk ratio) and inexpensive new programs, universal training under the new program is cost-effective (blue area). For a narrow range of moderately effective, fairly expensive new programs, screening prior to implementation becomes cost-effective (red area). When the new training program is expensive and less effective, it is less cost-effective than current training programs (green area).
Discussion
Our study compared the effectiveness and cost-effectiveness of two approaches to lower the risk of ACL injury in young athletes: training everyone or training high-risk athletes identified by screening. When a proposed screening and prevention protocol for any medical problem is evaluated, careful consideration has to be given to the costs and accuracy of screening as well as the cost and effectiveness of prevention in light of the economic and social burden of the disease. If the potential treatments cannot alter the course of disease, screening is ineffective, no matter how inexpensive or accurate. Likewise, potential treatments may be efficacious, but if there is no way to accurately identify at-risk patients, it can be difficult to efficiently allocate resources.
In this study, the universal neuromuscular training strategy was cost-effective in virtually all situations for several reasons. First, the injury is common, and is associated with a large cost due to a high rate of surgical treatment. Furthermore, the neuromuscular training programs have a relatively low cost coupled with a large demonstrable risk reduction, making primary prevention the dominant strategy.
There were virtually no scenarios in which universal screening was cost-effective. This makes intuitive sense, as the cost of training is low, the effectiveness of prevention is rather high, and the prevalence of injury is relatively high; in this context, it is unlikely that a screening test would be able to outperform universal training. This argues in favor of future investigations focusing on increasing the performance or efficiency of neuromuscular training regimens, rather than on improving or implementing screening tests.
The rationale for analyzing the hypothetical training scenario was done to determine if there was any scenario in which universal screening would be appropriate. The results of that analysis show that screening reduces costs only when there are extremely effective training interventions that are also expensive. Anecdotally, there are currently several different ACL prevention programs in use at more elite levels that are more resource intense in which this scenario might exist; however, as far as we know, there is currently no published literature on interventions of this type. Our hope is that these findings might help set goals for future research and act as a proof of concept that expensive, intense training techniques could be cost-effective if coupled with efficient screening programs.
One of the strengths of this analysis is the relatively large amount of data from prospective, high-quality studies, which can improve our confidence in the accuracy of the results. This was further confirmed by sensitivity analyses with respect to injury rate, reconstruction cost, and time horizon, which demonstrate the robustness of the model’s conclusions. Although a recent meta-analysis has shown prevention programs to be effective36, the magnitude of the effect remains debatable. While variability in the risk reduction may change the final incidence of ACL injury and cost savings, the most cost-effective strategy remains universal training for the entire range of values used in this model.
The post-ACL reconstruction utility was based on the current literature, but was fairly low in the reference case, so a broader range of utilities was tested in sensitivity analysis. In general, we made conservative estimates about the morbidity of ACL injury to avoid inflation of the apparent value of prevention tools. Additionally, we did not evaluate the possibility that some of the injured athletes may choose not to undergo ACL reconstruction. As all of the available literature currently suggests that reconstruction is more cost-effective7,8,46, patients choosing nonoperative treatment could have correspondingly worse outcomes, which would only further exaggerate the benefit of prevention. Despite these estimates, a widespread implementation of neuromuscular training programs continues to dominate as the most cost-effective strategy, although future research should continue to carefully scrutinize the loss of utility after ACL injury.
One of the limitations of the study was the lack of primary cost data for both training and screening programs. As a result, several assumptions were required to generate cost estimates to complete the model. Our sensitivity analysis shows that our conclusions are consistent for a wide variety of costs, including some that were more than an order of magnitude higher than our estimates. In fact, training costs would have to increase >$200 per player per season before cost-effectiveness came into question. Future research that generates more accurate cost estimates within the bounds of our sensitivity analyses for both training and screening protocols is needed to confirm our findings.
It should also be clarified that these results are applicable only to high school, college, and professional athletes who participate in regular, organized practices where formal alterations in warm-ups and additional training routines can be consistently implemented. Although we did not run subanalyses using sex or sport-specific injury rates, our sensitivity analysis showed that our results are consistent for a wide range of injury rates that would include these more specific estimates. However, we cannot make conclusions about how effective these programs would be in children under fourteen years old, those who participate in recreational-level sports, or older athletes.
