Abstract
Objective
The study was intended to document the clinical profile and treatment outcome of severe malaria caused by Plasmodium vivax (P.vivax) in children hospitalized in a tertiary care centre of northern India.
Methods
This prospective observational study was performed among children admitted with severe malaria at a tertiary care referral hospital of northern India from January 2012 to December 2012. Information was recorded pertaining to clinical symptoms at presentation, examination findings, biochemical and hematological investigation, and treatment outcome. Presence of malarial parasite on thick and thin smears and/or positive parasite lactate dehydrogenase (p-LDH) based rapid malaria antigen test was considered diagnostic of ‘malaria’. Based on the etiology, children were categorized into three groups: P.vivax, Plasmodium falciparum (P. falciparum) and mixed infection. Children diagnosed with ‘severe malaria’ (World Health Organization, 2000), were started on intravenous artesunate followed by artemether-lumefantrine combination.
Results
Thirty-five children with a diagnosis of severe malaria were enrolled [18 (51.4%) P. vivax, nine (25.7%) mixed infection, eight (22.8%) P. falciparum]. Clinical features of severe vivax malaria (n = 18) were abnormal sensorium [9 (50%)], multiple seizures [8 (44.4%)], jaundice [5 (27.8%)], severe anaemia [5 (27.8%)], and shock [3 (16.7%)]. Two children [2/18 (11.1%)] infected with P. vivax had died of cerebral malaria, acute respiratory distress syndrome, shock, and metabolic acidosis. The clinical presentation and outcome of severe vivax malaria was found to be similar to severe malaria caused by P. falciparum and mixed infection, except for higher chances of severe anaemia among the children infected with P. falciparum (P = 0.04).
Conclusion
The present study highlights P. vivax as an increasingly recognized causative agent for severe malaria in children from Rohtak, with similar clinical presentation and outcome to that caused by P. falciparum.
Keywords: Malaria, Plasmodium vivax, Plasmodium falciparum, Severe malaria, India
INTRODUCTION
Malaria continues to be a major public health problem in most tropical countries, with a high mortality in untreated severe forms of malaria. Traditionally, P. falciparum was considered to be the main cause of ‘severe or complicated malaria’ and P. vivax was considered to result in ‘benign tertian malaria’. Recently, P. vivax is being increasingly recognized as one of the etiological factors for severe malaria in children and adults.1–4
The clinical presentation of severe malaria caused by P. falciparum and P. vivax were similar among children from Papau New Guinea except for higher chances of respiratory distress among the latter.1 In a large prospective study from Bikaner district of North India, it was found that a proportion of under five children infected with P. vivax had a greater propensity for development of complications including severe anaemia, thrombocytopenia, cerebral malaria, acute respiratory distress syndrome, hepatic dysfunction, renal dysfunction, and abnormal bleeding.3 Similar observations were generated in another study from Delhi where children with severe malaria infected with P.vivax had more hepatic, renal, respiratory, and bleeding complications with less overall mortality when compared to falciparum malaria.4 P.vivax is being increasingly recognized as a causative agent of severe malaria from North India. Hence the present study was undertaken to outline the clinical profile and outcome of children with severe vivax malaria admitted to a tertiary hospital in Rohtak.
METHODS
A prospective observational study was conducted at the pediatric department of Pt. B.D. Sharma Postgraduate Institute of Medical Sciences, a tertiary care government-sponsored teaching hospital in Rohtak, Haryana. The study was conducted for a period of 12 months from January 2012 to December 2012. The patients seeking treatment in this hospital belong to the urban area of Rohtak and the surrounding rural districts of Haryana. Children between 1 month and 14 years diagnosed with severe malaria as per World Health Organization (WHO) (2000) criteria,5 were eligible to be included. Children with human immunodeficiency virus (HIV) infection, co-existing systemic illness (chronic renal failure, chronic liver disease, and known progressive neurological illness); and those who stayed in hospital for less than 6 hours were excluded. Clearance was obtained from the institutional ethics committee. The study protocol was fully explained to the parents/guardian, and written informed consent was obtained.
Demographic profile including the name, age, gender, and residential address were collected. A history of presenting complaints including fever, headache, vomiting, diarrhea, seizures, paleness of body, jaundice, and history of mucosal bleeding was elicited. Evaluation was done for anthropometric parameters, evidence of shock, prostration, and jaundice.
