The need for sophisticated anti-counterfeit technology is ever-growing as the practises of counterfeiters become increasingly advanced. Overt visible markers on a drug’s packaging have been commonly used to identify the genuine from the fake, but the holograms and distinguishing markers applied to the blister foil, film or paper substrates of the packaging are mimicked and imitated to a high level of accuracy. To the untrained eye, the genuine and fake examples can look identical (see Fig. 1).
Figure 1.
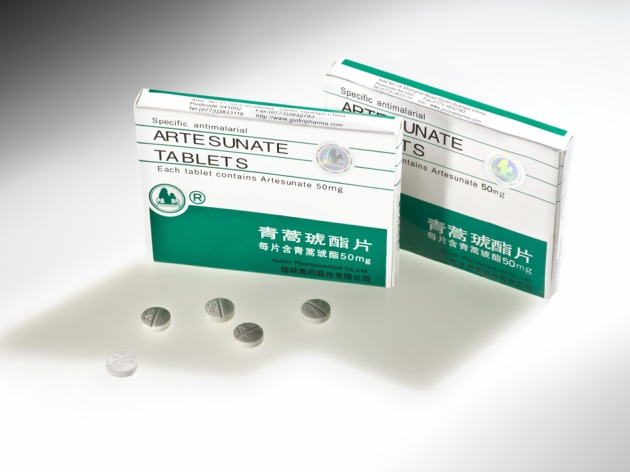
Artesunate packets: left, genuine, right, counterfeit. Courtesy of Wellcome collection.
Technology as a countermeasure must necessarily improve, especially in reaction to what is a multifarious problem. There is the manufacturing concern, to ensure brand protection and reinforce intellectual property rights for pharmaceutical companies, and the health concern, with the potential for counterfeit drugs to lead to mortality. A counterfeit medicine’s content is often substandard, containing fewer or no active ingredients or in incorrect measures, and it can contain contaminants or inappropriate excipients. The result of this is the possibility of treatment failure or death.
The technological needs resulting from these two problems and the increasing sophistication of the illicit manufacturers has been the impetus for new innovations or the combination of technologies. The overt markers are now often married with covert ones such as UV print or anti-tamper tape and solutions. These covert technologies are either barely visible or hidden entirely and can be of particular help in ensuring that genuine packaging is not reused with illicit content. While this strategy is certainly a deterrent, these overt and covert markers can be easily simulated, and to be effective require a knowledge and user education that is difficult to filter to the community and patient level, particularly in the developing world.
In recent years, the benchmark technology has shifted towards forensic techniques and systems that can carry unique authenticated information along the distribution line, right up to the point of dispensation to the patient. Barcodes, 2D datamatrix codes, tags and radio frequency indentifiction (RFID) can allow instantaneous remote authentication, making it much harder for counterfeit products to enter the supply chain. These systems rely on a scan-and-send system, meaning that, unlike the packaging markers, there is no interpretation of information by the user; the remote device does all the work. The technology is relatively young and often expensive, and the reliability of some of its systems is still questionable, but it offers a significant opportunity to truncate the spread of counterfeit products if government agencies and pharmaceutical manufacturers adopt variations of these strategies.
For anti-counterfeit technology to be at its most effective there is a need for various systems working in tandem, either on the same product, i.e. the combination of overt and covert markers or, indeed, remote authentication technology, or on different products. The combination of technologies adds heightened security to the individual product, and the use of various systems in a region can help to make the counterfeiters’ lives harder, as investing in fraudulent methods that can only be applied in a limited number of settings lessens the scope of their activity.
Inevitably, combined systems come at a price and even the more sophisticated systems of track and trace alone are enough for pharmaceutical companies to reconsider. In Europe, the EU serialisation takes effect in 2014, requiring pharmaceutical manufacturers to adopt serialisation systems. This directive will hope to produce a Europe-wide harmonisation of authentication and standards of protection. California’s centralised e-pedigree tracking requirement takes effect the following year. The practical hope of a global harmonisation of systems, however, is as yet elusive.