In conclusion, this model shows that universal prevention in focused neuromuscular training can represent a cost-effective strategy for reducing the morbidity and costs associated with ACL injuries. The results are based on a large body of high-quality published literature and are broadly robust when tested with sensitivity analyses. Screening for high-risk athletes is currently not cost-effective; however, if the costs are reduced and accuracy is improved, there may be a role for screening coupled with more focused and resource-intensive training programs. Future research should focus on improving and universally implementing these programs, rather than improving screening, which these analyses have shown not to be cost-effective.
Appendix
A figure showing a schematic representation of the decision tree model is available with the online version of this article as a data supplement at jbjs.org.
Supplementary Material
Disclosure of Potential Conflicts of Interest
A figure showing a schematic representation of the decision tree model
Footnotes
Disclosure: One or more of the authors received payments or services, either directly or indirectly (i.e., via his or her institution), from a third party in support of an aspect of this work. In addition, one or more of the authors, or his or her institution, has had a financial relationship, in the thirty-six months prior to submission of this work, with an entity in the biomedical arena that could be perceived to influence or have the potential to influence what is written in this work. No author has had any other relationships, or has engaged in any other activities, that could be perceived to influence or have the potential to influence what is written in this work. The complete Disclosures of Potential Conflicts of Interest submitted by authors are always provided with the online version of the article.
References
- 1. National Federation of State High School Associations. High school sports participation increases for 20th consecutive year. 2009 Sep 15. http://www.nfhs.org/. Accessed 2013 Feb 8.
- 2.Sutton KM, Bullock JM. Anterior cruciate ligament rupture: differences between males and females. J Am Acad Orthop Surg. 2013 Jan;21(1):41-50 [DOI] [PubMed] [Google Scholar]
- 3.Kaestner R, Xu X. Effects of Title IX and sports participation on girls’ physical activity and weight. Adv Health Econ Health Serv Res. 2007;17:79-111 [PubMed] [Google Scholar]
- 4.Frank JS, Gambacorta PL. Anterior cruciate ligament injuries in the skeletally immature athlete: diagnosis and management. J Am Acad Orthop Surg. 2013 Feb;21(2):78-87 [DOI] [PubMed] [Google Scholar]
- 5.Mihata LC, Beutler AI, Boden BP. Comparing the incidence of anterior cruciate ligament injury in collegiate lacrosse, soccer, and basketball players: implications for anterior cruciate ligament mechanism and prevention. Am J Sports Med. 2006 Jun;34(6):899-904 Epub 2006 Mar 27 [DOI] [PubMed] [Google Scholar]
- 6.Trentacosta NE, Vitale MA, Ahmad CS. The effects of timing of pediatric knee ligament surgery on short-term academic performance in school-aged athletes. Am J Sports Med. 2009 Sep;37(9):1684-91 Epub 2009 May 21 [DOI] [PubMed] [Google Scholar]
- 7.Gottlob CA, Baker CL, Jr, Pellissier JM, Colvin L. Cost effectiveness of anterior cruciate ligament reconstruction in young adults. Clin Orthop Relat Res. 1999 Oct;(367):272-82 [PubMed] [Google Scholar]
- 8.Lubowitz JH, Appleby D. Cost-effectiveness analysis of the most common orthopaedic surgery procedures: knee arthroscopy and knee anterior cruciate ligament reconstruction. Arthroscopy. 2011 Oct;27(10):1317-22 Epub 2011 Aug 19 [DOI] [PubMed] [Google Scholar]
- 9.Paxton ES, Kymes SM, Brophy RH. Cost-effectiveness of anterior cruciate ligament reconstruction: a preliminary comparison of single-bundle and double-bundle techniques. Am J Sports Med. 2010 Dec;38(12):2417-25 Epub 2010 Sep 09 [DOI] [PubMed] [Google Scholar]