Venous blood of patient was collected in an EDTA-containing vacutainer. A thin and a thick peripheral blood smear for malarial parasites were prepared and stained with Geimsa stain. Each slide was examined by light microscopy for a minimum of 100 high power fields or for a duration of at least 15 minutes. Simultaneously, a drop of blood was placed on the rapid malarial antigen detection kit [parasite lactate dehydrogenase (p-LDH) based immunochromatographic antigen detection assay] (S D Biostandard Diagnostic Pvt Ltd, Gurgaon, India). Presence of malarial parasite on thick and thin smear and/or positive p-LDH based rapid malaria antigen test was considered diagnostic of malaria. Parasite density was not estimated.
Patients were categorized into either of three groups: P. vivax monoinfection, P. falciparum monoinfection and mixed P. vivax, and P. falciparum infection. Diagnosis of mixed infection was based on microscopic presence of gametocytes for P. falciparum along with presence of schizonts and trophozoites for P. vivax.
The following laboratory tests were conducted: blood for hemoglobin levels, complete blood count, platelet count, blood glucose, serum creatinine, arterial blood gas (pH), prothrombin time, partial thromboplastin time, serum aminotransferase levels, bilirubin level, and arterial lactate. Other investigations included blood culture for evidence of bacterial sepsis, serum widal titres to exclude enteric fever, chest radiography to look for associated pneumonia among those presenting with respiratory distress, and cerebro-spinal fluid analysis performed among children presenting with multiple seizures and altered sensorium to exclude meningitis.
Diagnosis of ‘severe malaria’ was made in the presence of one of the following: severe anaemia (Hb<5 g/dl), multiple seizures (>2 episodes in 24 hours), impaired consciousness or unrousable coma (Glasgow coma scale<11), prostration (sudden complete exhaustion or weakness as described by parents), failure to feed, shock (systolic blood pressure<50 mmHg), jaundice, DIC, metabolic acidosis (plasma bicarbonate<15 mmol/l), acute kidney injury (serum creatinine>1.5 times the reference serum creatinine for age or urine output<0.5 ml/kg/hour) or hyperlactatemia (serum lactate>5 mmol/l).
Children diagnosed with ‘severe malaria’ were started on intravenous artesunate or quinine. Once it could be orally administered, children who were on IV artesunate were shifted to oral tablets of artemether-lumefantrine combination (3-day course); whereas those who were on IV quinine were shifted to oral quinine tablets to complete a 7-day course. Additional drugs including doxycycline or clindamycin were administered among those on oral quinine. The supportive management included fluid therapy, dextrose administration for hypoglycemia, nutritional support, intravenous antibiotics, blood product transfusion and paracetamol for hyperpyrexia, and anticonvulsants for seizures. Need for pediatric intensive care unit admission was decided on a case to case basis.
Children were preferably admitted in the pediatric ward for a minimum of 7 days. However, if the parents insisted on an early discharge, they were asked to return for a follow-up at 7 days. We looked for improvement in fever (temperature<100 F for 24 hours), improvement in sensorium (GCS of 15/15 for a minimum of 24 hours), and improvement in thrombocytopenia (two records of platelet count collected more than 24 hours apart>150×109/l). Time to improvement in sensorium was defined as time to GCS of 15/15.
Among the children who tested positive by peripheral smear, we performed a repeat peripheral smear examination on Day 2, Day 5, Day 7, and Day 28 for detection of early (Day 7 positive) and late (Day 28 positive) treatment failures. Data were entered in an MS Excel spreadsheet and all the entries were checked for any possible errors. Data were assessed for normality by plotting normal probability curves. All categorical variables were presented as proportions (%) and the comparison based on parasitological diagnosis was compared using chi square test. Continuous variables such as age were presented as Mean (SD) or Median (IQR) in skewed distribution. The clinical features of severe malaria were compared among three groups (P. vivax, P. falciparum, and mixed) by chi squared tests for categorical variables and ANOVA or Kruskal–Wallace testing for parametric or non-parametric data, respectively. Level of significance assumed in all tests was 5%. Statistical analysis was done using SPSS 17.0 version.
RESULTS
We screened 423 febrile children with suspected malaria (Fig. 1). A total of 35 enrolled children with severe malaria had an IQR age of 7 (3.5, 12) years. Among the 35 children [22 (62.9%) males and 13 (37.1%) females] enrolled, eight (22.8%) children were infected with P. falciparum, 18 (51.4%) children with P. vivax, and nine (25.7%) children had mixed infection. The SD age was comparable among P. vivax [6.51 (4.31)], P. falciparum [8.43 (3.95)], and mixed infection group [7.94 (4.92)] [P = 0.53].
