Abstract
Infection caused by intestinal parasites has significant public health consequences amongst children in the developing world. Street children are an under-studied group of society subjected to increased health risks when compared to their peers. To estimate the prevalence of intestinal parasitic infection and ascertain risk factors for parasitosis amongst this population, stool samples were collected from 258 children across four orphanages in three districts of Lima, Peru. Surveys were used to determine associations between risk factors and infection status. The prevalence of parasitic infection within the study sample was 66.3%, with 30.6% testing positive for pathogenic species. Entamoeba coli was the most commonly detected parasite (41.9%) and Giardia lamblia was the most commonly detected pathogenic parasite (17.1%). Of the group 15.1% had helminth infection. When testing for association, age and BMI were risk factors for infection. A notable difference in prevalence (P < 0.00001) based on orphanage was observed, but the duration of residence in an orphanage was not a predictor for infection. A sub-analysis conducted amongst children who were given anti-parasitic treatment 5 months beforehand found no significant difference in parasitosis between those who had been given treatment and those who had not (P = 0.218). It is suggested that a single dose of albendazole alone may not be effective in combating long-term infection rates.
Keywords: Peru, Homeless youth, Helminthiasis, Intestinal diseases, Parasitic, Cross-sectional studies
Introduction
The term ‘street children’ is used to describe vulnerable children who live or work on the streets. There is no universally accepted definition due to the social complexity of this population; one accepted definition by UNICEF makes a distinction between children who sleep on the street and those who make a living on the street and return to their families at night.1 Some of these children are taken in by organizations that provide amenities such as shelter and medical care, adopting the term ‘institutionalized’. It is acknowledged that population estimates of street children are unreliable,2 however a commonly used figure is the UNICEF estimation of 100 million worldwide. Of these, it is thought there are 40 million in Latin America alone.3 As with other marginalized populations, research on street children is often challenging. They are difficult to access and engage poorly with health services; consequently, there is little epidemiological data on them.
There are a number of reports highlighting the challenges faced by street children in Latin America.4–7 A study in La Paz, Bolivia, reported that up to 85% were victims of physical abuse, with children also reporting experience of sexual abuse (20%), serious medical conditions (53%), use of alcohol (58%), and inhalation of volatile solvents (88%).5 Qualitative studies in Brazil suggest that family breakdown combined with economic problems, peer pressure, and drug use are driving factors towards street life; these studies provide alarming insights into the risks these children face.6,7 Reports in Peru are similar; a survey of 134 street children in Lima found that 81% admitted alcohol consumption and 92% admitted to using ‘terokal’, a volatile solvent.8 These children often survive by working in the informal sector. This includes singing on buses, cleaning vehicles, shining shoes, begging, robbery, and prostitution.4,8
Peru’s orphan population was estimated to be 550 000 in 2009.9 Many of these children are living on the streets, although some move into orphanage residencies. These institutions have been shown to improve outcomes such as school attendance, problems with police, drug use, and sexual and physical abuse.5 Despite the potential benefits of these services, the proportion of children who use them and remain off the street is variable, in some cases as little as 3%10 and children can often be caught in a cycle between orphanage and street.
The limited available data consistently indicate that street children often suffer from poor health. Of the 134 street children surveyed in Lima, 46% reported a constant, productive cough, 28% reported abdominal pain, and 19% reported severe itching.8 In La Paz 53% of 124 abandoned street children reported health problems, five-fold more frequently than amongst those in institutions (OR = 4.6, 95% CI 1.8–11.2).5 Studies have also acknowledged the high HIV risk in this population.11,12 Due to the relative paucity of evidence available, little is known about the epidemiology or public health impact of disease amongst this population.