- 10.Genuario JW, Faucett SC, Boublik M, Schlegel TF. A cost-effectiveness analysis comparing 3 anterior cruciate ligament graft types: bone-patellar tendon-bone autograft, hamstring autograft, and allograft. Am J Sports Med. 2012 Feb;40(2):307-14 Epub 2011 Nov 15 [DOI] [PubMed] [Google Scholar]
- 11.Feller JA, Webster KE. A randomized comparison of patellar tendon and hamstring tendon anterior cruciate ligament reconstruction. Am J Sports Med. 2003 Jul-Aug;31(4):564-73 [DOI] [PubMed] [Google Scholar]
- 12.Marder RA, Raskind JR, Carroll M. Prospective evaluation of arthroscopically assisted anterior cruciate ligament reconstruction. Patellar tendon versus semitendinosus and gracilis tendons. Am J Sports Med. 1991 Sep-Oct;19(5):478-84 [DOI] [PubMed] [Google Scholar]
- 13.Matsumoto A, Yoshiya S, Muratsu H, Yagi M, Iwasaki Y, Kurosaka M, Kuroda R. A comparison of bone-patellar tendon-bone and bone-hamstring tendon-bone autografts for anterior cruciate ligament reconstruction. Am J Sports Med. 2006 Feb;34(2):213-9 Epub 2005 Nov 10 [DOI] [PubMed] [Google Scholar]
- 14.O’Neill DB. Arthroscopically assisted reconstruction of the anterior cruciate ligament. A follow-up report. J Bone Joint Surg Am. 2001 Sep;83(9):1329-32 [DOI] [PubMed] [Google Scholar]
- 15.Shaieb MD, Kan DM, Chang SK, Marumoto JM, Richardson AB. A prospective randomized comparison of patellar tendon versus semitendinosus and gracilis tendon autografts for anterior cruciate ligament reconstruction. Am J Sports Med. 2002 Mar-Apr;30(2):214-20 [DOI] [PubMed] [Google Scholar]
- 16.Hewett TE, Myer GD, Ford KR. Anterior cruciate ligament injuries in female athletes: Part 1, mechanisms and risk factors. Am J Sports Med. 2006 Feb;34(2):299-311 [DOI] [PubMed] [Google Scholar]
- 17.Arendt E, Dick R. Knee injury patterns among men and women in collegiate basketball and soccer. NCAA data and review of literature. Am J Sports Med. 1995 Nov-Dec;23(6):694-701 [DOI] [PubMed] [Google Scholar]
- 18.Lal S, Hoch AZ. Factors that affect the young female athlete. Phys Med Rehabil Clin N Am. 2007 Aug;18(3):361-83: vii [DOI] [PubMed] [Google Scholar]
- 19.Barber-Westin SD, Noyes FR, Smith ST, Campbell TM. Reducing the risk of noncontact anterior cruciate ligament injuries in the female athlete. Phys Sportsmed. 2009 Oct;37(3):49-61 [DOI] [PubMed] [Google Scholar]
- 20.Uhorchak JM, Scoville CR, Williams GN, Arciero RA, St Pierre P, Taylor DC. Risk factors associated with noncontact injury of the anterior cruciate ligament: a prospective four-year evaluation of 859 West Point cadets. Am J Sports Med. 2003 Nov-Dec;31(6):831-42 [DOI] [PubMed] [Google Scholar]
- 21.Huston LJ, Greenfield ML, Wojtys EM. Anterior cruciate ligament injuries in the female athlete. Potential risk factors. Clin Orthop Relat Res. 2000 Mar;(372):50-63 [DOI] [PubMed] [Google Scholar]
- 22.Hewett TE, Myer GD, Ford KR, Heidt RS, Jr, Colosimo AJ, McLean SG, van den Bogert AJ, Paterno MV, Succop P. Biomechanical measures of neuromuscular control and valgus loading of the knee predict anterior cruciate ligament injury risk in female athletes: a prospective study. Am J Sports Med. 2005 Apr;33(4):492-501 Epub 2005 Feb 08 [DOI] [PubMed] [Google Scholar]
- 23.Hewett TE, Stroupe AL, Nance TA, Noyes FR. Plyometric training in female athletes. Decreased impact forces and increased hamstring torques. Am J Sports Med. 1996 Nov-Dec;24(6):765-73 [DOI] [PubMed] [Google Scholar]
- 24.Irmischer BS, Harris C, Pfeiffer RP, DeBeliso MA, Adams KJ, Shea KG. Effects of a knee ligament injury prevention exercise program on impact forces in women. J Strength Cond Res. 2004 Nov;18(4):703-7 [DOI] [PubMed] [Google Scholar]
- 25.Myklebust G, Engebretsen L, Braekken IH, Skjølberg A, Olsen OE, Bahr R. Prevention of anterior cruciate ligament injuries in female team handball players: a prospective intervention study over three seasons. Clin J Sport Med. 2003 Mar;13(2):71-8 [DOI] [PubMed] [Google Scholar]
