SUMMARY
Functional variability (FV) of populations can be decomposed into three main features: the individual variability of multiple traits, the strength of correlations between those traits and the main direction of these correlations, the latter two being known as ‘phenotypic integration’. Evolutionary biology has long recognized that FV in natural populations is key to determining potential evolutionary responses, but this topic has been little studied in functional ecology.
Here we focus on the arctico-alpine perennial plant species Polygonum viviparum L.. We used a comprehensive sampling of seven functional traits in 29 wild populations covering the whole environmental niche of the species. The niche of the species was captured by a temperature gradient, which separated alpine stressful habitats from species-rich, competitive sub-alpine ones. We seeked to assess the relative roles of abiotic stress and biotic interactions in shaping different aspects of functional variation within and among populations, that is, the multi-trait variability, the strength of correlations between traits, and the main directions of functional trade-offs.
Populations with the highest extent of functional variability were found in the warm end of the gradient whereas populations exhibiting the strongest degree of phenotypic integration were located in sites with intermediate temperatures. This could reveal both the importance of environmental filtering and population demography in structuring FV. Interestingly, we found that the main axes of multivariate functional variation were radically different within and across population.
Although the proximate causes of FV structure remain uncertain, our study presents a robust methodology for the quantitative study of functional variability in connection with species’ niches. It also opens up new perspectives for the conceptual merging of intraspecific functional patterns with community ecology.
Keywords: alpine plants, ecological niche, functional traits, intraspecific variation, lines of least resistance, phenotypic integration, variance-covariance matrix
1. INTRODUCTION
Intraspecific phenotypic variability has recently emerged as an important topic in the field of plant community ecology (Violle et al. 2012). Several studies have shown that, contrary to previous expectations, plant functional traits that vary between species across environmental gradients and are related to community assembly could also be highly variable within species, and even within populations (Shipley & Almeida-Cortez 2003; Albert et al. 2010b). Accounting for this variability has proven to be crucial in answering various questions in plant ecology (see Jung et al. 2010 for community assembly; de Bello et al. 2011 for diversity measures; De Frenne et al. 2011 for functional strategies). To date the study of intraspecific phenotypic variability in community ecology has remained mainly univariate (i.e. traits were studied separately, Violle et al. 2012 but see Reich et al. 2003; Albert et al. 2010a), although it is the entire trait syndrome that influences individual’s fitness and can be linked with species’ environmental niches (Reich et al. 2003; Wilson & Nussey 2010). This lack of knowledge of the multivariate structure of functional traits at intraspecific level is particularly embarrassing. Indeed, there has been wide recognition in the field of evolutionary quantitative genetics that the variability of single traits as well as the correlations between them at the population level can be key in driving local adaptation, shaping the boundaries of species’ niches and determining their evolutionary potential (Kirkpatrick & Barton 1997; Gomulkiewicz & Houle 2009; Lavergne et al. 2010).
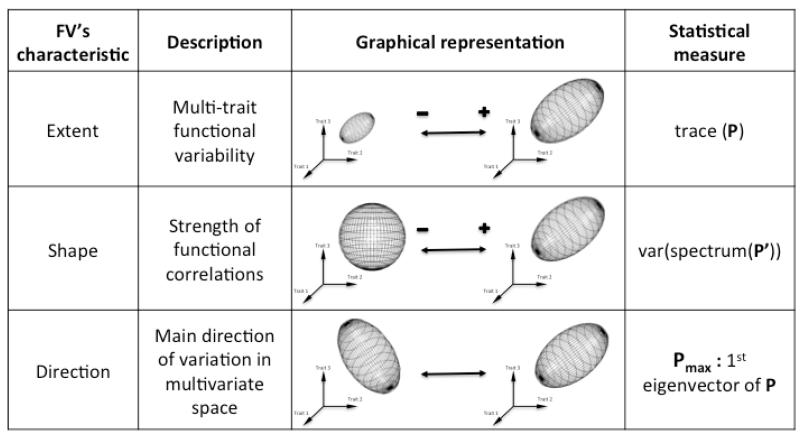
In this paper we use the term functional variability (hereafter FV) to jointly refer to the amount of variance in multiple functionally related traits (i.e. single trait variances) and to the pattern of covariation between these traits, this later characteristic being known as ‘phenotypic integration’ (Pigliucci 2003). The functional variability of a population can be summarized by its phenotypic variance-covariance matrix and visualised as an ellipsoid in a multidimensional trait space (Fig. 1). This ellipsoid has three main features: (i) the extent of Functional Variability (hereafter FV extent), which represents the overall amount of phenotypic variability, is the volume of the ellipsoid; (ii) the shape of Functional Variability (hereafter FV shape), measured as whether the ellipsoid is closer to a sphere or to a segment, which describes the strength of the correlations between the different traits (i.e. the intensity of phenotypic integration) and (iii) the direction of Functional Variability (hereafter FV direction), which represents the main direction of variation in the multi-trait phenotypic space, is the main direction of the ellipsoid.
Figure 1. Graphical representation of the functional variability of a population as an ellipsoid.
Each of the three characteristics of FV translates into different kinds of ellipsoids, as exemplified by the pictures. Statistical measures of each characteristic are presented. P is the variance-covariance matrix of the selected traits. P′ is their correlation matrix.
Based on this methodology, studying the link between multi-trait intraspecific FV and the ecological niche can be broken down into three main questions.
Firstly, concerning FV extent, it is crucial to understand how it varies within the niche from its core to its edge. Several hypotheses exist regarding the mechanisms driving FV extent. On the one hand, stressful abiotic environments should reduce intraspecific FV due to strong directional selective pressures resulting in the environmental filtering of adapted phenotypes (Keddy 1992; see Arnold et al. 2008 for the effect of selection on genetic variability). This kind of strong environmental filtering is frequently observed at the interspecific level in extremely arid or cold environments where functional diversity at the community level is reduced (Cowling et al. 1994; de Bello, Leps & Sebastia 2006). On the other hand, strong biotic interactions in species rich communities could result in larger intraspecific FV. Indeed, in such diverse communities many different kinds of competitors with varying ecological strategies and different functional traits are likely to be encountered by different individuals of a same species. This should drive divergent selection and character displacement in different directions for different individuals in order to reduce competition for resources with individuals from other species (Brown & Wilson 1956), thus resulting in a wider spectrum of functional strategies in the local population (Reich et al. 2003). In any case the effect of biotic interactions should be more important for traits that are related to coexistence mechanisms than for traits involved in the tolerance to abiotic conditions.
