Abstract
Visceral leishmaniasis (VL) is endemic in Northwest and southern Iran. Reports of cutaneous leishmaniasis (CL) in Northwest areas are rare, and its etiological agents are unknown. In the current study, we report six CL and two post kala-azar dermal leishmaniasis (PKDL) cases caused by Leishmania infantum from endemic areas of VL in the Northwest. Smears were made from skin lesions of 30 suspected patients in 2002–2011, and CL was determined by microscopy or culture. Leishmania spp. were identified by nested-PCR assay. The disease was confirmed in 20 out of 30 (66%) suspected patients by parasitological examinations. L. infantum was identified in eight and Leishmania major in 12 CL cases by nested-PCR. Cutaneous leishmaniasis patients infected with L. major had the history of travel to CL endemic areas. L. infantum antibodies were detected by direct agglutination test (DAT) at titers of 1:3200 in two cases with history of VL. Results of this study indicated that L. infantum is a causative agent of CL as well as PKDL in the VL endemic areas.
Keywords: Cutaneous leishmaniasis, Visceral leishmaniasis, Leishmania infantum, Post kala-azar dermal leishmaniasis, Nested-PCR, Iran
Introduction
Leishmaniasis occurs in 98 countries, varying considerably in severity and importance.1 Reports indicate 350–400 million people at risk and about 12 million victims worldwide.1,2 Leishmaniasis has been clinically classified into at least three primary categories including visceral (VL), cutaneous (CL), and mucocutaneous forms.2,3
Iran is an important focus of CL and VL in the Middle East.2,4 Rarely, mucosal and lymphatic leishmaniasis have also been reported in the country. Cutaneous leishmaniasis caused by Leishmania major and Leishmania tropica is a major health problem in Iran with incidence increasing in recent years.4,5 Leishmania infantum infections have been reported in canines,6,7 humans,8 Phlebotumus kandelakii,9 Phlebotumus major,10 and P. perfiliewi transcaucasicus11,12 but rarely in rodents.13 In recent years, cases of skin lesions similar to those in CL have been reported in northwestern (Ardabil and East Azerbaijan provinces) and southern parts (Fars province) of Iran, and the etiological agents are being sought.14,15 This study was conducted to determine parasitological, serological, and molecular aspects of CL in endemic areas of VL in Iran.
Materials and Methods
Sampling and patients
From April 2002 to August 2011, samples were collected from 30 suspected CL patients from Ardabil and East Azerbaijan provinces in Northwest Iran. The samples were collected by trained health workers from all suspected CL patients who had skin lesions for at least a duration of 2 weeks. Smears were prepared from the margins of scraped lesions. Fine-needle aspiration was performed with a sterile syringe with saline (0.1 ml) and a 26-gage needle. The needle was inserted into the outer border of the lesion, then saline was injected and the tissue fluid was aspirated back into the needle. Then the materials were air dried and fixed with 100% methanol prior, and stained with 10% Giemsa. Examinations (culture and microscopy) were used to confirm CL. Amastigote forms of Leishmania spp. were determined under light microscopy at high magnification (1000×). Fluid materials from skin lesions was cultured in NNN and RMPI1640.16 Only one case was positive in culture and the other cases (19) were microscopically positive. This study was reviewed and approved by the ethical committee of the Center of Diseases Control (CDC), health deputy, Ministry of Health and Medical Education, Islamic Republic of Iran.
Serology
Finger prick blood samples (∼50 ul) were taken by sterile lancets, and sera were separated immediately by centrifugation. The titer of anti-L. infantum antibodies were detected by the direct agglutination test (DAT).8,17,18
Molecular study
Smears wiped off with the xylol and paper tissue were then scraped with a sterile scalpel and the entire DNA in the smear was extracted by digestion, in a 1.5 ml micro tube with 200 μl lysis buffer. DNA was extracted by standard protocols with a DNA extraction and purification kit (Qiagen, Germany).19–21 The DNA samples were stored at 4°C. Nested-PCR was conducted on the 20 confirmed CL cases, following the method described by Ghasemian et al.,20 using two pairs of special primers of kDNA (external and internal primers). The primers CSB1XR and CSB2XF for the first round, and LiR and 13Z for the second, were used to amplify the variable segments on minicircles of kDNA from Leishmania parasites.