- 26.Mandelbaum BR, Silvers HJ, Watanabe DS, Knarr JF, Thomas SD, Griffin LY, Kirkendall DT, Garrett W., Jr Effectiveness of a neuromuscular and proprioceptive training program in preventing anterior cruciate ligament injuries in female athletes: 2-year follow-up. Am J Sports Med. 2005 Jul;33(7):1003-10 Epub 2005 May 11 [DOI] [PubMed] [Google Scholar]
- 27.Steffen K, Myklebust G, Olsen OE, Holme I, Bahr R. Preventing injuries in female youth football—a cluster-randomized controlled trial. Scand J Med Sci Sports. 2008 Oct;18(5):605-14 Epub 2008 Jan 14 [DOI] [PubMed] [Google Scholar]
- 28.Caraffa A, Cerulli G, Projetti M, Aisa G, Rizzo A. Prevention of anterior cruciate ligament injuries in soccer. A prospective controlled study of proprioceptive training. Knee Surg Sports Traumatol Arthrosc. 1996;4(1):19-21 [DOI] [PubMed] [Google Scholar]
- 29.Gilchrist J, Mandelbaum BR, Melancon H, Ryan GW, Silvers HJ, Griffin LY, Watanabe DS, Dick RW, Dvorak J. A randomized controlled trial to prevent noncontact anterior cruciate ligament injury in female collegiate soccer players. Am J Sports Med. 2008 Aug;36(8):1476-83 [DOI] [PubMed] [Google Scholar]
- 30.Heidt RS, Jr, Sweeterman LM, Carlonas RL, Traub JA, Tekulve FX. Avoidance of soccer injuries with preseason conditioning. Am J Sports Med. 2000 Sep-Oct;28(5):659-62 [DOI] [PubMed] [Google Scholar]
- 31.Hewett TE, Lindenfeld TN, Riccobene JV, Noyes FR. The effect of neuromuscular training on the incidence of knee injury in female athletes. A prospective study. Am J Sports Med. 1999 Nov-Dec;27(6):699-706 [DOI] [PubMed] [Google Scholar]
- 32.Petersen W, Zantop T, Steensen M, Hypa A, Wessolowski T, Hassenpflug J. [Prevention of lower extremity injuries in handball: initial results of the handball injuries prevention programme]. Sportverletz Sportschaden. 2002 Sep;16(3):122-6 [DOI] [PubMed] [Google Scholar]
- 33.Petersen W, Braun C, Bock W, Schmidt K, Weimann A, Drescher W, Eiling E, Stange R, Fuchs T, Hedderich J, Zantop T. A controlled prospective case control study of a prevention training program in female team handball players: the German experience. Arch Orthop Trauma Surg. 2005 Nov;125(9):614-21 [DOI] [PubMed] [Google Scholar]
- 34.Pfeiffer RP, Shea KG, Roberts D, Grandstrand S, Bond L. Lack of effect of a knee ligament injury prevention program on the incidence of noncontact anterior cruciate ligament injury. J Bone Joint Surg Am. 2006 Aug;88(8):1769-74 [DOI] [PubMed] [Google Scholar]
- 35.Hewett TE, Ford KR, Hoogenboom BJ, Myer GD. Understanding and preventing acl injuries: current biomechanical and epidemiologic considerations - update 2010. N Am J Sports Phys Ther. 2010 Dec;5(4):234-51 [PMC free article] [PubMed] [Google Scholar]
- 36.Sadoghi P, von Keudell A, Vavken P. Effectiveness of anterior cruciate ligament injury prevention training programs. J Bone Joint Surg Am. 2012 May 2;94(9):769-76 [DOI] [PubMed] [Google Scholar]
- 37.Noyes FR, Barber Westin SD. Anterior cruciate ligament injury prevention training in female athletes: a systematic review of injury reduction and results of athletic performance tests. Sports Health. 2012 Jan;4(1):36-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Padua DA, Marshall SW, Boling MC, Thigpen CA, Garrett WE, Jr, Beutler AI. The Landing Error Scoring System (LESS) Is a valid and reliable clinical assessment tool of jump-landing biomechanics: The JUMP-ACL study. Am J Sports Med. 2009 Oct;37(10):1996-2002 Epub 2009 Sep 02 [DOI] [PubMed] [Google Scholar]
