Abstract
Human malaria, a major public health burden in tropical and subtropical countries, is transmitted exclusively by the bite of a female Anopheles mosquito. Malaria control strategies aimed at inducing sexual sterility in natural vector populations are an attractive alternative to the use of insecticides. However, despite their importance as disease vectors, limited information is available on the molecular mechanisms regulating fertility in Anopheles mosquitoes. In the major malaria vector, An. gambiae, the full complement of sperm and seminal fluid required for a female’s lifelong egg production is obtained from a single mating event. This single mating has important consequences for the physiology and behavior of An. gambiae females: in particular, they become refractory to further insemination, and they start laying eggs. In other insects including Drosophila, similar post-copulatory changes are induced by seminal proteins secreted by the male accessory glands and transferred to the female during mating. In this review, we analyze the current state of knowledge on the function and characterization of male seminal proteins in An. gambiae, and provide a comparative assessment of the role of these male reproductive factors in other mosquito vectors of human disease in which female post-copulatory behavior has been studied. Knowledge of the factors and mechanisms regulating fertility in An. gambiae and other vectors can help the design of novel control strategies to fight the spread of disease.
Keywords: Anopheles, Fertility, Seminal fluid, Sperm, Post-mating response, Vector control, Malaria, Protease, Redox, Acps, Copulation, Reproduction, Sex, Sterile
Introduction
Mosquitoes transmit a variety of infectious diseases that severely affect human health. Malaria alone, transmitted by the bite of female Anopheles mosquitoes, annually infects more than 200 million people and causes nearly one million deaths. Infections by dengue and yellow fever virus, transmitted by Aedes mosquitoes, are a leading cause of illness and death in many tropical and subtropical countries. Current strategies aimed at targeting vector populations are mainly based on the use of insecticides; however, such efforts are hampered by the emergence of insecticide resistance in mosquitoes combined with the lack of novel chemicals. There is an urgent need for novel strategies to control mosquito disease-transmitting populations.
Among the hundreds of extant anopheline species, An. gambiae is the most important vector of human malaria. Plasmodium parasites, the causative agents of malaria, are transmitted when a female mosquito feeds on the blood of a host, releasing infective sporozoites into the blood stream.1 As blood feeding is necessary for egg production, the parasite exploits the mosquito’s reproductive needs to achieve its own transmission between vertebrate hosts. The high reproductive rate of An. gambiae mosquitoes is a major component of their capacity as malaria vectors. A female of this species can generate more than a hundred eggs from each blood meal, and can fertilize her lifetime egg production using sperm acquired from a single mating and stored in her sperm storage organ.
The acquisition of sperm by a female is a potential target for intervention aimed at vector control: An. gambiae females generally mate only once2 as mating with one male permanently switches off their receptivity to further insemination with other males and stimulates oviposition.3 This dependence of lifetime reproductive success on a single mating event offers an excellent target for intervention; interfering with insemination or oviposition would have a large impact on the size of natural mosquito populations. Fertility is a target in control strategies, such as the sterile insect technique (SIT),4 aimed at natural insect pests. SIT relies on the massive release of sterilized males into field populations. Females mated to sterile males lay infertile eggs, with a consequent decrease in population size. Despite the use of this technique for the control of many insect pests, to date SIT against Anopheles species has not been very successful.5 A deeper knowledge of mating and other processes underlying Anopheles fertility would definitely benefit the chances of SIT success, and would identify targets for the development of novel vector control strategies.
In this review, we describe the current understanding of reproductive biology in An. gambiae, with a particular focus on the mechanisms known to regulate female receptivity to mating and ability to lay eggs. In doing so, we provide a comparison of the events that influence female post-copulatory biology in other disease-transmitting mosquitoes, such as Aedes and Culex, and relate these factors to the primary model of insect reproductive biology, Drosophila melanogaster.