Secondly, it is important to understand what are the drivers of FV shape and in particular in which part of the environmental niche the most integrated phenotypes are found. Theory predicts that correlational selection should be the main driver of strongly integrated phenotypes (Arnold et al. 2008), even though other genetic mechanisms could also increase traits correlations (Armbruster & Schwaegerle 1996). At the intraspecific level, phenotypic integration in plants has mainly been studied on floral morphology, with the recognition that strong selective pressures imposed by pollinators are responsible for the high degree of integration in floral structures (Berg 1960; Ordano et al. 2008; Armbruster et al. 2009). Concerning vegetative traits, it has been observed that plant species living in harsh environments often exhibit suites of functional traits that are strongly correlated (Chapin, Autumn & Pugnaire 1993). Several experimental studies have supported this view at the intra-specific level. For example, Gianoli (2004) showed that traits related to resource acquisition and growth in Convolvulus arvensis are more tightly correlated when environmental stress increases, which might be due to stronger energetic trade-offs between several physiologic functions (see also Schlichting 1989). According to these observations, we would expect that the most integrated populations be found at the niche edges, and particularly where abiotic conditions are limiting. However, Tonsor & Scheiner (2007) have found an opposite result in Arabidopsis thaliana, where the overall degree of phenotypic integration does not change with CO2 availability.
Thirdly, examining FV direction provides interesting insights into the main drivers of functional trade-offs and the main axes of multivariate phenotypic variation at the population level. On the one hand, environmental factors could impose certain energetic constraints and thus settle trade-offs between several traits, resulting in natural selection shaping the main direction of phenotypic variation (Schluter 1996; Webb et al. 2010). This has been exemplified at interspecific level by the leaf economics spectrum, a single axis of variation that captures most of the variance in key foliar traits over thousands of plants from all around the World (Wright et al. 2004). However, if selection is the main driver of FV direction, there are no reasons why two populations that face different environments could not have different main axes of phenotypic variation. On the other hand, genetic factors like pleiotropic effects, random drift, asymmetric gene flow between source and sink populations or linkage disequilibrium between traits can increase correlations between certain pairs of traits and thus set the main directions of FV (Armbruster & Schwaegerle 1996; Gomulkiewicz & Houle 2009). In the case of extremely strong genetic control on FV direction, these directions should be the same among populations and within different populations (Sokal 1978; Armbruster & Schwaegerle 1996).
In this paper, we examine how these three different aspects of FV vary across the environmental niche of the widely distributed arctico-alpine plant species Polygonum viviparum L. Using robust statistical techniques borrowed from quantitative genetics, we studied the multivariate functional variability of different populations in natural conditions along an environmental gradient typical of alpine landscapes (i.e. temperature, see below). We specifically ask the following questions:
How does the extent of intraspecific FV vary across spatial scales, i.e. what is the importance of intra-population trait variability compared to inter-population trait variability?
Does the extent of intraspecific FV increase from the warm to the cold edge of the species’ niche due to the shift from environments dominated by competition to environments dominated by environmental filtering?
Is phenotypic integration higher at the edges of the niche due to more stressful conditions that impose stronger energetic trade-offs?
Do different populations share the same FV direction? And how does these directions relate to the environmental gradients and to the main direction of FV at the inter-population level?
2. MATERIAL AND METHODS
(a) Study species and site
We chose Polygonum viviparum L. as a model species because of its large environmental niche. This herbaceous perennial occurs in all arctico-alpine regions of the Northern Hemisphere. In the Alps, it can be found from the montane belt (starting around 1000m of altitude), where plant biomass is high and competition for light and nutrients severe, to the upper alpine level (ending at c.a. 3000m a.s.l.), where the environment is harsher and physiological limitations are stronger (Körner 1999, see Appendix S2). It has a preference for relatively moist habitats. The species has the specificity of bearing both flowers and bulbils (clonal reproductive organs) on the flowering spike.
We studied the species in the central French Alps Guisane Valley (Fig. S1) where it occurs in a variety of ecological contexts (from forests dominated by Larix decidua Mill. to alpine screes). In order to maximise the environmental differences between sites (Albert et al. 2010c), we stratified the sampling design following two independent gradients known to have high impact on the physiology of alpine plants (Körner 1999): mean annual temperature and solar radiation in June. These two variables were selected from a set of climatic variables interpolated at 50m resolution (Aurelhy model, (Benichou & Le Breton 1987) extracted from all known occurrence points for P. viviparum in the Guisane valley (data collected by the National Botanical Alpine Conservatory, http://www.cbn-alpin.fr/). The selection was made by choosing two orthogonal gradients that strongly correlated with the two first axes of the Principal Component Analysis conducted on this set of variables (results not shown).
Temperature was the main environmental gradient and the primary determinant for P. viviparum’s environmental niche in our study area (Thuiller et al. 2010; Boulangeat, Gravel & Thuiller 2012). This climatic variable acts on plant physiology and phenology, with colder sites being subject to more frequent frost events even during the summer and experiencing a shorter growing season. Temperature also plays an indirect biotic role in conjunction with soil by discriminating between warm productive species-rich habitats and cold unproductive species-poor habitats (Körner 1999). Using botanical surveys to estimate species richness per site as well as a spectral measure of overall biomass per area (NDVI, see Appendix S2), we confirmed that mean annual temperature was indeed positively correlated to both species richness (R2=0.10, p=6e-5) and biomass per area (R2=0.28, p=0.0005).
This led us to interpret the temperature gradient as a climatic gradient influencing plant physiology and phenology but also as a gradient discriminating between sites mainly dominated by biotic vs. abiotic constraints. Such a contrast between the limiting role of abiotic stress at the cold end of the distribution and the primary importance of biotic interactions at the warm end of the distribution has recently been confirmed for several alpine plant species, including P. viviparum (Boulangeat, Gravel & Thuiller 2012). In contrast, even if it is usually an important gradient for alpine vegetation and although we explicitly sampled along it, solar radiation did not explain any FV characteristic at the population level: its influence is therefore not discussed in the following of this article.