Figure 1.
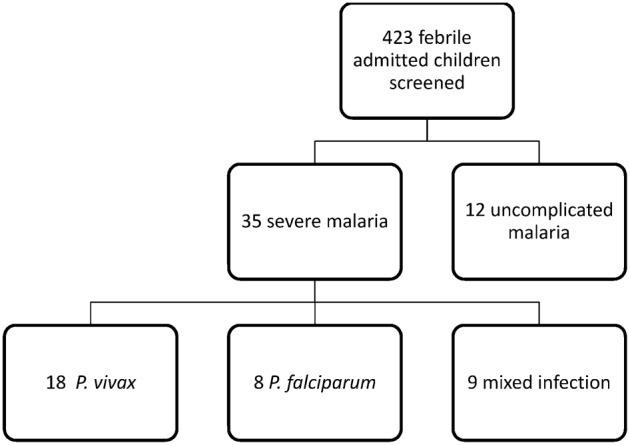
Flow diagram of patients screened for malaria among the admitted febrile patients.
Among 18 children diagnosed with P. vivax monoinfection, six children were under 5 years of age, six in the age group of 5–10 years, and six were above 10 years of age. The majority [21(60%)] of children had presented with prolonged fever of more than 5 days. Other clinical presentation were headache [13 (72.2%)], vomiting [10 (55.6%)], diarrhea [6 (33.3%)], seizures [8 (44.4%)], altered sensorium [8 (44.4%)], abnormal behavior [7 (38.9%)], pallor [14 (77.8%)], jaundice [5 (27.8%)], and bleeding [3 (16.7%)]. Clinical examination findings in children infected with P. vivax were pallor [14 (77.8%)], icterus [5 (27.8%)], hepatomegaly [14 (77.8%)], and splenomegaly [5 (27.8%)]. Diagnosis of P. vivax monoinfection was made in 18 children of whom seven (38.8%) were diagnosed positive by rapid antigen test, 11 (61.1%) were positive by smear examination, and four (22.2%) were positive by both. Clinical features of severe malaria (World Health Organization, 2010) were compared by parasitological diagnosis (Table 1).
Table 1. Comparison of clinical characteristics of severe malaria (WHO definition) caused by Plasmodium falciparum, Plasmodium vivax and mixed infection.
| Clinical characteristics | P. falciparum (N = 8) | P. vivax (N = 18) | Mixed infection (N = 9) | P value |
| Severe anaemia (Hb<5 g/dl) N (%) | 6 (75%) | 5 (27.8%) | 2 (22.2%) | 0.04 |
| Multiple seizures (>2 episodes in 24 hours) N (%) | 2 (25%) | 8 (44.4%) | 4 (44.4%) | 0.61 |
| Abnormal sensorium (GCS*<11) N (%) | 3 (37.5%) | 9 (50%) | 5 (55.6%) | 0.74 |
| Prostration N (%) | 8 (100%) | 12 (66.7%) | 8 (88.9%) | 0.11 |
| Failure to feed N (%) | 8 (100%) | 14 (77.8%) | 8 (88.9%) | 0.31 |
| Shock (SBP ˆ<50 mmHg) N (%) | 0 | 3 (16.7%) | 2 (22.2%) | 0.39 |
| Jaundice N (%) | 5 (62.5%) | 5 (27.8%) | 2 (22.2%) | 0.15 |
| DIC N (%) | 0 | 1 (5.6%) | 1 (11.1%) | 0.61 |
| Metabolic acidosis (Bicarbonate <15 mmol/l) N(%) | 0 | 2 (11.1%) | 1 (11.1%) | 0.61 |
| Acute kidney injury# N (%) | 1 (12.5%) | 3 (16.7%) | 1 (11.1%) | 0.91 |
| Hyperlactatemia (serum lactate >5 mmol/l) N (%) | 0 | 1 (5.6%) | 0 | 0.61 |
*GCS-Glasgow Coma Scale; ˆSBP-Systolic Blood Pressure; #Acute kidney injury–serum creatinine>1.5 times the reference serum creatinine for age or urine output<0.5 ml/kg/hour
All 18 (100%) children with P.vivax monoinfection were initiated on intravenous artesunate followed by a 3-day course of artemether-lumefantrine combination therapy once the patient could be allowed oral medications. Two [2/18 (11.1%)] children infected with P. vivax died. The first child presented with features of cerebral malaria, acute respiratory distress syndrome, hypoglycemia, metabolic acidosis, and catecholamine refractory shock. The second child presented with cerebral malaria, hepatitis, deranged coagulation profile, and acute kidney injury. The outcome was favorable (survival at 7 days) among the other 16 (88.8%) children.