Mating Behavior and Physiology In Anopheles and Other Mosquitoes
Mosquitoes are members of a family of the nematocerid flies: the Culicidae. This family consists of three subgroubs: the toxorhynchitinae, the anophelinae, and the culicinae. Blood feeding mosquitoes, including vectors of human diseases, belong to the two latter groups. Anophelines mate predominantly in crepuscular station-keeping swarms formed by large aggregations of males above inanimate markers.6–12 Virgin females enter the swarm, are captured by a male, and leave the swarm while in copula. Most male culicines also aggregate in the proximity of visual markers, although members of the Aedes subgenus are known to swarm and mate in the vicinity of the host.13–18 There is strong evidence that males and females recognize each other by the wing beat frequency specific to each species19,20 and interact acoustically by shifting their harmonic overtones to match.21,22 Furthermore, spatial segregation of the swarms may contribute to reproductive isolation of different species, as observed for the incipient M and S forms of An. gambiae species.23,24
Anopheline and culicine females are generally monandrous as after mating they become refractory to further insemination.2,25–29 Field studies show that remating does not occur in anophelines,29 or is observed at very low rates.2,27,28 During mating, male mosquitoes transfer sperm, and seminal secretions from the male accessory glands (MAGs) (seeFig. 1 for a representation of the male and female reproductive tracts). Sperm are stored by the female in a dedicated sperm storage organ named the spermatheca. While Anopheles mosquitoes have a single spermatheca, Aedes and Culex have three, like Drosophila.30 Seminal secretions from the MAGs are transferred to the female reproductive tract, and in some anophelines including the major malaria vectors (An. gambiae, An. arabiensis, and An. funestus), these secretions are coagulated into a gelatinous mating plug that is deposited in the atrium (sometimes called the uterus).31
Figure 1.
Cartoon representing male and female reproductive tracts in An. gambiae. (A) The male reproductive tract, showing the testes (T) and male accessory glands (MAG). (B) The reproductive tract of a freshly mated female, showing the ovaries (Ov), the atrium (A) containing a mating plug, and the spermatheca (Sp) filled with sperm.
The Role of MAG Secretions in Modulating Female Behavior after Mating
In many insect species, the MAGs exert powerful control over female reproduction and behavior. In D. melanogaster, a wealth of studies has demonstrated that MAG secretions, composed of proteins, carbohydrates, and lipids,32 play a major role in inducing behavioral and physiological changes in the female. These changes include loss of mating receptivity, increased oogenesis and oviposition, increased feeding and sleeping activity, induction of immune responses, and decreased longevity (reviewed in Refs. 33 and 34). Seminal fluid proteins are also associated with the behavioral and physiological changes (namely, the loss of mating receptivity and increased oogenesis and oviposition) observed in females of some mosquito species after mating (reviewed in Refs. 32 and 35). The physical act of copulation is not always required to induce these post-mating responses; in Aedes and Culex species, simply transplanting the MAGs or injecting MAG extracts into the hemolymph of virgin females is sufficient to induce life-long mating refractoriness and to trigger oviposition.26,36–40 Early studies showed some promise in the identification of MAG factors modulating oviposition and female remating.26,38–40,41 However, in spite of recent advances in characterizing the components of the seminal fluid in Aedes mosquitoes,42,43 the nature of the molecule(s) responsible for inducing post-mating behavior remains elusive.
The mode of action of key seminal fluid proteins appears to be conserved across several species; heterologous transplant of MAGs between Ae. aegypti, C. pipiens, and D. melanogaster stimulates oviposition in virgins of all species.44 Similarly, oviposition can be triggered in virgin Ae. aegypti by the transplantation of MAGs from Ae. triseriatus.45 However, some functions of the seminal fluid may be species specific. Yeh and Klowden46 found that implanting MAGs or injecting MAG homogenates from a conspecific male stimulated pre-oviposition behavior (i.e. the attraction of gravid mosquitoes to mating sites) in Ae. aegypti. However, injection of MAG extracts from other mosquito species failed to induce pre-oviposition behavior.