(b) Field trait measurements
We sampled 29 populations at altitudes ranging from 1500m to 2950m, covering a large proportion of the climatic space occupied by the species in the study area (99% of the temperature gradient and 53% of the radiation gradient, Fig. S1). Measurements were made at each population’s flowering peak in order to sample each population at the same phenological stage (July 2010). In each population (10m × 10m), three subpopulations of 1m × 1m were selected in order to represent contrasted microenvironmental conditions, using the same methodology as Albert et al. (2010b).
In each subpopulation, the following functional traits were measured on 5 randomly selected individuals: maximum vegetative height (Hmax, top of plant photosynthetic tissue); total length of the inflorescence (Hinflo); ratio of sexual reproduction (SEX, ratio of the length of flowers divided by the total length of the spike); leaf dry matter content (LDMC, the ratio of leaf dry mass over fresh mass); specific leaf area (SLA, the ratio of leaf surface over fresh mass); leaf nitrogen content (LNC, the percentage of nitrogen in the dry mass of the leaf) and carbon to nitrogen ratio (C:N, the ratio of carbon over nitrogen in the leaf dry mass). These traits relate to various aspects of plant functional strategy (Westoby, Falster & Moles 2002), like resource acquisition and growth rate (LDMC, SLA, LNC, C:N), ability for light competition (Hmax) and reproductive effort (Hinflo and SEX). Foliar traits are known to be physiologically correlated due to leaf economics constraints (Wright et al. 2004) and are thus suited to studying phenotypic integration. However, energetic trade-offs could also arise at the whole plant level due to resource allocation conflicts between growth, longevity and reproduction (Chapin 1993; Enquist et al. 1999; see Diggle 1997 for allocation in P. viviparum); our decision to include Hmax, Hinflo and SEX was intended to include this higher-level trade-off.
(c) Characterising functional variability in wild populations
Overall trait variability
To quantify the extent of intraspecific functional variability in the whole dataset and understand the structure of intraspecific FV across spatial scales, we first broke down the variability of each trait at different hierarchical levels using mixed effects regression models. In order to do this, we used intercept models with random effects corresponding to the different levels of hierarchy (i.e. population and subpopulation nested in population). We then extracted the percentage of variance explained by each hierarchical level for each trait. Variance components were estimated using restricted maximum likelihood (REML).
To get a finer understanding of trait variation across our study area, we also examined the response of all traits against the temperature gradient, using linear or quadratic models with the same random effects as above to account for the hierarchical structure of the dataset. P-values for such models were obtained by likelihood ratio tests, using an R function provided by Christopher Moore (http://blog.lib.umn.edu/moor0554/canoemoore/2010/09/lmer_p-values_lrt.html). In order to quantify FV extent for each population, all traits were transformed to have a mean of zero and a standard deviation of one across the whole sample. Thus, all traits have equal importance in the subsequent analyses. For each population, a variance-covariance matrix for the seven traits was built (P-matrices). Overall trait variability (i.e. FV extent) in a population was measured as the trace of P (i.e. the sum of its diagonal elements), a measure commonly used for genetic variance matrices (Revell 2007).
Phenotypic integration: patterns and causes
A matrix of correlations between the seven traits was built for each population (P′-matrices) and the variance of the eigenvalues of P′ was taken as an index of integration (i.e. FV shape, Cheverud, Wagner & Dow 1989), higher values meaning stronger correlations between traits.
The direction of phenotypic integration was compared between populations by determining the axis of maximum phenotypic variation, Pmax (first eigenvector of P, also known as ‘the line of least resistance’, Schluter 1996), for each population. For each couple of populations, one minus the correlation between their Pmax was used to measure the functional distance between them, producing a matrix of functional distances between populations (P-dist).
To test whether and how environmental or genetic constraints drive FV direction, we compared P-dist to different environmental distance matrices (Euclidean distance on the climatic plane defined by temperature and radiation, and Euclidean distance on the temperature gradient only) and geographic distances using Mantel tests. As the influence of gene flow between populations was expected to mainly play a role at small geographic scales (of the same order of magnitude as the species’ dispersal distance), we also used Mantel correlograms to unravel these small-scale dependencies.
We conducted the same analysis on FV direction using Random Skewers (Cheverud 1996) to measure functional distances between populations. Although Random Skewers were originally designed to compare the responses of different populations to putative selection events, they can also be used to compare all kinds of variance-covariance matrices (e.g. Kolbe et al. 2011) and have advantages over Pmax methods in that they compare the properties of entire matrices. This additional procedure was used to back up the results obtained with the Pmax analysis and led to the same conclusions (detailed method, R code and results for Random Skewers available in Appendix S3).
Robustness of matrix estimation
Given our sampling implied a low number of measured individuals within each population (i.e. 15), it could impede robust estimation of the P and P′ of each population. We measured the robustness of matrix estimation using a bootstrapping procedure (Cheverud, Wagner & Dow 1989) and found that on average there are 7.1% of chances that differences between two P matrices are not meaningful and 14.3% of chances for P′ matrices (detailed description in Appendix S5). This uncertainty is however counterbalanced by the two main strengths of our approach which are that (1) we studied FV within and among numerous (i.e. 29) populations of the same species, thereby rendering our analyses less sensible to this matrix estimation error, and (2) followed a stratified hierarchical sampling along in situ and continuous environmental gradients. This should provide a more comprehensive picture of trait variability and integration across the whole niche of the study species than what is generally done under controlled conditions on few discrete environmental conditions.
3. RESULTS
(a) Extent of trait variability
Variance decomposition revealed two different cases. In the case of vegetative height (Hmax), most of the variation (73%) was found between populations. Conversely, for all other traits included in our study, around half of the variance occurred between individuals of the same sub-population (Table 1). Overall, there was little variation between different sub-populations (1-21% depending on the trait). The subsequent analyses carried out at population level were then justified, as FV was rather high within populations.
Table 1. Variance decomposition of the different traits.