Intestinal parasitic infection is associated with poor sanitation and limited access to drinking water, both of which are more common in resource-constrained settings. Soil-transmitted helminthiasis is a WHO listed ‘Neglected Tropical Disease’, with over one billion people infected worldwide. Protozoan intestinal infections are the cause of over 58 million cases of diarrhoea in children every year.13 Symptoms can include anorexia, diarrhoea, and abdominal pains, and if left untreated can lead to malnutrition, cognitive impairment, and a failure to thrive.14,15
The prevalence of parasites amongst street children has been reported in other countries; however, these reports are few. The aim of this study is to estimate the prevalence of intestinal parasitic infection in street children in Lima, and to identify factors associated with infection. Such data could inform whether strategies of blanketed or targeted empiric treatment are warranted in this population, either in an outreach context or opportunistically at the moment of entry into an institution.
Methods
Study design
In this cross-sectional study participants were eligible if they were under 18 years of age and were residing within an institution. Four orphanage administrators were contacted through the LimaKids programme (www.limakids.org) for enrolment. In all but one orphanage, every child was approached and included in the study irrespective of symptoms. For the remaining orphanage, two male and two female houses of 16 were consented.
Data collection
Data were collected between May and June 2011 in four orphanages located in the Zapallal, Jesus Maria (2), and Cieneguilla districts of Lima. Zapallal is located in the Puente Piedra district of northern Lima. It has a high proportion of slums and informal settlements, representing one of the poorest areas in the city. Jesus Maria is an urbanized, middle class district within central Lima. Cieneguilla is a rural district in eastern Lima. The orphanages were subsequently anonymized as A–D for data analysis. The use of distinct districts allowed identification of regional variance as a potential influencing factor to the outcome. The children and orphanage administrators provided information through standardized questionnaires, which obtained information on age, duration of residence in the orphanage, type of water consumed, diet, and management of excretion. Missing data were excluded from the relevant analyses. In one orphanage, a single dose of albendazole (400 mg) was provided 5 months beforehand as part of an eradication programme; this information was used for sub-analysis. Participant height and weight were recorded and BMI was calculated. A single stool sample collected from each child was transported to the laboratory for analysis. Treatment was provided to children where pathological parasites were detected.
Stool examination
Specimens were stored at 4°C until analysis and fixed using a merthiolate–iodine–formaline solution. Helminths were detected using the formalin–ether concentration method (as described by Ritchie), and fixed smears were stained by the modified Ziehl–Neelsen technique to identify coccidia. Direct microscopy was used to confirm protozoal infection, with immuno-fluorescence antigen assays used to detect antibodies to trophozoites and cysts to Giardia lamblia.
Statistical methods
The outcome was defined as the presence of one or more parasites within a stool sample. This was sub-grouped into pathological and non-pathological parasites. The data were analyzed using Epi Info Version 3.5.3 statistical software. The prevalence of parasitic infection was analyzed in relation to BMI (<17, 17–19, 20–22, >22), age group, gender, region, and time spent living in the orphanage. Children residing in orphanage B were given anti-parasitic treatment with a single dose of albendazole (400 mg) 5 months prior to the study. Those who had been residing less than 5 months were assigned to a non-treatment group and the effects of anti-parasitic medication on parasitic infection was also analysed. A chi-square test was used to compare differences in proportions and identify trends within categorical variables. Logistic regression analysis was used to ascertain the odds of parasitic detection as determined by these variables. All P values <0.05 were considered as significant. Any missing values were excluded from the analysis.
Ethics review
Ethical approval for the study protocol and informed consent and assent forms were obtained from the ethics committee of the Universidad Peruana Cayetano Heredia. All institutions reviewed the study procedures and agreed to participate.
Results
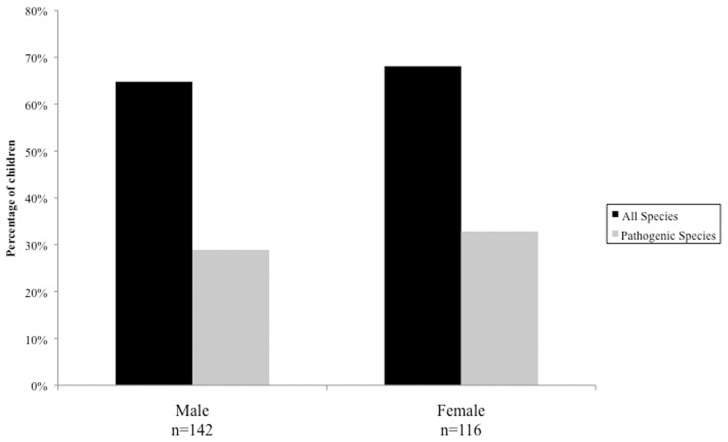
A total of 258 samples were examined; 142 participants (55%) were male, 116 (45%) were female (Fig. 1), with an overall mean age of 10.5 years (SD = 2.88).