The role of MAG secretions in shaping two of the major female post-mating responses in Anopheles, such as the acquisition of sexual refractoriness and the induction of oviposition, is controversial (see Table 1 for a summary of the experiments). Early studies, based on hybrid mating, suggested a role for the MAGs in triggering oviposition. Virgin An. gambiae, which rarely lay eggs,47 can be induced to oviposit unfertilized eggs if mated to hybrid An. gambiae/An. melas males with degenerate testes but normal accessory glands.48 However, attempts to replicate the MAG transplant and injection experiments that provide such clear results in other Diptera have produced mixed results in anophelines. Intraspecific MAG implant induced loss of mating receptivity in virgin An. quadrimaculatus26 but not in An. gambiae and An. albimanus.49 Furthermore, Klowden found that intra-abdominal injections of MAG extracts had no effect on mating receptivity or oviposition behavior of virgin An. gambiae.49 In contrast, recent experiments by Shutt et al.50 showed that intra-thoracic injections of MAG extracts into virgin An. gambiae females reduced their likelihood of subsequently becoming inseminated. To explain this discrepancy, Shutt et al. suggested that putative MAG protein receptors in An. gambiae might be located in the thorax, and that this would reduce the efficacy of abdominal injections.50 There might be several explanations for these conflicting results. In the field, Anopheles remating occurs at very low rates2,27–29 while it is much higher under laboratory conditions.51–53 A number of parameters can influence the frequency of mating (and remating) — as well as oviposition — in laboratory cages. These parameters include cage size, female age, female fitness after the injection (MAG homogenates contain many proteolytic enzymes that when injected may interfere with normal female physiology), and length of time that females are exposed to males.51 Further studies are needed to clarify the role of MAG secretions in modulating female post-mating changes. MAG products may need to be processed by atrial proteases in order to stimulate ovulation and oviposition, explaining why MAG implants or extract injections are not effective. In support of this hypothesis, proteases associated with processing of peptide hormones54 are highly expressed and synthesized in virgin atria.55
Table 1. Induction of post-mating response experiments in Anopheles.
| Reference | Species | Mating status | Operation | Behavioral change |
| 26 | An. quadrimaculatus | Virgin | MAG implant | Refractoriness |
| 48 | An. gambiae | Mated | Mated males with abnormal testes and normal MAGs | Refractoriness,* oviposition |
| 64 | An. gambiae | Mated | Mated males with abnormal testes and abnormal MAGs | No refractoriness, no oviposition† |
| 49 | An. gambiae/An. albimanus | Virgin | MAG implant | No refractoriness, no oviposition |
| 49 | An. gambiae | Virgin | Injection of MAG homogenate (intra-abdominal) | No refractoriness, no oviposition |
| 49 | An. gambiae | Virgin | Injection of MAG homogenate (into the genital tract) | No refractoriness |
| 49,51 | An. gambiae | Virgin | Implant of spermatheca from a mated female | No refractoriness, no oviposition |
| 49,51 | An. gambiae | Mated | Removal of spermatheca | No oviposition |
| 50 | An. gambiae/An. stephensi | Virgin | Injection of MAG homogenate (intra-thoracic) | Refractoriness |
| 53 | An. gambiae | Mated | Mated males with no sperm cells and functional MAGs | Refractoriness, oviposition |
Notes: MAG, male accessory gland.
Behavioral changes include the ability to lay eggs (oviposition) or the inhibition of remating (refractoriness) after treatment.
*Further mating was performed by forced copulation. No insemination was detected.
†Oviposition occurred at very low levels similar to those observed in virgins.