The percentage of variance explained by the different hierarchical levels is shown for each of the seven functional traits. Coefficients of variation are presented in the last row.
| Hierarchical level/Trait | Hmax | SEX | Hinflo | LDMC | SLA | C:N | LNC |
|---|---|---|---|---|---|---|---|
| Population | 73 | 17 | 46 | 42 | 56 | 38 | 42 |
| Sub-population | 6 | 21 | 8 | 6 | 10 | 1 | 3 |
| Individuals | 21 | 62 | 46 | 51 | 34 | 61 | 55 |
| Coefficient of variation | 0.43 | 1.52 | 0.34 | 0.14 | 0.28 | 0.26 | 0.24 |
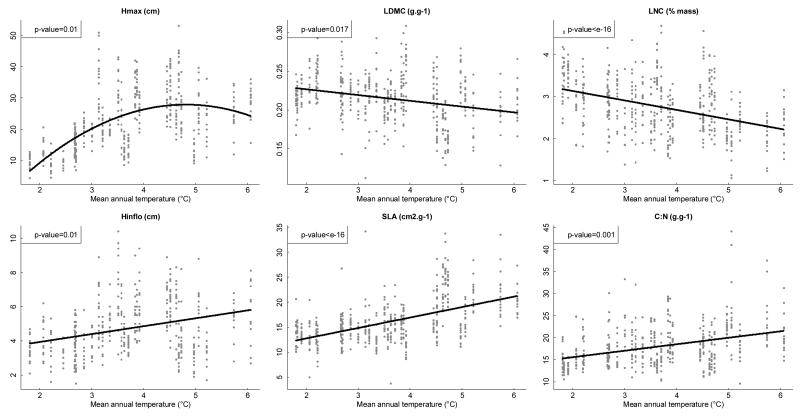
All of the traits we studied except SEX showed a significant relationship with mean annual temperature (see Fig. 2). Mean population values of LDMC and LNC decreased with temperature, while SLA, C:N and Hinflo increased with temperature. Hmax showed a quadratic relationship, reaching a maximum value for intermediate temperatures.
Figure 2. Response of functional traits to the temperature gradient.
Individual trait measures for all traits except SEX are plotted in grey. Black lines show the regression lines (quadratic regression in the case of Hmax).
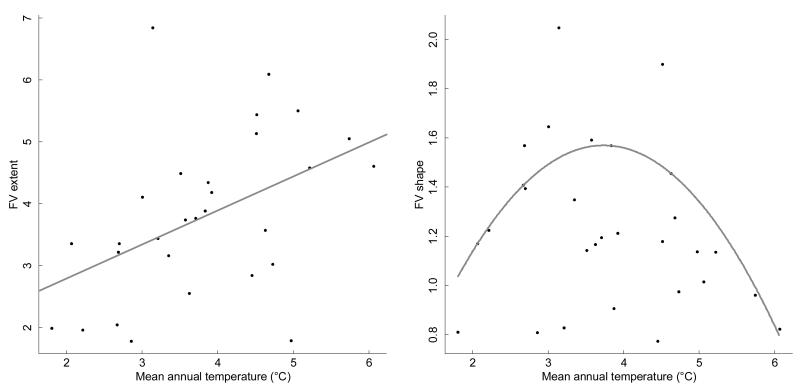
FV extent (overall trait variability) in each population positively correlated with the mean annual temperature of the site (R2 = 18%, p = 0.012, see Fig. 3). No significant relationship was found with solar radiation.
Figure 3.
Left-panel: Relation between overall trait variability (FV extent) and mean annual temperature. Black dots represent each of the 29 populations sampled. The regression line is drawn in grey (p=0.012). Overall trait variability increases with temperature. To get an idea of the unit, the extent of FV across the 29 populations equals 7. Right panel: Relationship between the strength of phenotypic integration (FV shape) and mean annual temperature. The parable represents the quadratic regression for the 75% percentile (p=0.022). The most strongly integrated populations are found on the middle of the gradient. All values are above 0.75, and thus represent significant integration.
(b) Phenotypic integration
The strength of phenotypic integration was in general relatively high for all populations. Indeed, under the assumption of no correlation between the seven traits studied and given that we sampled 15 individuals per population, the expected value for the integration index is 0.4 (Wagner 1984). To evaluate a confidence interval for that value, we built a null distribution for the integration index by randomly sampling seven trait values for 15 individuals (Gaussian traits, 100,000 resamples) and computing the integration index. We obtained a 95% quantile of 0.75. Observed values of phenotypic integration across the 29 populations were always significantly stronger than randomly expected (min=0.77) and were on average rather high (mean=1.23).
This integration showed a triangular relationship with mean annual temperature (Fig. 3). This result was not dependent on the traits included in P (results not shown). Quantile regressions confirmed that the most integrated populations were found at the middle of the temperature gradient, which corresponds to the niche core: the 75% percentile of the distribution of integration values shows a quadratic relationship with temperature (p-value=0.022).
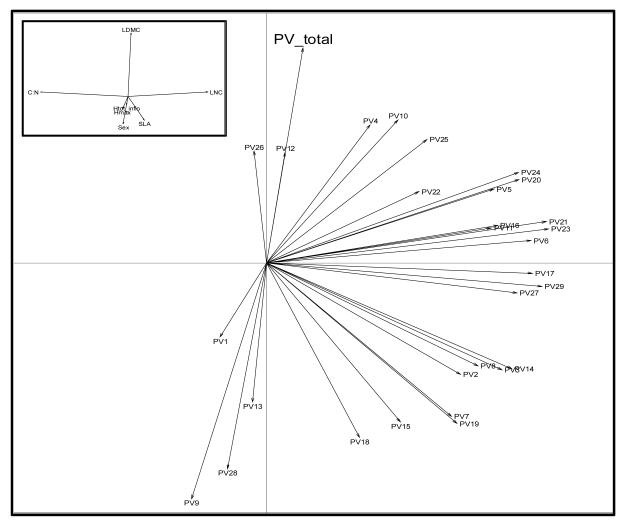
The main directions of phenotypic integration, estimated by Pmax, generally correlated between populations (cor=0.48 ±28). No general line of least resistance emerged although most of the Pmax were directed towards high variance in LNC. Interestingly, the main direction of phenotypic variation for all 29 populations pooled together is orthogonal to this dominant intra-population direction (results from a PCA, see Fig. 4). The differences in the main directions of phenotypic integration between populations were not explained by environmental nor geographic distance, as all the Mantel tests were non-significant (p-values=0.328; 0.793 and 0.668 for environmental, temperature and geographic distances respectively). However FV direction in populations tended to be positively correlated at short distances (<200m, cor= 0.083, p=0.043, Figure S4), but no relation between functional and geographic distances was found for larger distances.