After PCR amplification, amplicons (PCR products) of the second round were analyzed on 2% (w/w) agarose gel under UV light. DNA extracted from promastigote cultures of reference strains of L. infantum (MCAN/IR/07/Moheb-gh.), L. major (MHOM/IR/75/ER), and L. tropica (MHOM/IR/02/Mash10) were run on each gel as positive controls. Negative controls (the products of PCR in which ultrapure water replaced the template DNA) were also run. The size of each amplicon detected was estimated by comparison with a 100–1500 bp molecular-weight ladder (Roche) run on the same gel (Fig. 1).19–21
Figure 1.
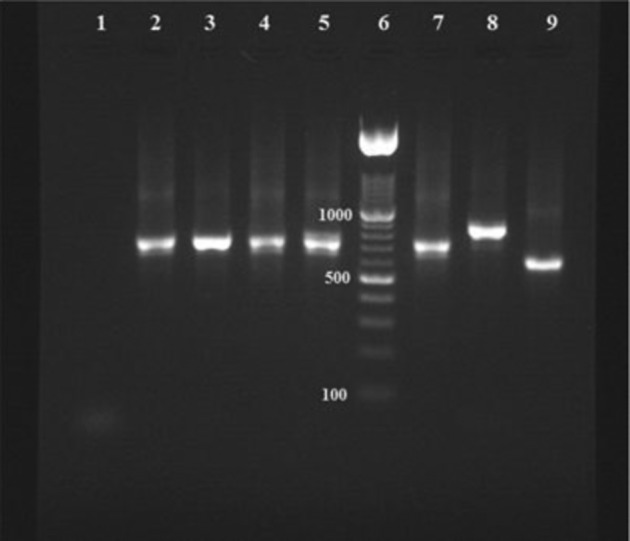
Nested-PCR-based amplification of kDNA extracted from Giemsa-stained lesion smears; lane 1: negative control; lanes 2, 3, 4, and 5: positive samples of CL patients due to Leishmania infantum; lane 6: 100–1500 bp molecular-weight ladder (Roche); lanes 7, 8, and 9: positive control of L. infantum (680 bp); L. tropica (750 bp); and Leishmania major (560 bp), respectively.
Results
Twenty of the 30 (66%) samples were positive for Leishmania spp. The positive smears were examined by nested-PCR, and L. infantum was identified as the causative agent in eight children aged ≤5 years (Table 1). Leishmania major was indicated as the agent in the remaining 12 patients. Cases with L. major had a history of travel to endemic regions of zoonotic CL in Iran. Post kala-azar dermal leishmaniasis (PKDL) cases caused by L. infantum were identified in two boys lesser than 5 years of age with a history of VL (∼1.5 years ago) who showed anti-L. infantum antibodies at titers of 1:3200.
Table 1. Characteristics of patients with Leishmania infantum infections.
| Patient | Age (year) | Sex | History of visceral leishmaniasis (VL) | No. of lesions | Location of lesions | Type of lesions | Treatment of Lesions• | Cured/failure | Diagnosis | ||
| Parasitology•• | Serology | Nested-PCR | |||||||||
| 1 | 2 | Male | Negative | 1 | Face | Nodule | Yes | Cured | 1+ | Negative | L. infantum |
| 2 | 1 | Female | Negative | 1 | Face | Ulcer | Yes | Cured | 4+ | Negative | L. infantum |
| 3 | 4 | Male | Positive | 1 | Face | Ulcer | Yes | Cured | 2+ | 1:3200 | L. infantum |
| 4 | 4 | Female | Positive | 1 | Face | Ulcer | Yes | Cured | 4+ | 1:3200 | L. infantum |
| 5 | 5 | Male | Negative | 1 | Face | Ulcer | Yes | Cured | 2+ | Negative | L. infantum |
| 6 | 3 | Male | Negative | 1 | Face | Ulcer | Yes | Cured | 2+ | Negative | L. infantum |
| 7 | 4 | Female | Negative | 1 | Face | Ulcer | Yes | Cured | 3+ | Negative | L. infantum |
| 8 | 1 | Male | Negative | 1 | Face | Ulcer | Yes | Cured | 2+ | Negative | L. infantum |
• Treatment with meglumine antimoniate (Glucantime®) drug (20 mg/kg for 14–28 days IM and/or intra lesions).
••Parasitemia grade.
Bold indicates innovations.