- 39.Myer GD, Ford KR, Hewett TE. New method to identify athletes at high risk of ACL injury using clinic-based measurements and freeware computer analysis. Br J Sports Med. 2011 Apr;45(4):238-44 Epub 2010 Nov 16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Myer GD, Ford KR, Khoury J, Succop P, Hewett TE. Development and validation of a clinic-based prediction tool to identify female athletes at high risk for anterior cruciate ligament injury. Am J Sports Med. 2010 Oct;38(10):2025-33 Epub 2010 Jul 01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Myer GD, Ford KR, Khoury J, Succop P, Hewett TE. Clinical correlates to laboratory measures for use in non-contact anterior cruciate ligament injury risk prediction algorithm. Clin Biomech (Bristol, Avon). 2010 Aug;25(7):693-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Myer GD, Ford KR, Hewett TE. Tuck jump assessment for reducing anterior cruciate ligament injury risk. Athl Ther Today. 2008 Sep 1;13(5):39-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gold MR, Siegel JE, Russell LB, Weinstein MC, editors. Cost-effectiveness in health and medicine. New York: Oxford University Press; 1996 [Google Scholar]
- 44.Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007 Oct;35(10):1756-69 Epub 2007 Aug 29 [DOI] [PubMed] [Google Scholar]
- 45.Gillquist J, Messner K. Anterior cruciate ligament reconstruction and the long-term incidence of gonarthrosis. Sports Med. 1999 Mar;27(3):143-56 [DOI] [PubMed] [Google Scholar]
- 46.Farshad M, Gerber C, Meyer DC, Schwab A, Blank PR, Szucs T. Reconstruction versus conservative treatment after rupture of the anterior cruciate ligament: cost effectiveness analysis. BMC Health Serv Res. 2011;11:317 Epub 2011 Nov 19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barrera Oro F, Sikka RS, Wolters B, Graver R, Boyd JL, Nelson B, Swiontkowski MF. Autograft versus allograft: an economic cost comparison of anterior cruciate ligament reconstruction. Arthroscopy. 2011 Sep;27(9):1219-25 Epub 2011 Aug 04 [DOI] [PubMed] [Google Scholar]
- 48.Gottlob CA, Baker CL., Jr Anterior cruciate ligament reconstruction: socioeconomic issues and cost effectiveness. Am J Orthop (Belle Mead NJ). 2000 Jun;29(6):472-6 [PubMed] [Google Scholar]
- 49.Mather RCK, 3rd, Koenig L, Kocher MS, Dall TM, Gallo P, Scott DJ, Bach BR, Jr, Spindler KP; MOON Knee Group. Societal and economic impact of anterior cruciate ligament tears. J Bone Joint Surg Am. 2013 Oct 2;95(19):1751-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tashman S, Kopf S, Fu FH. The Kinematic Basis of ACL Reconstruction. Oper Tech Sports Med. 2008 Jul 1;16(3):116-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Siegel L, Vandenakker-Albanese C, Siegel D. Anterior cruciate ligament injuries: anatomy, physiology, biomechanics, and management. Clin J Sport Med. 2012 Jul;22(4):349-55 [DOI] [PubMed] [Google Scholar]
- 52.Lawrence JT, Argawal N, Ganley TJ. Degeneration of the knee joint in skeletally immature patients with a diagnosis of an anterior cruciate ligament tear: is there harm in delay of treatment? Am J Sports Med. 2011 Dec;39(12):2582-7 Epub 2011 Sep 14 [DOI] [PubMed] [Google Scholar]
- 53.Ladenhauf HN, Graziano J, Marx RG. Anterior cruciate ligament prevention strategies: are they effective in young athletes - current concepts and review of literature. Curr Opin Pediatr. 2013 Feb;25(1):64-71 [DOI] [PubMed] [Google Scholar]
- 54. US Bureau of Labor Statistics. May 2011 National Occupational Employment and Wage Estimates. 2011. http://www.bls.gov/oes/2011/may/oes_nat.htm. Accessed 2013 Feb 23.
- 55.Braithwaite RS, Meltzer DO, King JT, Jr, Leslie D, Roberts MS. What does the value of modern medicine say about the $50,000 per quality-adjusted life-year decision rule? Med Care. 2008 Apr;46(4):349-56 [DOI] [PubMed] [Google Scholar]
- 56.Biau DJ, Tournoux C, Katsahian S, Schranz P, Nizard R. ACL reconstruction: a meta-analysis of functional scores. Clin Orthop Relat Res. 2007 May;458:180-7 [DOI] [PubMed] [Google Scholar]
- 57.Tibor LM, Long JL, Schilling PL, Lilly RJ, Carpenter JE, Miller BS. Clinical outcomes after anterior cruciate ligament reconstruction: a meta-analysis of autograft versus allograft tissue. Sports Health. 2010 Jan;2(1):56-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosure of Potential Conflicts of Interest
A figure showing a schematic representation of the decision tree model