Figure 1.
Percentage of children tested positive for intestinal parasitic infection according to sex. (chi square for differences in proportions; P = 0.669 when accounting for all species, P = 0.591 when accounting for pathogenic species).
Ten species of intestinal parasites were detected; of those, G. lamblia, Ascaris lumbricoides, Trichuris trichiura, Ancylostoma/Necator, Hymenolepis nana, and Cyclospora cayetanensis were considered pathogenic, and Entamoeba coli, Endolimax nana, Iodamoeba butschlii, and Chilomastix mesnilli were considered non-pathogenic.
Intestinal parasites (pathogenic and non-pathogenic) were detected in the stool of 171 children (66.3%) of whom 79 (30.6% overall) were found to have pathogenic parasites. E. coli was the most commonly detected parasite (41.9%), with G. lamblia being the most commonly detected pathogenic parasite (17.1%) (Table 1). Amongst the children 62.8% were found to have protozoan infection, and 15.1% had helminth infection. Of the sample, 80 (31.0%) were infected by a single species, while 91 (35.3%) tested positive for more than one parasite.
Table 1. Prevalence of parasites detected by species.
| Number infected | Percentage | |
| Protozoa | 162 | 62.8% |
| Entamoeba coli | 108 | 41.9% |
| Endolimax nana | 83 | 32.2% |
| Giardia lamblia | 44 | 17.1% |
| Chilomastix mesnilli | 30 | 11.6% |
| Cyclospora cayetanensis | 9 | 3.5% |
| Iodamoeba butschlii | 1 | 0.4% |
| Helminths | 39 | 15.1% |
| Hymenolepis nana | 20 | 7.8% |
| Trichuris trichiura | 13 | 4.7% |
| Ascaris lumbricoides | 9 | 3.5% |
| Ancylostoma/Necator | 3 | 1.2% |
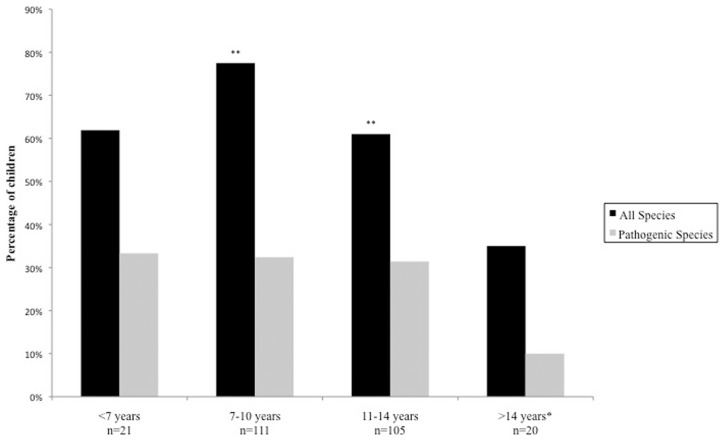
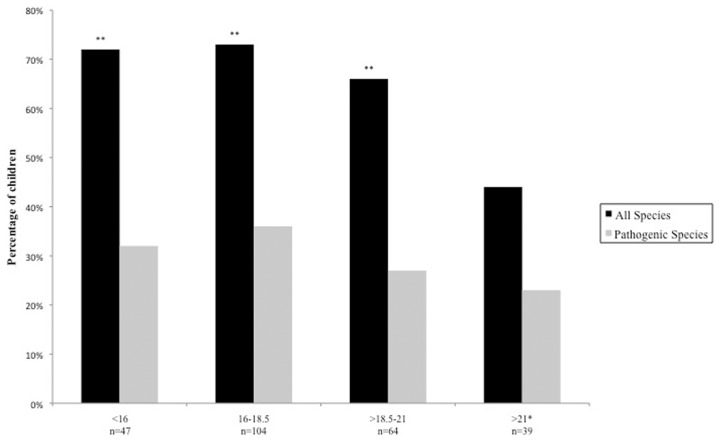
The prevalence of parasitosis was highest amongst children aged 7–10 years (77.5%), and lowest amongst those aged >14 years (10.0%) (Fig. 2). A chi-squared test for trend was statistically significant (P = 0.003), but only when both pathogenic and non-pathogenic species were accounted for. Parasitosis was also associated with decreasing BMI (P = 0.0025, accounting for all species), occurring in 72% of children with a BMI <16 and in 44% of children with a BMI >21 (Fig. 3). When testing for all species, the logistic regression analysis found that lower age groups and low BMI were statistically significant predictive factors associated with parasitic infection.