Their skin lesions caused by L. infantum were single, relatively ulcerative, occurred on the face, and persisted for about 1 year (Fig. 2). On average, the amastigote forms of L. infantum were smaller than L. major, usually less than 3 μm and they were not vacuolated. Both cases of PKDL were successfully treated by meglumine antimoniate (Glucantime®, Rorer Rhone-Poulenc Specia, Paris, France) administered intramuscularly at 20 mg/kg body weight daily for 28 days.
Figure 2.
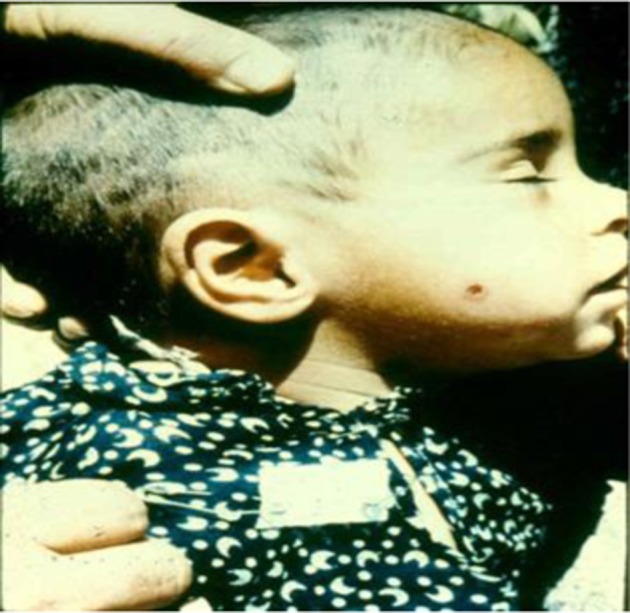
Post kala-azar dermal leishmaniasis (PKDL) due to Leishmania infantum, DAT positive and history of visceral leishmaniasis (VL) from Ardabil province.
Discussion
Leishmaniasis is an emerging disease in immunosuppressed patients and travelers with a wide clinical spectrum. Clinical forms of leishmaniasis in developed and non-endemic countries have increased sharply over the past decade.1 This can be associated with increasing international tourism, military operations, and immigration from endemic countries.1 Post kala-azar dermal leishmaniasis due to Leishmania donovani is prevalent in India and the Sudan, while PKDL caused by L. infantum is rare, with few reported cases. Dereure et al. identified L. infantum from a PKDL case,22 and an additional report of PKDL caused by L. infantum occurring 13 months after a diagnosis of VL was confirmed by molecular methods in an AIDS patient.23 Stark et al. reported the first case of PKDL due to L. infantum in a human immunodeficiency virus type 1-infected patient in Australia.24
From 2002–2011, we confirmed eight CL cases caused by L. infantum, two of which had a history of VL. Previously, three cases of PKDL of unknown causative agent were reported from Northwest Iran by Mohebali et al.14 Five cases of PKDL of unknown etiology have been reported from Shiraz City in southern Iran where VL is endemic.15 All the PKDL cases due to L. infantum in the present study showed a history of VL 5 years earlier and were DAT positive. Post kala-azar dermal leishmaniasis caused by L. donovani in India and Sudan have been reported in at least 10–15% of VL cases.2 Ulcerative lesions are rare in Indian PKDL, because the lesions are usually closed and present as macular or popular, and nodular shapes,25 while skin lesions in the present study were usually open, restricted to the face, and of nearly a year duration.
Leishmania infantum has been previously reported as a causative agent of CL in the Middle East. Most cases of CL in Tunisia are associated with L. infantum named sporadic cutaneous leishmaniasis and CL caused by L. infantum was reported from Italy.26–28
Our findings indicate that L. infantum is a causative agent of CL and PKDL in VL endemic areas of Iran. International travels to Mediterranean countries are suggested as a risk factor for leishmaniasis. Leishmaniasis should be considered in patients presenting with a clinical symptoms consistent with the disease and a history of travel to an endemic area, even if the travel was several months or years earlier.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Acknowledgments
This study received financial support from Tehran University of Medical Sciences (Project No: 89-04-27-11828) and also National Institute of Health Research (NIHR), Islamic Republic of Iran (Meshkin-Shahr research station). The present work was a part of MSc thesis. We wish to thank Dr MM Gooya and Dr MR Shirzadi from the Center of Communicable Diseases Control, Ministry of Health, Treatment and Medical Education, Tehran, Iran. We thank Dr F Pourfarzi, Dr Barak, Mr Rafei from Ardabil University of Medical Sciences, as well as Mrs N Mirsamadi from the East Azerbaijan Health Centre and Mrs S Charehdar from the Leishmaniasis laboratory at the School of Public Health, Tehran University of Medical Sciences.