Figure 4. Results of a PCA on the Pmax of the 29 populations studied as well as the general Pmax for all populations combined (PV_total).
The ‘line of least phenotypic resistance’ for each population is projected on the plane defined by the two first PCA axes. The top-left plot shows the different trait variances in relation to the PCA axes. Note that most of the Pmax for populations are directed towards high variance in LNC and low variance in C:N, while the general Pmax is orthogonal to most of them and directed towards high variance in LDMC.
4. DISCUSSION
Our study reveals some general patterns of functional variability in P. viviparum. The primary observation is that trait values are highly variable and that a large proportion of this variation is found between individuals of the same population, confirming previous observations on plants (Albert et al. 2010b; Messier, McGill & Lechowicz 2010). This could be due to high levels of phenotypic plasticity but the fact that our attempt to distinguish sub-populations does not explain much FV may also reveal that microenvironmental heterogeneity plays a role at a smaller scale than the one we chose (1×1m), possibly at individual scale. This highlights the importance of studying FV within populations. However, populations do exhibit some differences and temperature strongly influences mean trait values at the population level, as already observed for various types of alpine plants including P. viviparum (Albert et al. 2010b). High values of LDMC and LNC along with low values of Hmax and SLA for populations at the cold end of the gradient are characteristic of stressful environments (Chapin, Autumn & Pugnaire 1993) and indicate cold tolerance in these populations (Reich et al. 2003).
When trying to understand how FV extent is structured across P. viviparum’s niche, we found that the overall trait variability increases with mean temperature. This pattern supports the hypothesis that habitat filtering prevails in environments dominated by abiotic constraints (cold edge of the niche) and that functional divergence may be high within communities with a high number of interspecific biotic interactions (warm edge of the niche). Such a pattern of increased trait variability in richer communities has already been observed for morphological traits in grasshoppers of the genus Melanoplus (Roff & Mousseau 2005). In the case of P. viviparum, the very harsh conditions experienced in alpine habitats should lead to strong directional selection pressures for increased resistance to cold, drought and high solar radiation, whereas in the warmer subalpine meadows plant competition is expected to be stronger and lead to phenotypic divergence for niche partitioning between interacting individuals, thus resulting in increased trait variance within species (Weiher & Keddy 1995; Cornwell, Schwilk & Ackerly 2006, but see Spasojevic & Suding 2011).
We also observed that phenotypic integration is in general relatively high within P. viviparum populations. This is primarily due to the strong correlations between the four foliar traits included in this study (cor=0.45 ±0.32 in absolute value over the 29 populations), which are known to reflect the worldwide leaf economics spectrum (Wright et al. 2004). The allometric correlation between Hmax and Hinflo explains the rest of this pattern. Yet, no systematic trade-off was detected between reproductive, growth and persistence functions (the mean correlation for Hinflo with any of the foliar traits is always <0.08 in absolute value). Note that the allocation to sexual reproduction (SEX) is a very idiosyncratic trait that does not correlate to the environmental gradients in our study area (contrary to what has been observed in the Arctic by Dormann, Albon & Woodin 2002), nor to any other trait measured.
One important finding is that the most integrated populations are found at the centre of the niche (i.e. middle of the temperature gradient, see Fig. 3). This result is contrary to our expectations and contrasts with some studies in controlled conditions where phenotypic integration has been found to increase with stress (e.g. Schlichting 1989; Gianoli 2004). We suggest that this pattern could be due to demographic asymmetries between the centre and the margins of the niche. Indeed, many theoretical models suggest that the larger population sizes at the centre of the niche lead to a better response to natural selection (e.g. Kirkpatrick & Barton 1997), which could produce integrated, more ‘optimised’ phenotypes. Conversely, marginal populations could be subject to high levels of both genetic drift and gene flow from the central populations, rendering selection inefficient, and leading to low integration (Sexton et al. 2009). In our study area we verified that population size is on average higher in the middle of the temperature gradient (F. Boucher, field observation).
The last attribute of FV that we intended to study was its direction. We found that the main axis of trait variation within populations is often related to variance in leaf nitrogen content, a trait linked to soil nitrogen uptake efficiency in fertile environments (Zatylny & St-Pierre 2006) and which also strongly affects the plant’s photosynthetic rate (Reich, Walters & Ellsworth 1991). This high variation in LNC within populations might be explained by the heterogeneity of the nitrogen supply in soils. This heterogeneity is both qualitative and quantitative: nitrogen can be present either in its organic form, which is costly to acquire, or in the form of ammonia or nitrates, and the amount of these alternative forms varies spatially. Indeed, it has been revealed that fine-scale factors like soil characteristics have a great influence on the functional diversity of plant communities in the Guisane valley (de Bello et al. In press). The slightly lower variances in LNC and C:N found in colder populations of P. viviparum could be due to the predominance of organic nitrogen in high altitude sites (Averill & Finzi 2011) or to less spatial heterogeneity in soil nitrogen concentrations in these habitats, relative to subalpine ones. Interestingly, the main direction of phenotypic variation at the inter-population level is almost orthogonal to this general intra-population direction and lines up with traits that are more directly related to the abiotic environment, supporting the main axis of variation observed at the inter-specific level in plants. Indeed, Hmax strongly correlates with temperature in our study area (Fig. 2), ranging from more than 50cm in subalpine meadows to less than 5cm in the alpine sites. LDMC is also strongly affected by climatic conditions since it is expected to increase leaf longevity and thus resource conservation, which are likely to be favoured in stressful habitats. This suggests that the main functional trade-offs revealed for plants at the interspecific level (e.g. Leaf Economics Spectrum) over large geographic gradients might not be reflected at finer spatial scales (e.g. population level). This finding could have profound implications for the study of local coexistence in community ecology. A similar result has already been found for two forest herb species that show an opposite pattern of correlation between plant height and seed mass at the intraspecific level than the one observed at the interspecific level (De Frenne et al. 2011). The fact that environmental distances do not correlate with functional distances between populations (measured either by Pmax or Random Skewers correlations) confirms that climate is not the main driver of FV direction at the population level. On the contrary, the strong spatial auto-correlation that we found at small distances in the functional structure of populations suggests that high genetic similarity between close populations could result in very similar integration patterns (Stone, Nee & Felsenstein 2011). However, the fact that the main direction of integration differs significantly between populations shows that genetic correlations between traits are not excessively strong (Armbruster & Schwaegerle 1996).