Figure 2.
Percentage of children tested positive for intestinal parasitic infection according to age. (chi square for trend; P = 0.003 when accounting for all species, P = 0.17 when accounting for pathogenic species.) *Baseline reference for logistic regression analysis, **statistically significant predictors associated with parasitic infection.
Figure 3.
Percentage of children tested positive for intestinal parasitic infection according to BMI. (chi square for trend; P = 0.0025 when accounting for all species, P = 0.169 when accounting for pathogenic species.) *Baseline reference for logistic regression analysis, **statistically significant predictors associated with parasitic infection.
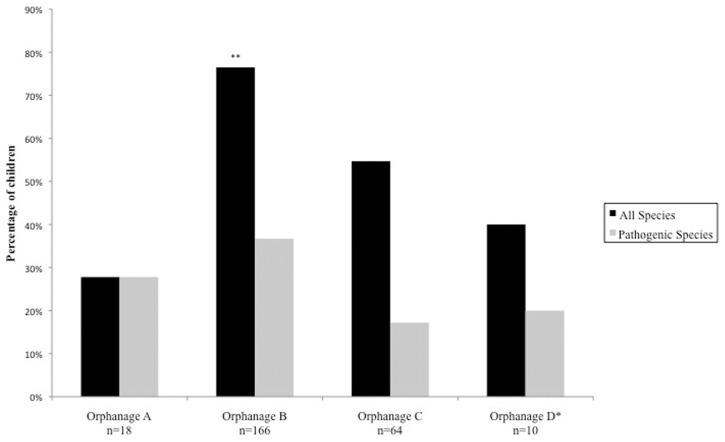
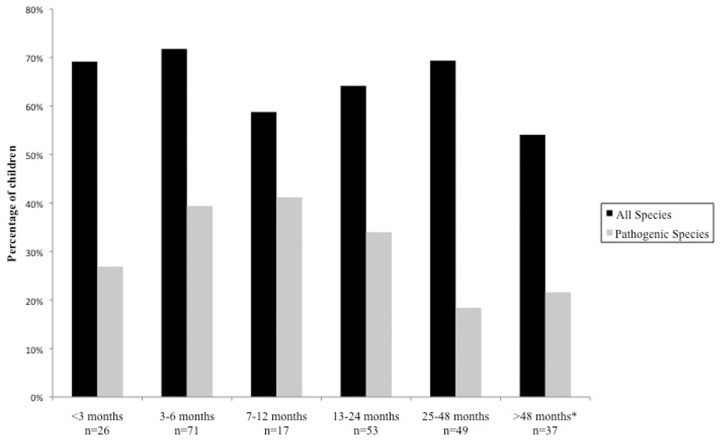
There was significant variability amongst orphanages in the prevalence of parasitosis of any species (Fig. 4). The population sample tested in orphanage B had the highest prevalence (76.5%) with the sample in orphanage A recording the lowest prevalence (27.8%). When observing the effect of the time spent in an orphanage on the prevalence of parasitosis, both chi-squared and logistic regression analysis found no evidence that the length of stay in an orphanage had any effect on parasitosis when accounting for all species (Fig. 5), and when the children who did not receive prior treatment were analysed separately. There was evidence to suggest that a trend is seen when adjusting for prevalence of pathogenic species only (P = 0.04).