References
- 1.Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, et al. Leishmaniasis worldwide and global estimates of its incidence. PloS one. 2012;7(5):356–71. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. 2010. Report of a meeting of the WHO expert committee on the control of leishmaniases, 22–26 March 2010. Geneva. [Google Scholar]
- 3.Reithinger R, Dujardin JD, Louzir H, Pirmez C, Alexander B, Brooker S. Cutaneous leishmaniasis. Lancet Infect Dis. 2007;7:581–96. doi: 10.1016/S1473-3099(07)70209-8. [DOI] [PubMed] [Google Scholar]
- 4.Edrissian GhH. Visceral leishmaniasis in Iran and the role of serological tests in diagnosis and epidemiological studies. Parasitology for 21st Century (ICOPA VIII) Izmir, Turkey: CAB International; 1996. pp. 63–78. [Google Scholar]
- 5.Mohebali M, Javadian E, Yaghoobi-Ershadi MR, Akhavan AA, Hajjaran H, Abaei MR. Characterization of Leishmania infection in rodents from endemic areas of the Islamic Republic of Iran. East Mediterr Health J. 2004;10(4/5):591–9. [PubMed] [Google Scholar]
- 6.Mohebali M, Hajjaran H, Hamzavi Y, Mobedi I, Arshi S, Zarei Z, et al. Epidemiological aspects of canine visceral leishmaniasis in the Islamic Republic of Iran. Vet Parasitol. 2005;129(3–4):243–51. doi: 10.1016/j.vetpar.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 7.Parvizi P, Mazloumi-Gavgani A, Davies C, Courtenay O, Ready P. Two Leishmania species circulating in the Kaleybar focus of infantile visceral leishmaniasis, northwest Iran: implications for deltamethrin dog collar intervention. Trans R Soc Trop Med Hyg. 2008;102(9):891–7. doi: 10.1016/j.trstmh.2008.04.026. [DOI] [PubMed] [Google Scholar]
- 8.Mohebali M, Edrissian Gh H, Shirzadi MR, Akhoundi B, Hajjaran H, Zarei Z, et al. An observational study on the current distribution of visceral leishmaniasis in different geographical zones of Iran and implication to health policy. Travel Med Infect Dis. 2011;9:67–74. doi: 10.1016/j.tmaid.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Rassi Y, Javadian E, Nadim A, Zahraii A, Vatandoost H, Motazedian H, et al. Phlebotomus (Larroussius) kandelakii the principal and proven vector of visceral leishmaniasis in north west of Iran. Pak J Biol Sci. 2005: 8(12); pp. 1802–6. [Google Scholar]
- 10.Azizi K, Rassi Y, Javadian E, Motazedian M, Asgari Q, Yaghoobi-Ershadi M. First detection of Leishmania infantum in Phlebotomus (Larroussius) major (Diptera: Psychodidae) from Iran. J Med Entomol. 2008;45(4):726–31. doi: 10.1603/0022-2585(2008)45[726:fdolii]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 11.Rassi Y, Javadian E, Nadim A, Rafizadeh S, Zahraii A, Azizi K, Mohebali M. Phlebotomus perfiliewi transcaucasicus, a vector of Leishmania infantum in northwestern Iran. J Med Entomol. 2009;46(5):1094–8. doi: 10.1603/033.046.0516. [DOI] [PubMed] [Google Scholar]
- 12.Oshaghi MA, Ravasan NM, Hide M, Javadian EA, Rassi Y, Sadraei J, et al. Phlebotomus perfiliewi transcaucasicus is circulating both Leishmania donovani and L. infantum in northwest Iran. Exp Parasitol. 2009;123(3):218–25. doi: 10.1016/j.exppara.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Mohebali M, Poormohammadi B, Kanani A, Hajjaran H, Edrissian G, Rodents H: another group of animal reservoir hosts of visceral leishmaniasis in Meshkin-Shahr district, the Islamic Republic of Iran. East Mediterr Health J 199842376–8. [Google Scholar]
- 14.Mohebali M, Nadim A, Tahvildar-Bidruni G. Report of cutaneous leishmaniasis after kala-azar in Moghan city from Ardebil province. Hakim (in persian) 1998;1(1):57–61. [Google Scholar]