Sperm Effect in Female Post-Mating Responses
Injection of MAG secretions or purified components into female Drosophila induces post-mating changes for 1–2 days.56 The maintenance of the response for up to a week, as seen in natural matings, requires transfer of sperm,57–59 a phenomenon known as the ‘sperm effect’. The sperm effect is mediated by the binding of MAG peptides to the sperm cell flagella; seminal proteins bound to sperm tails are carried into the female sperm storage organs, where their gradual release maintains the post-mating response.60–62
Early studies suggested that sperm transfer could play an important role in influencing female behavior in mosquitoes. Gwadz found that refractoriness to mating was induced within 15 seconds of mating in female Ae. aegypti,63 in contrast to the 4 hour latency arising from MAG transplants described by Craig.26 This difference could be due to the time required by the injected females to recover from the operation or can be interpreted as an evidence for a sperm effect with the immediate post-mating response triggered by the filling of a female’s bursa by sperm.63 If a sperm effect is present in Ae. aegypti, it would only be effective over the short-term, unlike the long-term maintenance observed in D. melanogaster. Removal of the aedine spermathecae, where sperm migrate after their initial deposition in the bursa, prior to copulation did not interfere with the induction of oviposition upon mating.51
Due to the ambiguous results of MAG transplantation and injection experiments, sperm has often been viewed as a key trigger of post-mating changes in anophelines (Table 1). As with studies of the effects of MAG secretions, early studies of the sperm effect examined hybrid matings. Hybrid An. gambiae/An. melas males with degenerate testes but with well-developed MAGs were still capable of inducing post-mating behavior,48 whereas hybrid males lacking both MAGs and testes were not,64 suggesting that sperm are not required to trigger female behavioral changes. In a recent study, the role of sperm in modulating female behavior in An. gambiae has been unambiguously established by RNA interference-mediated silencing of zero population growth, a germ cell differentiation gene whose knockdown results in males lacking sperm cells but with functional accessory glands. These spermless males were capable of inducing oviposition and inhibiting remating in females,53 confirming the original findings by Bryan that in this species sperm have no role in triggering post-mating behavior. Furthermore, spermless males induced female transcriptional responses similar to those triggered by normal males.53
Although sperm do not play a major role in triggering behavioral changes in female An. gambiae, an intact (and possibly innervated) spermatheca may be needed for such responses to take place. Surgical removal of the spermatheca from mated An. gambiae results in inhibition of oviposition, while implantation of a mated spermatheca into virgin females does not stimulate egg laying or loss of sexual receptivity.49,51 In the grasshopper Gomphocerus rufus, microinjection of MAG secretion into the spermatheca induces female mating refractoriness and sperm have no role in the process.65 In this insect spermathecal gland cells digest and resorb seminal secretions, and the neural pathway between the spermatheca and the ventral nerve cord is required to maintain sexual refractoriness.66 Recently, it has been shown in Drosophila that spermathecal secretory cells attract sperm into the female sperm storage organ and participate in modulating sperm motility and stimulating oviposition.67 A possible role of spermathecal cells in Anopheles post-mating behavior needs to be fully established.
Mag Proteins in An. Gambiae and Other Insects