Figure 4.
Percentage of children tested positive for intestinal parasitic infection according to orphanage. (chi square for differences in proportions; P < 0.00001 when accounting for all species, P = 0.028 when accounting for pathogenic species.) *Baseline reference for logistic regression analysis, **statistically significant predictors associated with parasitic infection.
Figure 5.
Percentage of children tested positive for intestinal parasitic infection according to the time residing within an orphanage. (chi square for trend; P = 0.172 when accounting for all species, P = 0.04 when accounting for pathogenic species.) *Baseline reference for logistic regression analysis, **statistically significant predictors associated with parasitic infection.
The analysis for antiparasitic treatment is shown in Table 2. All children who resided in orphanage B (n = 166) were included in the analysis. There was no statistically significant difference in parasitosis of any species between the children who had been given treatment and those who had not (P = 0.218). There was no difference observed when adjusting for protozoa (P = 0.725), and the reduction in positive samples for helminths amongst the children taking antiparasitic medication was also not statistically significant (P = 0.087).
Table 2. Effect of prior treatment 5 months earlier on prevalence of intestinal parasitosis in children residing in orphanage B.
| Positive | Negative | chi square for differences in proportions | |
| Total | |||
| Given treatment | 65 (72.2%) | 25 (27.8%) | |
| No treatment | 62 (81.6%) | 14 (18.4%) | P = 0.22 |
| Helminths | |||
| Given treatment | 14 (15.6%) | 76 (84.4%) | |
| No treatment | 21 (27.6%) | 55 (72.4%) | P = 0.09 |
| Protozoa | |||
| Given treatment | 63 (70.0%) | 27 (30.0%) | |
| No treatment | 56 (73.7%) | 20 (26.3%) | P = 0.73 |
Discussion
There are few studies that have looked at the prevalence of parasitosis in street children, and these have been conducted in other countries. In comparison to a prevalence of 66.3% reported here, a study amongst 106 orphans in Thailand found a prevalence of 81%,16 and in the Philippines a study of 284 samples found 62.0% positive for one or more intestinal parasites.17 The high rates observed in these countries are consistent with the figures observed in this study, and highlight the public health implications for this population all over the world.
Studies in Peru have estimated figures for parasitosis amongst other populations of children. The prevalence found in this study (66.3%) does not differ greatly from the data from studies conducted in Trujillo in 2004 and 2005 (66.3%, n = 489; 68%, n = 845),18,19 Surco, Lima (54.7%, n = 192),20 and Puno (91%, n = 91).21 When adjusting for individual species, a similarly high prevalence of G. lamblia was seen in the Trujillo studies (23.8%, 26.4%),18,19 and prevalence of helminthiasis was comparable to the study conducted in Surco (14.6%),20 however in Paijan, a study of 491 school children found helminths in 46.8%.22 Unlike other reports, we did not find any evidence of Entamoeba histolytica, Strongyloides or Cryptosporidium infection, despite acknowledgement of the morphological similarities between Entamoeba species. A high prevalence of E. coli was also noted when compared with other studies.16,17,19,20 Though coupled with the high prevalence of G. lamblia and E. nana, the figures may be explained by a high risk of exposure to fecally contaminated food and water. Nevertheless, a study amongst six rural communities in Puno estimated prevalence of E. coli at 78%.21 These figures suggest, as reported elsewhere, that non-street children in poor areas may be subjected to similar environmental and economic factors determining rates of infection as street children.23
The results of the study indicate that age, BMI, and variance between orphanages are factors associated with parasitic infection. Using the age group of >14 years as a reference, the odds of parasitic infection increase between <7 years and 7–11 years, and then decrease with increasing age group. Studies in Pakistan24 and Côte d'Ivoire25 have also shown that age is an important risk factor for parasitic infection, with school-age children being a particularly high risk group.26 The present study affirms the link between low BMI and parasitic infection. Pathogenic parasites are associated with diarrhoea, malnutrition, stunting, and cognitive impairment.15,26 Non-pathogenic parasites are often associated with faecal–oral contamination and are indicators of poor hygiene. The variance seen amongst orphanages may stem from the characteristics of the different districts of the city. Poorer areas of the city with increased exposure to contaminated water would be at risk of parasites such as Giardia and Entamoeba, however those in more rural settings may see a higher prevalence of soil-transmitted helminth infection. This presents an issue when determining the applicability of prevalence studies to other cities, or even areas within a city. Studies have shown that infection rates are often dependent on economic wellbeing, living conditions, and community infrastructure within a catchment population.24