The duration of hospitalization was more than 5 days in nine children (50%). Half of the children with P. vivax infection [9/18 (50%)] had improvement in fever in 2–5 days time frame. Among children with cerebral malaria, five [5/8 (62.5%)] children had improvement in sensorium within 48 hours. Among smear positive P. vivax monoinfection (n = 11), parasitemia clearance took 48 hours in two (18.2%), 5 days in seven (63.6%), and two (18.2%) had cleared by 7 days. Antibiotics were initiated in seven [7/18 (38.9%)] children pending the blood culture report. The cultures were sterile after 48 hours of incubation with all 18 children and the antibiotics were omitted following a sterile culture report. Three (16.7%) children required platelet transfusion and six (33.3%) required packed red cell transfusion.
DISCUSSION
Malaria imposes a great burden on humanity with infants, young children, and pregnant women being identified as high-risk groups with a propensity to develop severe malaria. Among cases of severe malaria, P vivax has emerged as an important causative agent in recent years. In the present series, more than half of the cases with severe malaria (51.4%) were due to P. vivax monoinfection, while P. falciparum and mixed infections contributed to the rest. The emergence of P. vivax as a causative agent of severe malaria could have significant implications in the management of malaria in northern India. The present study highlights the clinical presentation and outcome of severe vivax malaria to be similar to severe malaria caused by P. falciparum, except for higher chances of severe anaemia among the latter.
P. vivax is increasingly recognized as an important cause of severe malaria in children with clinical manifestations like cerebral malaria, anaemia, and thrombocytopenia.1–4 In a large prospective study from Bikaner district of North India, 150 children admitted with severe malaria were analyzed, and it was observed that P. vivax monoinfection contributed to one third of cases.3 It was also seen that children under 5 years infected with P. vivax infection had higher risk of developing severe malaria when compared to those infected with P. falciparum [OR 2.3 (1.4–3.76)]. They observed that children with vivax monoinfection had a higher chance of severe anaemia, cerebral malaria, acute respiratory distress syndrome, hepatic and renal dysfunction.3
In another study from Delhi, it was observed that severe anaemia, cerebral malaria, and shock were more frequent among children infected with P. falciparum.4 These results were consistent with our findings of severe anaemia among P. falciparum group. In addition, there are a number of case series and retrospective data on severe vivax malaria among adults and children that have been reported from other parts of North India.6–8
In a large study of 91 patients from Pakistan with severe malaria,9 P. vivax contributed to 11% (9/91) of cases. Severe anaemia was present in 42% of their patients, consistent with our findings [severe anaemia: 37.1% (13/35)]. In contrast, the authors had observed a higher prevalence of MODS (70%) and thrombocytopenia (84%). This could be attributed to the majority of their patients being young and infected with P. falciparum as compared to our study, hence leading to larger mortality among them (12.5% vs. 5.7% in our study). Younger children with severe falciparum malaria have been reported to have a higher risk of severe anaemia and cerebral malaria.10
The possible mechanisms for anaemia include destruction of parasitized red cells, complement mediated lysis, phagocytosis of non-parasitized red cells, increased splenic clearance, reduction of red cell survival even after disappearance of parasitemia, dyserythropoeisis in the bone marrow, and drug-induced hemolysis. Cerebral malaria in patients with P. vivax has been postulated to sequestration of infected erythrocytes in vascular beds of the cerebrovascular system.
Profound thrombocytopenia is well-documented in both P. vivax and P. falciparum malaria. A recent study by Yadav et al.4 reported thrombocytopenia in 83.2% cases with P. vivax malaria, however severe thrombocytopenia requiring platelet transfusion was seen in only 13%. In contrast, our study saw 50% (9/18) of the P. vivax severe malaria cases having low platelet count, of whom three (16.7%) had profuse bleeding requiring platelet transfusion. In a large prospective study from the Bikaner district of North India, they found thrombocytopenia in 73.16% (278/380) of children with P. vivax monoinfection, which was found to be significantly higher than either P. falciparum monoinfection [55.34%] or mixed infection [55.88%].11 The possible mechanisms for thrombocytopenia include direct lysis of platelets by P. vivax, immunological destruction by platelet-associated IgG antibody, and oxidative stress damage of thrombocytes.