Despite the crucial role of MAG components in regulating many aspects of mosquito reproduction, elucidation of the seminal proteomes of mosquitoes has only been established in the last few years.42,43,68,69 The molecular composition of mosquito MAG secretions remained entirely unknown until Dottorini et al.’s69 bioinformatic comparison of D. melanogaster and An. gambiae identified 46 MAG-expressed anopheline genes. Since then, further components have been identified in An. gambiae68 and the Ae. aegypti seminal proteome has been well characterized.42,43 These analyses have identified 71 genes expressed in the MAGs of An. gambiae.68–70 Moreover, for the purpose of this review, we have identified 50 additional An. gambiae MAG-specific genes by analyzing the data produced in a recent whole genome microarray study where gene expression was determined in multiple male and female tissues71 (Table 2).
Table 2. Genes identified in the MAGs of An. gambiae.
| Functional class | Anopheles | Drosophila | Aedes | Expression data (%) |
| Acps | ||||
| AGAP001510 | nd | AAEL017145 | 100 | |
| AGAP006362 | CG13699 | AAEL014767 | 100 | |
| AGAP006581 | Acp62F* | AAEL000356 | 13 | |
| AGAP006583 | Acp63F* | nd | 100 | |
| AGAP006585 | Acp63F* | nd | 100 | |
| AGAP006586 | Acp62F* | AAEL000356 | 17 | |
| AGAP006587 | Acp62F* | nd | 100 | |
| AGAP006589 | nd | nd | 100 | |
| AGAP008116 | nd | AAEL010264 | 100 | |
| AGAP008968 | CG31704 | nd | nd | |
| AGAP009352 (AGAP012681) | Acp70A* | nd | 100 | |
| AGAP009353 (AGAP012680) | Msopa* | nd | 100 | |
| AGAP009354 | Mst57Da* | nd | 100 | |
| AGAP009355 | Dro-PA | nd | 100 | |
| AGAP009356 | Mst57Da* | nd | 100 | |
| AGAP009357 | Dro-PA | nd | 100 | |
| AGAP009358 (AGAP012682/AGAP012830) | Nplp4 | nd | 100 | |
| AGAP009359 | Mst57Da* | nd | 100 | |
| AGAP009360 (AGAP012807) | CG13230 | nd | 98 | |
| AGAP009361 | Acp95EF* | nd | nd | |
| AGAP009362 | CG6409 | nd | 100 | |
| AGAP009367 | Acp26Ab* | nd | 100 | |
| AGAP009368 | CG14770 | nd | 100 | |
| AGAP009369 | Acp53Ea* | nd | 100 | |
| AGAP009370 (AGAP012706) | Acp53Ea* | nd | 100 | |
| AGAP009371 | CG14302 | nd | 100 | |
| AGAP009372 | CG32726 | nd | nd | |
| AGAP009373 | vsg | Supp02310* | 100 | |
| AGAP013714 | nd | nd | 100 | |
| AGAP013731 | CG13230 | nd | 98 | |
| AGAP013734 | CG15065 | nd | 100 | |
| AGAP013776 | Nplp4 | nd | 100 | |
| Redox | ||||
| AGAP001039 | spo | AAEL009762 | 100 | |
| AGAP003067 | Cyp304a1 | AAEL014413 | 100 | |
| AGAP005784 | PAM | AAEL007732♮ | 100 | |
| AGAP007420 | PHM | AAEL001394 | 0 | |
| AGAP007491 | CG4670 | AAEL012054 | 35 | |
| AGAP008019 | Cyp12b2 | AAEL002031♮ | 99 | |
| AGAP008203 | Cyp6a2 | AAEL009120 | 100 | |
| AGAP009363 | Cyp9f2 | AAEL001312♮ | 0 | |
| AGAP009584 | Trx-2 | AAEL010777 | 24 | |
| AGAP012855 | Cyp6a2 | AAEL009120 | 100 | |
| CYP302A1 | dib | AAEL015655 | 85 | |
| CYP306A1 | phm | AAEL004888 | 88 | |
| CYP314A1 | shd | AAEL011850 | 98 | |
| CYP315A1 | sad | AAEL010946 | 100 | |
| Proteases | ||||
| AGAP005791 | CG12951 | nd | 100 | |
| AGAP005792 | CG12951 | nd | 100 | |
| AGAP008276 | Try29F | nd | 100 | |
| AGAP008277 | Try29F | nd | 100 | |
| AGAP008997 | CG8172 | AAEL000238 | 100 | |
| AGAP012315 | CG34290 | AAEL012447♮ | 100 | |
| AGAP013150 | CG9806* | AAEL009108 | 100 | |
| ENSP017764 | nd | nd | 100 | |
| ZCP1 | nd | nd | 100 | |
| ZCP3 | nd | nd | 100 | |
| ZCP4 | nd | nd | 100 | |
| ZCP6 | nd | nd | nd | |
| ZCP7 | nd | nd | 100 | |
| ZCP9 | nd | nd | 100 | |
| Nucleic acid binding | ||||
| AGAP000355 | Mkrn1 | AAEL007476 | 100 | |
| AGAP000754 | CG15439 | AAEL003032 | 100 | |
| AGAP000916 | pAbp | nd | 100 | |
| AGAP000918 | pAbp | nd | 100 | |
| AGAP000920 | pAbp | nd | 100 | |
| AGAP003844 | cwo | AAEL010513 | 100 | |
| AGAP009339 | CG6654 | AAEL005029 | 100 | |
| AGAP009699 | sens-2 | AAEL001243 | 100 | |
| AGAP010358 | gsb | nd | 100 | |
| AGAP010359 | prd | nd | 100 | |
| Hydrolases | ||||
| AGAP001649 | CG31414 | AAEL014321♮ | 100 | |
| AGAP005255 | Rab3 | AAEL006267 | 100 | |
| AGAP006425 | CG9701 | nd | 100 | |
| AGAP010720 | pom1 | AAEL013237 | 100 | |