The length of time spent residing in an orphanage had no effect on the number of parasitic infections detected when accounting for all species, which may be explained by high re-infection rates. There was some evidence to suggest that the amount of pathogenic parasites detected decreased with increasing length of residency. The provision of drinking water and cooked food combined with medical input within the institutions may account for the decrease observed, however to better clarify this, a study comparing those who are currently living on the street with those in institutions is needed.
The lack of reduction in positive cases observed 5 months following treatment with antiparasitic medication suggests that either re-infection rates are high or the initial treatment is ineffective. The provision of a single dose of albendazole alone in the de-worming regime did not reduce rates of protozoan infection after 5 months, but may account for the small decline in positive cases of helminth infection. A larger study sample is required to clarify this effect, and this should consider issues of compliance that were beyond the time frame of this study. Some reports suggest effective de-worming should include a follow-up of at least 6 months.17 More evidence is required to establish the effect of de-worming programmes on protozoan infections and whether modifying these programmes to better cover intestinal protozoa amongst high risk populations would be valuable. Successful eradication initiatives need to meet the sanitary, medical, and educational needs of the target population.27
Like other cross-sectional studies involving similar populations, the logistical and resource limitations must be considered. Only one stool sample was obtained per child, and as a consequence the prevalence of G. lamblia reported may be an underestimate since the parasite is intermittently shed in stool.28 The samples were taken during May and June, and consequently seasonal variation may impact the figures presented. However, Lima has an unusually stable climate for its subtropical location and so the effects of seasonality may not be as strong as those reported in other locations.29,30 A larger study over a longer period of time would be required to further clarify these effects.
Other factors to consider are the applicability of the population to all street children. The differences seen in institutions may be due to the nature of the children taken in to a particular institution; some orphanages take children without families or homes, whereas others take children previously housed, but unwanted by families. Despite being included under the umbrella term of ‘street children’, there may be a difference in social, health, and economic factors, which should be considered in future work with this population. There is however, a significant logistical hurdle in locating, interviewing, and testing children who currently reside on the street. The street environment holds harsher living conditions, poorer community infrastructure, and limited access to drinking water when compared to institutions. Although many children within the study were recently subjected to such conditions, the effect of institutional residency on infection rates is yet to be tested.
The present study describes a high prevalence rate of intestinal parasitic infection in an understudied and marginalized population in a major Latin American city, though rates were perhaps surprisingly not markedly greater than previously described for the general population in the region. It identifies factors associated with infection and suggests that prevention programmes using single dose albendazole may not be effective in combating long-term infection rates.
Acknowledgments
This study was funded by Shine-A-Light Children Foundation. We thank Stugart Maza, Ivor Munoz, Miguel Candia and Maritza Sáenz Pachas for their support in our data collection, Bob Gilman for the use of his laboratory, and the Laureus Sport for Good Foundation, which supports much of LimaKids’ activities.