There is a greater need for collaboration between government agencies and pharmaceutical manufacturers to address the concern of cost, for the manufacturer so that anti-counterfeit technology is not a barrier to their production of quality medicines for those who need it, and for the patient. While these technologies go some way towards preventing counterfeit medicines from entering the formal supply chain, the drug peddlers and the informal sector thrive in many parts of the world because of the direct relationship with the poverty of its citizens. Patients in poor underdeveloped and developing countries seek a cheaper alternative to the genuine brands sold in regulated pharmacists, simply because of the high cost of drugs relative to income. They turn instead to non-regulated outlets, allowing counterfeiters to bypass the authentication process without the need for advanced hacking of the authentication systems or counterfeiting techniques. So, while innovation to tackle counterfeit medicine is vital, making it and the medicines it is trying to protect available at affordable prices is just as important in preventing related mortality.
Technology profiles
GPHF-Minilab
The GPHF-Minilab is a low-cost, on-the-spot, screening mini-laboratory designed to help low-income countries detect counterfeit and substandard medicine (Fig. 2). The kit verifies label claims on drug identity and content, and detects counterfeit medicines containing the wrong, much too high, much too low or zero levels of active ingredients. It can be used outside a laboratory environment by those having some understanding of analytical chemistry.
Figure 2.

Global Pharma Health Fund’s Minilab TLC TestKit.
Transportable lab that tests drugs for their content
The kit covers 57 drug compounds, including antimalarial and anti-tuberculosis
Total material cost for one test run not in excess of €2
Currently has units in 80 countries, and 267 individual units in Africa today
Richard Jähnke, project manager, Global Pharma Health Fund reports: ‘The key is that our Minilabs are reaching people where protection against counterfeit and substandard quality medicines is needed instantly. Hence, in countries and drug supply organisations where appropriate testing capacities are still lacking.
Testing the quality of drugs by means of the GPHF-Minilab involves a four-stage test plan that employs very simple physical and chemical analytical techniques. The first step in identifying potential counterfeit drugs is the careful visual inspection of the product, and its packaging and labelling for an early rejection of the more crudely presented counterfeits. The first step is followed by a simple tablet and capsule disintegration test performed in water for a preliminary assessment of deficiencies related to drug solubility and availability. The third step employs simplified colour reactions for a quick check of any drug present, thus ensuring that the drug is actually there before tackling the final step, a thinlayer chromatographic run for a quick check whether the quantities of drug claimed on the label are actually in the product. The results obtained by a simple visual inspection of the chromatoplates produced can be as accurate as 10% if great care is taken and skill executed. In order to achieve this accuracy training of staff might be required before using the Minilab’s procedures first time.’
TruScan
The Thermo Scientific TruScanTM analyzer allows non-expert users to perform quick, reliable and repeatable analyses of pharmaceuticals (Fig. 3). It examines the chemical composition of all components of a pharmaceutical dosage – APIs, excipients, fillers, dyes, and coatings – to create a chemical fingerprint, representing the specific authentic material. Any slight deviation from the original formulation will lead to a detectable change in the measured spectrum.
Figure 3.

Thermo Scientific TruScanTM analyzer.
Performs remote rapid analyses on medicines without risk of contamination
Mobile and light-weight device, weighing less than 4 pounds (1.8 kg)
No on-going costs for consumables or services
Duane Sword, former Senior Director of Thermo Fisher, explains: ‘The TruScan analyser is a handheld spectroscopic analyser that confirms raw materials are what they claim to be. The idea was to provide an analyser that could be used by anyone (including people who are not chemists), but still provide technical analytical results. Regulatory bodies around the world are heightening quality measures and increasing the levels of scrutiny of raw material inspection. If a company is being told to go from 10% sampling to 100% sampling, that is a big game-changer, and it normally means extensive extra cost to employ more legacy methods of laboratory testing.
We turned the process upside-down, so when the material arrives at the factory or warehouse on a truck, whether it’s liquid or solid, the forklift truck driver can unload it, scan the barcode on the drum’s label to identify what the material claims to be, and simply press the analyser onto the packaging to confirm the material’s identity.
Utilising Raman spectroscopy, the analyser compares the spectrum of the contents with the spectrum of the compound stored inside the analyser’s library. There is no need to touch the material, so there is no contamination, no risk of exposure to hazardous chemicals, and the identification is confirmed in a matter of seconds’.1
TruTagTM
TruTag Technologies, Inc.’s TruTagTM is an edible, low-cost, covert, heat-resistant microtag that can help to prevent counterfeiting, to assure quality, and provide informatics for product/component tracking and authentication throughout any supply chain (Fig. 4).
Figure 4.

TruTag Technologies Inc.’s TruTag microtag tablets.