Given that additive genetic variances were not measured and that the level of heritability for each studied trait is uncertain (Ackerly et al. 2000; Geber & Griffen 2003), any potential evolutionary interpretations of the patterns we report must be cautious. It is indeed possible that differences in intraspecific FV structure are only due to differences in genetic diversity in our populations arising from past demographic fluctuations (Wright 1969). However, these differences might as well be largely due to phenotypic plasticity (Pigliucci 2003) and in particular to the fact that individuals living in high-resource environments are expected to be more plastic than their stressed conspecifics (Chapin, Autumn & Pugnaire 1993; Grassein, Till-Bottraud & Lavorel 2010). Integrating direct measurements of genetic diversity and the relatedness of populations in the kind of ecological study proposed herein might constitute a promising avenue for future research (e.g. Martin, Chapuis & Goudet 2008) and will help to disentangle the relative effects of ecology, demography and genetics on the functional variability of populations.
Conclusion
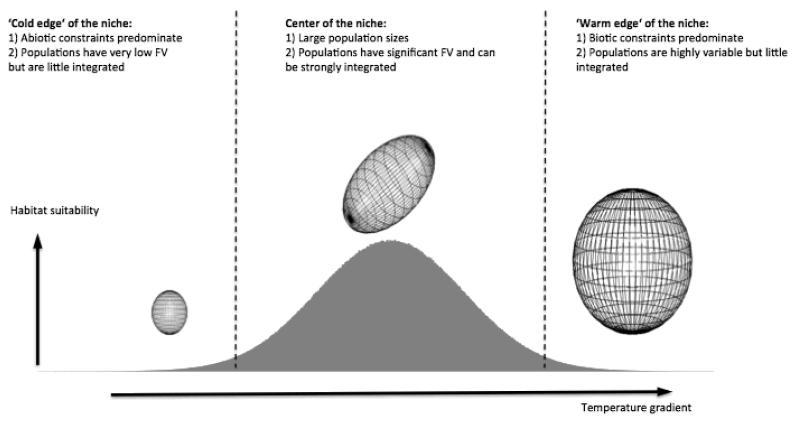
Put together, our results give a clearer picture of how intraspecific FV is structured in different parts of P. viviparum’s environmental niche (Fig. 5). Indeed, populations at the ‘cold end’ of the niche have low FV but are mildly integrated. Populations located at the centre of the niche have intermediate trait variability and varying degrees of integration, but some are subject to strong trade-offs between traits. Finally, populations of the ‘warm end’ of the niche are both highly variable and little integrated. Our study thus shows the importance of the environment in setting FV extent and reveal substantial asymmetry in the species’ environmental niche between its ‘biotic’ and ‘abiotic’ edges. Interestingly enough, this finding corroborates hypotheses and observations traditionally made in community ecology that strong abiotic filters lead to greater functional convergence between species coexisting within natural communities (Webb et al. 2002). Finally, we demonstrate that the main functional trade-offs differ within and among populations and that the idea that phenotypic integration increases in stressful environments cannot be considered a rule.
Figure 5. FV structure in populations located in different parts the environmental niche.
This illustration is meant to summarize the main findings of our study and differences between populations have been exaggerated for clarity. The environmental niche of P. viviparum can be symbolically represented along the temperature gradient, the grey Gaussian curve representing values of habitat suitability. The niche has been cut into three main parts for simplicity, according to the results: the niche centre and the ‘cold’ and ‘warm’ edges. The size and shape of the ellipsoids represent respectively FV extent and shape: smaller volumes meaning low FV, and volumes close to spheres representing less integrated populations.
Supplementary Material
Acknowledgements
We would like to thank the Ecrins National Park, the National Alpine Botanical Conservatory and the Joseph Fourier Alpine Station for their help and the data they provided. J. Renaud provided much help with GIS data and illustrations. We also thank P-A. Jachiet, L. Gallien and C. Roquet for assistance in the field. I. Boulangeat, A. Estoup, M. Evans, O. Gaggiotti, L. Gallien, N. Legay, T. Münkemüller, K. Schiffers as well as five anonymous reviewers provided thoughtful comments on this work. Thanks to Version Originale for checking the English spelling. This work was funded by the ANR EVORANGE (ANR-09-PEXT-011) project. The grant to FB was provided by the Ecole Polytechnique (AMX 2010-2013). CHA was supported by a Marie Curie International Outgoing Fellowship under the 7th European Community Framework Programme (DYVERSE project, no. 272284). WT received funding from the European Research Council under the European Community’s Seven Framework Programme FP7/2007-2013 Grant Agreement no. 281422 (TEEMBIO).