References
- 1.Lusk M. Street children programs in Latin America. J Sociol Soc Welf. 1989;16(1):55–77. [Google Scholar]
- 2.UNICEF. 2006. Excluded and invisible: state of the world’s children; p. 2005. [Google Scholar]
- 3.World Health Organization. A one-way street? Report on phase I of the street children project. In: Programme on Substance Abuse. Geneva: WHO; 1993. [Google Scholar]
- 4.Scanlon TJ. Street children in Latin America. BMJ. 1998;316(7144):1596–600. doi: 10.1136/bmj.316.7144.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang CC, Barreda P, Mendoza V, Guzman L, Gilbert P. A comparative analysis of abandoned street children and formerly abandoned street children in La Paz, Bolivia. Arch Dis Child. 2004;89(9):821–6. doi: 10.1136/adc.2003.042911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abdelgalil S, Gurgel R, Theobald S, Cuevas L. Household and family characteristics of street children in Aracaju, Brazil. Arch Dis Child. 2004;89(9):817–20. doi: 10.1136/adc.2003.032078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inciardi JA, Surratt HL. Children in the streets of Brazil: drug use, crime, violence, and HIV risks. Subst Use Misuse. 1998;33(7):1461–80. doi: 10.3109/10826089809069809. [DOI] [PubMed] [Google Scholar]
- 8.Vara Horna AA. Informe Estadístico del Primer Censo de los niños de la calle Lima. Vol. 2001 Lima: Asociación por la Defensa de las Minorías (ADM); [Google Scholar]
- 9.UNICEF. 2009. At a glance: Peru – statistics. http://www.childinfo.org/hiv_aids_orphanestimates.ph. [Google Scholar]
- 10.Huang CC, Huang K. Caring for abandoned street children in La Paz, Bolivia. Arch Dis Child. 2008;93(7):626–7. doi: 10.1136/adc.2007.122663. [DOI] [PubMed] [Google Scholar]
- 11.Kayembe PK, Mapatano MA, Fatuma AB, Nyandwe JK, Mayala GM, Kokolomami JI, et al. Knowledge of HIV, sexual behavior and correlates of risky sex among street children in Kinshasa, Democratic Republic of Congo. East Afr J Public Health. 2008;5(3):186–92. [PubMed] [Google Scholar]
- 12.Wutoh AK, Kumoji EK, Xue Z, Campusano G, Wutoh RD, Ofosu JR. HIV knowledge and sexual risk behaviors of street children in Takoradi, Ghana. AIDS Behav. 2006;10(2):209–15. doi: 10.1007/s10461-005-9038-6. [DOI] [PubMed] [Google Scholar]
- 13.Savioli L, Smith H, Thompson A. Giardia and Cryptosporidium join the ‘Neglected Diseases Initiative’. Trends Parasitol. 2006;22(5):203–8. doi: 10.1016/j.pt.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 14.Berkman DS, Lescano AG, Gilman RH, Lopez SL, Black MM. Effects of stunting, diarrhoeal disease, and parasitic infection during infancy on cognition in late childhood: a follow-up study. Lancet. 2002;359(9306):564–71. doi: 10.1016/S0140-6736(02)07744-9. [DOI] [PubMed] [Google Scholar]
- 15.Pierce KK, Kirkpatrick BD. Update on human infections caused by intestinal protozoa. Curr Opin Gastroenterol. 2009;25(1):12–7. doi: 10.1097/mog.0b013e32831da7dd. [DOI] [PubMed] [Google Scholar]
- 16.Saksirisampant W, Nuchprayoon S, Wiwanitkit V, Yenthakam S, Ampavasiri A. Intestinal parasitic infestations among children in an orphanage in Pathum Thani province. J Med Assoc Thai. 2003;86(Suppl 2):S263–70. [PubMed] [Google Scholar]