The tags, made of clear, edible high-purity silica are ingested by the patient with the pill
Covert or semi-covert use available
Can be applied via inks, dyes, paints, or by using industry-standard pan or spray coaters
Can cost as little US$0.01 per drug
We asked TruTag about its benefits over other alternative solutions, such as RFID: and Peter Wong, Chief Operating Officer, TruTag Technologies, Inc. responded: ’Our microtags are significantly smaller than most RFID tags – our tags are 100 microns by 20 microns, while most RFIDs are measured in millimeters, so they can be used on very small items like tablets or individual computer chips. Second, we can tag a pill at a cost of US$0.01 or less, while most RFIDs cost between US$0.05 to US$0.15. Third, our microtags are edible, because they are made of high purity silica (silicon dioxide, which is ’generally recognized as safe’ by the U.S. Food and Drug Administration) so they can be applied directly on the drug product and be ingested with the pill. The silica passes through the digestive tract and is not absorbed. And because we can serialize the codes associated with our tags, they can serve as ‘covert, edible bar codes.’ Ultimately, our on-dose authentication solution is complementary to a packaging level solution like RFID.
Our microtags can not only help confirm whether a pill is real or fake, but it can provide so much more information about that particular pill: When was it made? Where was it made? Has is expired? In which country is it supposed to be sold? Currently, product security measures are at the packaging level (which is where RFIDs are placed). This is insufficient, and counterfeiters have been defeating packaging level security for years. Authentic packaging does not guarantee that what is inside the package is real. On-dose authentication measures like the TruTag solution helps to close this security gap.’
NanoGuardian
Using NanoEncryptionTM, NanoGuardian delivers forensic, multilayered authentication and tracing capability on each dose. The NanoEncryptionTM process incorporates NanoCodes directly onto tablets, capsules and vial caps. These codes may be associated with an unlimited amount of manufacturer-determined data, including product information (strength and expiration date), manufacturing information (location, date, batch and lot number) and distribution information (country, distributor, wholesaler and chain).
Unlimited information can be encrypted into the drug
Can be linked with track and trace technologies, including RFID and 2D Barcodes
Can encrypt overt or covert features
Can cost as little as from US$0.005 to $0.01 per drug
‘NanoEncryption technology is an on-dose brand protection weapon that can be implemented immediately by manufacturers to protect brands and patients, as it does not require an investment by downstream supply partners to be effective’. Developed by NanoGuardian, NanoEncryption enables manufacturers to trace and authenticate every single dosage from plant to patient. NanoEncryption technology adds no additional chemicals or materials to the dose, and is achieved by making purposeful manipulations in the coatings and gelatine used in the manufacture of tablets and capsules.
The overt and covert NanoEncrypted features allow in-field authentication of every dose at any point in the supply chain, while the forensic-level, nano-sized NanoCodes provide comprehensive tracing information on each and every dose. Given their nano-scale size – 350 NanoCodes fit into the width of a human hair – the reading of the NanoCodes requires specialised equipment and software housed at Nanoguardian’s Product Integrity Centre. The process to decrypt the Nanocodes is non-destructive and can be completed within minutes’ – Dean Hart, Chief Commercial Officer, NanoGuardian.2
AuthentiTrack
COVECTRA’s AuthentiTrack offers serialisation, track and trace and authentication that provide unique identification down to the unit level. This is made possible through proprietary imaging technologies, variable data printing of unique numbers or barcodes with specialty inks, including invisible inks, and with RFID.
1 or 2D barcodes with speciality inks, including invisible inks
Instant authentication and track and trace capability using RFID technology
Like most track and trace technologies, not yet universally affordable but attractive still to larger pharmaceutical companies
‘With applications providing the opportunity for traceability throughout the supply chain, manufacturers, customs officials and law enforcement can identify counterfeit and diverted product and detect how and where the product entered the supply chain,’ reports Steve Wood, President and CEO, COVECTRA.3
For more information on Anti-counterfeit technologies, see the latest WHO and IMPACT report: http://www.who.int/impact/events/IMPACT-ACTechnologiesv3LIS.pdf.
References
- 1. http://www.manufacturingchemist.com/technical/article_page/In_the_business_of_winning/62168. [Google Scholar]
- 2. http://www.iptonline.com/articles/public/NanoGuardian.pdf. [Google Scholar]
- 3. http://www.drugs.com/news/fda-guideline-calls-more-secure-pharmaceutical-supply-chain-covectra-offers-robust-solutions-27974.html. [Google Scholar]