| COEBE1D | EST-6 | nd | nd | |
| COEBE4D | EST-7 | nd | nd | |
| Protein folding | ||||
| AGAP000831 | CG7872 | AAEL013114 | 100 | |
| AGAP001424 | CG5520 | AAEL012827 | nd | |
| AGAP001502 | CG11267 | AAEL001052 | 10 | |
| AGAP007088 | CG2852 | AAEL013279 | 3 | |
| AGAP008822 | CG9847 | AAEL004313 | 0 | |
| AGAP012407 | PDI* | AAEL000641* | 4 | |
| Transferases | ||||
| AGAP000843 | CG4774 | AAEL012719♮ | 100 | |
| AGAP009099 | CG7356 | nd | 100 | |
| AGAP009190 | CG4688 | AAEL007955 | 100 | |
| AGAP009191 | CG16936 | AAEL007954 | 100 | |
| AGAP009365 | CG5973 | AAEL001297 | 100 | |
| AGAP009377 | CG5973 | AAEL001297 | 93 | |
| Protein binding | ||||
| AGAP000927 | sqh | AAEL008921 | 100 | |
| AGAP004761 | mwh | AAEL008269 | 100 | |
| AGAP005684 | Syx17 | AAEL000282♮ | 100 | |
| AGAP007041 | fibrinogen | AAEL001713* | 100 | |
| AGAP012986 | spi | AAEL011205 | 100 | |
| Transmembrane proteins | ||||
| AGAP000107 | pyx | AAEL004179 | 98 | |
| AGAP002824 | Takr86C | AAEL017414 | 100 | |
| AGAP010637 | Toll6 | nd | 100 | |
| AGAP010861 | CG1698 | AAEL003626 | 100 | |
| Immune peptides | ||||
| AGAP007049 | CG10433 | AAEL009861 | 2 | |
| AGAP009429 | Anp* | nd | 98 | |
| TEP15 | CG10363 | AAEL014755 | nd | |
| Serpins | ||||
| AGAP005246 | CG9334 | AAEL007765 | 17 | |
| SRPN9 | CG10956 | AAEL008364 | nd | |
| Nucleic acid metabolism | ||||
| AGAP000139 | tam | AAEL015671 | 100 | |
| AGAP009842 | CG8194 | AAEL001159 | 36 | |
| Protein metabolism | ||||
| AGAP000926 | l(1)G0196 | AAEL008950♮ | 100 | |
| AGAP009673 | QPCT | AAEL010727 | 100 | |
| Lipases | ||||
| AGAP003083 | CG17097 | AAEL012343♮ | 100 | |
| AGAP003749 | CG6296 | AAEL012790 | 100 | |
| CRISPs | ||||
| AGAP006418 | CG17575* | AAEL009239*♮ | 100 | |
| Kinases | ||||
| AGAP002181 | CG5644 | AAEL010062 | 100 | |
| Others | ||||
| AGAP004428 | CG3359 | AAEL014917 | 3 | |
| AGAP005239 | CG8323 | AAEL006262 | 93 | |
| AGAP005504 | scramb1 | AAEL010661 | 100 | |
| AGAP007339 | TpnC47D | AAEL000744 | 96 | |
| AGAP009001 | Hdc | AAEL014632 | 100 | |
| AGAP009189 | Eps-15 | AAEL007950 | 100 | |
| AGAP009364 | CG5793 | AAEL001308 | 0 | |
| CALRETICULIN | Crc | AAEL001005♮ | nd | |
| Unknown | ||||
| AGAP003736 | CG30053 | nd | 100 | |
| AGAP005859 | nd | nd | 100 | |
| AGAP008439 | CG31705 | AAEL005017 | 100 |
Notes:</emph> Acps, accessory gland proteins; CRISPs, cysteine-rich secretory proteins.
The table contains all 121 genes identified to date in the accessory glands of An. gambiae (Anopheles) males, identified by a number of strategies (as described in the text).68–71 Genes are organized based on their functional class. When known, the putative D. melanogaster (Drosophila) and Ae. aegypti (Aedes) orthologues are indicated. In the Anopheles column, identifiers in bold represent genes whose products have been detected in the male accessory glands (MAGs) by mass spectrometry68 and/or reverse transcription PCR (RT-PCR) data.68–70 The ‘*’ symbol indicates D. melanogaster and Ae. aegypti genes that are expressed in the MAGs of these species. The ‘♮’ symbol in the Aedes column indicates the presence of multiple putative orthologues (only the most similar orthologue is reported). The expression data column refers to the degree of MAG specificity of the An. gambiae genes, as obtained from MozAtlas website (http://www.tissue-atlas.org/); the degree of specificity is reported as the percentage of expression observed in the MAGs relative to all male and female tissues where expression was present in a minimum of 3/4 calls.71 Numbers are rounded to the nearest integer. Numbers in bold represent expression data obtained by transcriptional and/or immuno blotting analyses.68–70 Please note that An. gambiae genes in the following groups share a common probeset in mozatlas: (1) AGAP009354, AGAP009356, AGAP009359; (2) AGAP009355, AGAP009357; (3) AGAP009358, AGAP0013776; (4) AGAP009360, AGAP0013731; (5) AGAP009365, AGAP009377; and (6) AGAP009369, AGAP009370.