LITERATURE CITED
- Ackerly DD, Dudley SA, Sultan SE, Schmitt J, Coleman JS, Linder R, Sandquist DR, Geber MA, Evans AS, Dawson TE, Lechowicz MJ. The evolution of plant ecophysiological traits: recent advances and future directions. BioScience. 2000;50:979–995. [Google Scholar]
- Albert CH, Thuiller W, Yoccoz NG, Douzet R, Aubert S, Lavorel S. A multi-trait approach reveals the structure and the relative importance of intra- vs. interspecific variability in plant traits. Functional Ecology. 2010a;24:1192–1201. [Google Scholar]
- Albert CH, Thuiller W, Yoccoz NG, Soudant A, Boucher F, Saccone P, Lavorel S. Intraspecific functional variability: extent, structure and sources of variation. Journal of Ecology. 2010b;98:604–613. [Google Scholar]
- Albert CH, Yoccoz NG, Edwards TC, Graham CH, Zimmermann NE, Thuiller W. Sampling in ecology and evolution - bridging the gap between theory and practice. Ecography. 2010c;33:1028–1037. [Google Scholar]
- Armbruster WS, Pelabon C, Hansen TF, Bolstad GH. Macroevolutionary patterns of pollination accuracy: a comparison of three genera. New Phytologist. 2009;183:600–617. doi: 10.1111/j.1469-8137.2009.02930.x. [DOI] [PubMed] [Google Scholar]
- Armbruster WS, Schwaegerle KE. Causes of covariation of phenotypic traits among populations. Journal of Evolutionary Biology. 1996;9:261–276. [Google Scholar]
- Arnold SJ, Burger R, Hohenlohe PA, Ajie BC, Jones AG. Understanding the evolution and stability of the G-matrix. Evolution. 2008;62:2451–2461. doi: 10.1111/j.1558-5646.2008.00472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averill C, Finzi AC. Increasing plant use of organic nitrogen with elevation is reflected in nitrogen uptake rates and ecosystem delta N-15. Ecology. 2011;92:883–891. doi: 10.1890/10-0746.1. [DOI] [PubMed] [Google Scholar]
- Benichou P, Le Breton O. Prise en compte de la topographie pour la cartographie des champs pluviométriques statistiques. La Météorologie. 1987;7:23–34. [Google Scholar]
- Berg RL. The ecological significance of correlation pleiades. Evolution. 1960;14:171–180. [Google Scholar]
- Boulangeat I, Gravel D, Thuiller W. Accounting for dispersal and biotic interactions to disentangle the drivers of species distributions and their abundances. Ecology Letters. 2012;15:584–593. doi: 10.1111/j.1461-0248.2012.01772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown WL, Wilson EO. Character displacement. Systematic Zoology. 1956;5:49–64. [Google Scholar]
- Chapin FS, Autumn K, Pugnaire F. Evolution of suites of traits in response to environmental stress. The American Naturalist. 1993;142:S78–S92. [Google Scholar]
- Cheverud JM. Quantitative genetic analysis of cranial morphology in the cotton-top (Saguinus oedipus) and saddle-back (S-fuscicollis) tamarins. Journal of Evolutionary Biology. 1996;9:5–42. [Google Scholar]
- Cheverud JM, Wagner GP, Dow MM. Methods for the comparative analysis of variation patterns. Systematic Zoology. 1989;38:201–213. [Google Scholar]
- Cornwell WK, Schwilk DW, Ackerly DD. A trait-based test for habitat filtering: Convex hull volume. Ecology. 2006;87:1465–1471. doi: 10.1890/0012-9658(2006)87[1465:attfhf]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Cowling RM, Esler KJ, Midgley GF, Honig MA. Plant functional diversity, species diversity and climate in arid and semi-arid southern Africa. Journal of Arid Environments. 1994;27:141–158. [Google Scholar]
- de Bello F, Leps J, Sebastia MT. Variations in species and functional plant diversity along climatic and grazing gradients. Ecography. 2006;29:801–810. [Google Scholar]
- de Bello F, Lavorel S, Albert CH, Thuiller W, Grigulis K, Dolezal J, Janecek S, Leps J. Quantifying the relevance of intraspecific trait variability for functional diversity. Methods in Ecology and Evolution. 2011;2:163–174. [Google Scholar]
- de Bello F, Lavorel S, Lavergne S, Albert CH, Boulangeat I, Mazel F, Thuiller W. Hierarchical effects of environmental filters on the functional structure of plant communities: a case study in the French Alps. Ecography. 2013;36(3):393–402. [Google Scholar]
- De Frenne P, Graae BJ, Kolb A, Shevtsova A, Baeten L, Brunet J, Chabrerie O, Cousins SAO, Decocq G, Dhondt R, Diekmann M, Gruwez R, Heinken T, Hermy M, Oester M, Saguez R, Stanton S, Tack W, Vanhellemont M, Verheyen K. An intraspecific application of the leaf-height-seed ecology strategy scheme to forest herbs along a latitudinal gradient. Ecography. 2011;34:132–140. [Google Scholar]
- Diggle PK. Extreme preformation in alpine Polygonum viviparum: An architectural and developmental analysis. American Journal of Botany. 1997;84:154–169. [PubMed] [Google Scholar]
- Dormann CF, Albon SD, Woodin SJ. No evidence for adaptation of two Polygonum viviparum morphotypes of different bulbil characteristics to length of growing season: abundance, biomass and germination. Polar Biology. 2002 doi:10.1007/s00300-002-0417-4. [Google Scholar]
- Enquist BJ, West GB, Charnov EL, Brown JH. Allometric scaling of production and life-history variation in vascular plants. Nature. 1999;401:907–911. [Google Scholar]
- Geber MA, Griffen LR. Inheritance and natural selection on functional traits. International Journal Of Plant Sciences. 2003;164:S21–S42. [Google Scholar]
- Gianoli E. Plasticity of traits and correlations in two populations of Convolvulus arvensis (Convolvulaceae) differing in environmental heterogeneity. International Journal Of Plant Sciences. 2004;165:825–832. [Google Scholar]
- Gomulkiewicz R, Houle D. Demographic and Genetic Constraints on Evolution. American Naturalist. 2009;174:E218–E229. doi: 10.1086/645086. [DOI] [PubMed] [Google Scholar]
- Grassein F, Till-Bottraud I, Lavorel S. Plant resource-use strategies: the importance of phenotypic plasticity in response to a productivity gradient for two subalpine species. Annals of Botany. 2010;106:637–645. doi: 10.1093/aob/mcq154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung V, Violle C, Mondy C, Hoffmann L, Muller S. Intraspecific variability and trait-based community assembly. Journal of Ecology. 2010;98:1134–1140. [Google Scholar]
- Keddy PA. Assembly and response rules - two goals for predictive community ecology. Journal of Vegetation Science. 1992;3:157–164. [Google Scholar]
- Kirkpatrick M, Barton NH. Evolution of a species’ range. The American Naturalist. 1997;150:1–23. doi: 10.1086/286054. [DOI] [PubMed] [Google Scholar]