In the present study, all 18 children were treated with intravenous artesunate followed by a course of artemether-lumefantrine combination and we observed a reasonable clinical response with no evidence of early or late treatment failures. Moreover, the majority of the children had improvement in fever, sensorium with return of normal platelet count by the fifth day of admission. This probably justifies use of artemesinin-based combination therapy for management of severe vivax malaria in children from North India. But the rising concern with the use of artemisinin-based combination therapy is empirical and often rampant use in peripheral health facilities for all febrile children in an endemic zones might consequently result in emergence of drug resistance to the effective antimalarial drug.
Our study has a limitation of its small sample size, although the data is being prospectively collected to further describe the evolving pattern of severe malaria at our centre. To conclude, our study highlights P. vivax as an increasingly recognized causative agent for severe malaria in children from Rohtak district of Haryana, northern India with a similar clinical presentation and outcome to that caused by P. falciparum. However, a larger sample size and a longer follow-up period would be reasonable to predict the outcome of this emerging trend.
REFERENCES
- 1.Manning L, Laman M, Law I, Bona C, Aipit S, Teine D, et al. Features and prognosis of severe malaria caused by Plasmodium falciparum, Plasmodium vivax and mixed Plasmodium species in Papua New Guinean children. PLoS One. 2011;6(12):e29203. doi: 10.1371/journal.pone.0029203. doi:10.1371/journal.pone.0029203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kochar DK, Das A, Kochar SK, Saxena V, Sirohi P, Garg S, et al. Severe Plasmodium vivax malaria: a report on serial cases from Bikaner in northwestern India. Am J Trop Med Hyg. 2009;80:194–8. [PubMed] [Google Scholar]
- 3.Kochar DK, Tanwar GS, Khatri PC, Kochar SK, Sengar GS, Gupta A, et al. Clinical features of children hospitalized with malaria–A study from Bikaner, Northwest India. Am J Trop Med Hyg. 2010;83:981–9. doi: 10.4269/ajtmh.2010.09-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yadav D, Chandra J, Aneja S, Kumar V, Kumar P, Dutta AK. Changing profile of severe malaria in North Indian children. Indian J Pediatr. 2012;79:483–7. doi: 10.1007/s12098-011-0603-x. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Severe falciparum malaria. Trans R Soc Trop Med Hyg. 2000;94:S1–90. [PubMed] [Google Scholar]
- 6.Singh H, Parakh A, Basu S, Rath B. Plasmodium vivax malaria: is it actually benign? J Infect Public Health. 2011;4:91–5. doi: 10.1016/j.jiph.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Srivatsava S, Ahmad S, Shirazi N, Verma SK, Puri P. Retrospective analysis of vivax malaria patients presenting to tertiary referral center of Uttarakhand. Acta Trop. 2011;117:82–5. doi: 10.1016/j.actatropica.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Parakh A, Aggarwal N, Aggarwal A, Aneja A. Plasmodium vivax malaria in children: uncommon manifestations. Ann Trop Paediatr. 2009;29:253–6. doi: 10.1179/027249309X12547917868844. [DOI] [PubMed] [Google Scholar]
- 9.Ahmed S, Adil F, Shahzad T, Yahiya Y. Severe malaria in children: factors predictive of outcome and response to quinine. J Pak Med Assoc. 2011;61:54–8. [PubMed] [Google Scholar]
- 10.Kumar A, Shrivatsava AK, Taksande AM, Singh DK, Rai R. Severe P. Falciparum malaria in children in a tertiary care center of Allahabad region of India. Internet J Pediatr Neonatol. 2010;12 DOI: 10.5580/11e1. [Google Scholar]
- 11.Tanwar GS, Khatri PC, Chahar CK, Sengar GS, Kochar A, Tanwar G, et al. Thrombocytopenia in childhood malaria with special reference to P. vivax monoinfection: a study from Bikaner (Northwestern India). Platelets. 2012;23:211–6. doi: 10.3109/09537104.2011.607520. [DOI] [PubMed] [Google Scholar]