- 17.Baldo ET, Belizario VY, De Leon WU, Kong HH, Chung DI. Infection status of intestinal parasites in children living in residential institutions in Metro Manila, the Philippines. Korean J Parasitol. 2004;42(2):67–70. doi: 10.3347/kjp.2004.42.2.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cordova Paz Soldan O, Vargas Vásquez F, Gonzalez Varas A, Peréz Cordón G, Velasco Soto JR, Sánchez-Moreno M, et al. Intestinal parasitism in Peruvian children and molecular characterization of Cryptosporidium species. Parasitol Res. 2006;98(6):576–81. doi: 10.1007/s00436-005-0114-7. [DOI] [PubMed] [Google Scholar]
- 19.Peréz Cordón G, Cordova Paz Soldan O, Vargas Vásquez F, Velasco Soto JR, Sempere Bordes L, Sánchez Moreno M, et al. Prevalence of enteroparasites and genotyping of Giardia lamblia in Peruvian children. Parasitol Res. 2008;103(2):459–65. doi: 10.1007/s00436-008-1007-3. [DOI] [PubMed] [Google Scholar]
- 20.Iannacone J, Julia Benites M, Chirinos L. Prevalencia de infección por parásitos intestinales en escolares de primaria de Santiago de Surco, Lima, Perú. Parasitol Latinoam. 2006;1–2(61):54–62. [Google Scholar]
- 21.Maco Flores V, Marcos Raymundo LA, Terashima Iwashita A, Samalvides Cuba F, Gotuzzo Herencia E. Distribution of entero-parasitic infections in the Peruvian Highland: study carried out in six rural communities of the department of Puno, Peru. Rev Gastroenterol Peru. 2002;22(4):304–9. [PubMed] [Google Scholar]
- 22.Liñan-Abanto R, Jara C. Frecuencia y aspectos epidemiológicos del parasitismo por helmintos intestinales en la población infantil de Paiján, La Libertad-Perú. Bol Peruano Parasitol. 1995;11:46–50. [Google Scholar]
- 23.Greska LP, Rie NI, Islam AB, Maki U, Omori K. Growth and health status of street children in Dhaka, Bangladesh. Am J Hum Biol. 2007;19(1):51–60. doi: 10.1002/ajhb.20573. [DOI] [PubMed] [Google Scholar]
- 24.Mehraj V, Hatcher J, Akhtar S, Rafique G, Beg MA. Prevalence and factors associated with intestinal parasitic infection among children in an urban slum of Karachi. PLoS One. 2008;3(11):e3680. doi: 10.1371/journal.pone.0003680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raso G, Luginbuhl A, Adjoua CA, Tian-Bi NT, Silue KD, Matthys B, et al. Multiple parasite infections and their relationship to self-reported morbidity in a community of rural Côte d’Ivoire. Int J Epidemiol. 2004;33(5):1092–102. doi: 10.1093/ije/dyh241. [DOI] [PubMed] [Google Scholar]
- 26.Bethony J, Brooker S, Albonico M, Geiger SM, Loukas A, Diemert D, et al. Soil-transmitted helminth infections: ascaris, trichuriasis and hookworm. Lancet. 2006;367:1521–32. doi: 10.1016/S0140-6736(06)68653-4. [DOI] [PubMed] [Google Scholar]
- 27.Dancesco P, Abeu J, Akakpo C, Iamandi I, Kacou E, Quenou F, et al. Les parasitoses intestinales dans un village de Côte d’Ivoire. I: essai de mise en place d’une stratégie de lutte et de prévention. Sante. 2005;15(1):5–10. [PubMed] [Google Scholar]
- 28.Danciger M, Lopez M. Number of Giardia in the feces of infected children. Am J Trop Med Hyg. 1975;24(2):237–42. doi: 10.4269/ajtmh.1975.24.237. [DOI] [PubMed] [Google Scholar]
- 29.Siwila J, Phiri IG, Enemark HL, Nchito M, Olsen A. Seasonal prevalence and incidence of Cryptosporidium spp. and Giardia duodenalis and associated diarrhoea in children attending pre-school in Kafue, Zambia. Trans R Soc Trop Med Hyg. 2011;105(5):298–300. doi: 10.1016/j.trstmh.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 30.Araj GF, Musharrafieh UM, Haydar A, Ghawi A, Itani R, Saliba R, et al. Trends and prevalence of intestinal parasites at a tertiary care center in Lebanon over a decade. J Med Liban. 2011;59(3):143–8. [PubMed] [Google Scholar]