- Kolbe JJ, Revell LJ, Szekely B, Brodie ED, III, Losos JB. Convergent evolution of phenotypic integration and its alignment with morphological diversification in Caribbean Anolis ecomorphs. Evolution. 2011;65:3608–3624. doi: 10.1111/j.1558-5646.2011.01416.x. [DOI] [PubMed] [Google Scholar]
- Körner C. Alpine Plant Life. Springer-Verlag; Berlin: 1999. [Google Scholar]
- Lavergne S, Mouquet N, Thuiller W, Ronce O. Biodiversity and climate change: Integrating evolutionary and ecological responses of species and communities. Annual Review of Ecology Evolution and Systematics. 2010;41:321–350. [Google Scholar]
- Martin G, Chapuis E, Goudet J. Multivariate Q(st)-F(st) Comparisons: A Neutrality Test for the Evolution of the G Matrix in Structured Populations. Genetics. 2008;180:2135–2149. doi: 10.1534/genetics.107.080820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messier J, McGill BJ, Lechowicz MJ. How do traits vary across ecological scales? A case for trait-based ecology. Ecology Letters. 2010;13:838–848. doi: 10.1111/j.1461-0248.2010.01476.x. [DOI] [PubMed] [Google Scholar]
- Ordano M, Fornoni J, Boege K, Dominguez CA. The adaptive value of phenotypic floral integration. New Phytologist. 2008;179:1183–1192. doi: 10.1111/j.1469-8137.2008.02523.x. [DOI] [PubMed] [Google Scholar]
- Pigliucci M. Phenotypic integration: studying the ecology and evolution of complex phenotypes. Ecology Letters. 2003;6:265–272. [Google Scholar]
- Reich PB, Walters MB, Ellsworth DS. Leaf age and season influence the relationships between leaf nitrogen, leaf mass per area and photosynthesis in maple and oak trees. Plant Cell and Environment. 1991;14:251–259. [Google Scholar]
- Reich PB, Wright IJ, Cavender-Bares J, Craine JM, Oleksyn J, Westoby M, Walters MB. The evolution of plant functional variation: traits, spectra, and strategies. International Journal of Plant Science. 2003;164:143–164. [Google Scholar]
- Revell LJ. The G matrix under fluctuating correlational mutation and selection. Evolution. 2007;61:1857–1872. doi: 10.1111/j.1558-5646.2007.00161.x. [DOI] [PubMed] [Google Scholar]
- Roff DA, Mousseau T. The evolution of the phenotypic covariance matrix: evidence for selection and drift in Melanoplus. Journal of Evolutionary Biology. 2005;18:1104–1114. doi: 10.1111/j.1420-9101.2005.00862.x. [DOI] [PubMed] [Google Scholar]
- Schlichting CD. Phenotypic plasticity in Phlox. 2. Plasticity of character correlations. Oecologia. 1989;78:496–501. doi: 10.1007/BF00378740. [DOI] [PubMed] [Google Scholar]
- Schluter D. Adaptive radiation along genetic lines of least resistance. Evolution. 1996;50:1766–1774. doi: 10.1111/j.1558-5646.1996.tb03563.x. [DOI] [PubMed] [Google Scholar]
- Sexton JP, McIntyre PJ, Angert AL, Rice KJ. Evolution and Ecology of Species Range Limits. Annual Review of Ecology Evolution and Systematics. 2009:415–436. [Google Scholar]
- Shipley B, Almeida-Cortez J. Interspecific consistency and intraspecific variability of specific leaf area with respect to irradiance and nutrient availability. Ecoscience. 2003;10:74–79. [Google Scholar]
- Sokal RR. Population differentiation: Something new or more of the same? In: Brussard PF, editor. Ecological Genetics: The Interface. Springer-Verlag; New York: 1978. pp. 215–239. [Google Scholar]
- Spasojevic MJ, Suding KN. Inferring community assembly mechanisms from functional diversity patterns: the importance of multiple assembly processes. Journal of Ecology. 2012;100(3):652–661. [Google Scholar]
- Stone GN, Nee S, Felsenstein J. Controlling for non-independence in comparative analysis of patterns across populations within species. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences. 2011;366:1410–1424. doi: 10.1098/rstb.2010.0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuiller W, Albert CH, Dubuis A, Randin C, Guisan A. Variation in habitat suitability does not always relate to variation in species’ plant functional traits. Biology Letters. 2010;6:120–123. doi: 10.1098/rsbl.2009.0669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonsor SJ, Scheiner SM. Plastic trait integration across a CO2 gradient in Arabidopsis thaliana. American Naturalist. 2007;169:E119–E140. doi: 10.1086/513493. [DOI] [PubMed] [Google Scholar]
- Violle C, Enquist BJ, McGill BJ, Jiang L, Albert CH, Hulshof C, Jung V, Messier J. The return of the variance: intraspecific variability in community ecology. Trends in Ecology & Evolution. 2012;27:244–252. doi: 10.1016/j.tree.2011.11.014. [DOI] [PubMed] [Google Scholar]
- Wagner GP. On the eigenvalue distribution of genetic and phenotypic dispersion matrices - Evidence for a nonrandom organization of quantitative character variation. Journal of Mathematical Biology. 1984;21:77–95. [Google Scholar]
- Webb CO, Ackerly DD, McPeek MA, Donoghue MJ. Phylogenies and community ecology. Annual Review of Ecology and Systematics. 2002;33:475–505. [Google Scholar]
- Webb CT, Hoeting JA, Ames GM, Pyne MI, Poff NL. A structured and dynamic framework to advance traits-based theory and prediction in ecology. Ecology Letters. 2010;13:267–283. doi: 10.1111/j.1461-0248.2010.01444.x. [DOI] [PubMed] [Google Scholar]
- Weiher E, Keddy P. The assembly of experimental wetland plant communities. Oikos. 1995;73:323–335. [Google Scholar]
- Westoby M, Falster DS, Moles AT. Plant ecological strategies: Some Leading Dimensions of Variation Between Species. Annu. Rev. Ecol. Syst. 2002;33:125–159. [Google Scholar]
- Wilson AJ, Nussey DH. What is individual quality? An evolutionary perspective. Trends in Ecology & Evolution. 2010;25:207–214. doi: 10.1016/j.tree.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, Cavender-Bares J, Chapin T, Cornelissen JHC, Diemer M, Flexas J, Garnier E, Groom PK, Gulias J, Hikosaka K, Lamont BB, Lee T, Lee W, Lusk C, Midgley JJ, Navas M-L, Niinemets U, Oleksyn J, Osada N, Poorter H, Poot P, Prior L, Pyankov VI, Roumet C, Thomas SC, Tjoelker MG, Veneklaas EJ, Villar R. The worldwide leaf economics spectrum. Nature. 2004;428:821–827. doi: 10.1038/nature02403. [DOI] [PubMed] [Google Scholar]
- Wright S. Evolution and the Genetics of Populations, the Theory of Gene Frequencies. University of Chicago Press; Chicago: 1969. [Google Scholar]
- Zatylny A, St-Pierre R. Nitrogen uptake, leaf nitrogen concentration, and growth of saskatoons in response to soil nitrogen fertility. Journal of Plant Nutrition. 2006;29:209–218. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.