- 15.Kumar PV, Sadeghi E, Torabi S. Kala-azar with disseminated dermal leishmaniasis. Am J Trop Med Hyg. 1989;40(2):150–3. doi: 10.4269/ajtmh.1989.40.150. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization. Basic laboratory methods in medical parasitology. 1st ed. Geneva: 1991. [Google Scholar]
- 17.Fakhar M, Motazedian M, Hatam G, Asgari Q, Kalantari M, Mohebali M. Asymptomatic human carriers of Leishmania infantum: possible reservoirs for Mediterranean visceral leishmaniasis in southern Iran. Ann Trop Med Parasitol. 2008;102(7):577–83. doi: 10.1179/136485908X337526. [DOI] [PubMed] [Google Scholar]
- 18.Abass E, Mahamoud AH, Mansour D, Mohebali M, Harith A. Validation of a β-ME ELISA for detection of anti Leishmania donovani antibodies in eastern Sudan. Iran J Immunol. 2011;8(3):150–8. [PubMed] [Google Scholar]
- 19.Yokota M, Tatsumi N, Tsuda I, Yano I. DNA extraction and amplification from Giemsa-stained blood smears. J Clin Lab Anal. 1995;9(6):387–91. doi: 10.1002/jcla.1860090609. [DOI] [PubMed] [Google Scholar]
- 20.Ghasemian M, Maraghi S, Samarbafzadeh A, Jelowdar A, Kalantari M. The PCR-based detection and identification of the parasites causing human cutaneous leishmaniasis in the Iranian city of Ahvaz. Ann Trop Med Parasitol. 2011;105(3):209–15. doi: 10.1179/136485911X12899838683520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Motazedian H, Karamian M, Noyes H, Ardehali S. DNA extraction and amplification of Leishmania from archived, Giemsa-stained slides, for the diagnosis of cutaneous leishmaniasis by PCR. Ann Trop Med Parasitol. 2002;96(1):31–4. doi: 10.1179/000349802125000484. [DOI] [PubMed] [Google Scholar]
- 22.Dereure J, El-Safi SH, Bucheton B, Boni M, Kheir MM, Davoust B, et al. Visceral leishmaniasis in eastern Sudan: parasite identification in humans and dogs; host-parasite relationships. Microbes Infect. 2003;5:1103–8. doi: 10.1016/j.micinf.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 23.Ridolfo AL, Gervasoni C, Antinori S, Pizzuto M, Santambrogio S, Trabattoni D, et al. Post-kala-azar dermal leishmaniasis during highly active antiretroviral therapy in an AIDS patient infected with Leishmania infantum. J Infect. 2000;40:199–202. doi: 10.1053/jinf.1999.0630. [DOI] [PubMed] [Google Scholar]
- 24.Stark D, Pett S, Marriott D, Harkness J. Post-kala-azar dermal leishmaniasis due to Leishmania infantum in a human immunodeficiency virus type 1-infected patient. J Clin Microbiol. 2006;44(3):1178–80. doi: 10.1128/JCM.44.3.1178-1180.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zijlstra EE, Musa AM, Khalil EAG, El Hassan IM, El Hassan AM. Post-kala-azar dermal leishmaniasis. Lancet Infect Dis. 2003;3(2):87–98. doi: 10.1016/s1473-3099(03)00517-6. [DOI] [PubMed] [Google Scholar]
- 26.BenSaid M, Guerbouj S, Saghrouni F, Fathallah-Mili A, Guizani I. Occurrence of Leishmania infantum cutaneous leishmaniasis in central Tunisia. Trans R Soc Trop Med Hyg. 2006;100(6):521–6. doi: 10.1016/j.trstmh.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 27.Ben-Ismail R, Smith D, Ready P, Ayadi A, Gramiccia M, Ben-Osman A. Sporadic cutaneous leishmaniasis in north Tunisia: identification of the causative agent as Leishmania infantum by the use of a diagnostic deoxyribonucleic acid probe. Trans R Soc Trop Med Hyg. 1992;86(5):508–10. doi: 10.1016/0035-9203(92)90087-s. [DOI] [PubMed] [Google Scholar]
- 28.Gramiccia M, Gradoni L, Pozio E. Leishmania infantum as an agent of cutaneous leishmaniasis in Abruzzi region (Italy). Trans R Soc Trop Med Hyg. 1987;81(2):235–7. doi: 10.1016/0035-9203(87)90225-2. [DOI] [PubMed] [Google Scholar]