These studies allow comparative analysis of the MAG proteomes in An. gambiae, Ae. aegypti, and D. melanogaster. Many of the functional classes of MAG proteins are shared between the two mosquito species and D. melanogaster (Fig. 2 and Table 2). In all three species, accessory gland proteins (Acps) form the most abundant category. Acps are defined as MAG-specific proteins that are secreted and do not contain known functional domains. In the fruitfly, many of these proteins are known to be transferred to females and control a number of responses to mating. For instance, sex peptide (Acp70A) has been implicated in the inhibition of remating,56,61,62,72 increased egg production,73,74 decreased longevity,75 alteration of locomotion and feeding behaviors,76,77 and stimulation of the immune system.78,79 Sex peptide is detected by sensory neurons in the female reproductive tract80,81 where it binds to a G protein coupled receptor82 leading to the alteration of female physiology and behavior. Another category abundant in An. gambiae and D. melanogaster comprises peptides that have putative hormonal function. For instance, the hormone ovulin (Acp26Aa) is an important regulator of ovulation in D. melanogaster.83 Although functions have yet to be ascribed to MAG peptide hormones in An. gambiae, putative orthologues of Acp53Ea, another peptide hormone that in Drosophila is involved in sperm competition,84,85 were localized in close proximity to the spermatheca, suggesting a role in sperm function.86 In Ae. aegypti, a head peptide expressed primarily in the MAGs87 has been implicated in the short-term inhibition of host-seeking behavior in females.88
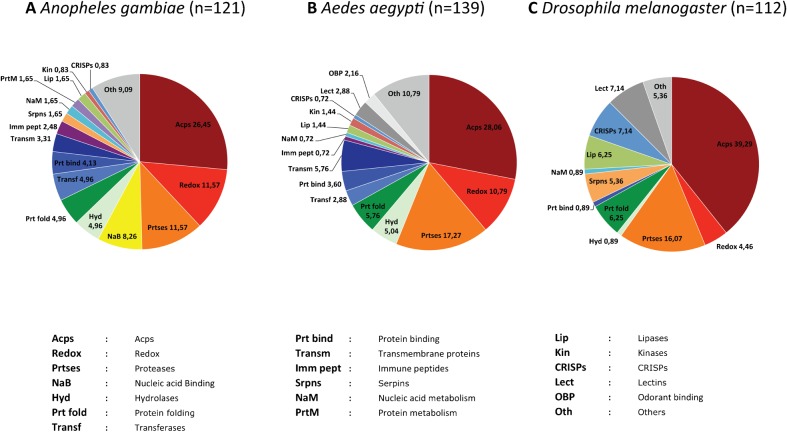
Figure 2.
Functional classes of MAG-specific genes in An. gambiae, Ae. aegypti, and D. melanogaster. The pie charts represent the functional classes of genes that are expressed in the male accessory glands of An. gambiae (A), Ae. aegypti (B), and D. melanogaster (C). Values indicate the percentage of genes that belongs to each class in the three species. These charts are based on published data derived from bioinformatics,34,42,69 transcriptional34,42,68,69,71 and proteomic34,43,68 analyses.
Proteases and peptidases are represented at high levels in all three species. These enzymes can be involved in the activating cleavage of many seminal fluid protein.83,89 In Drosophila, ovulin is transferred to females as a preprohormone where it is processed by a seminal astacin-like protease.89 Other proteases can play roles in controling the activity of these processing proteases.90,91 Similarly abundant are serpins and other protease inhibitors, which have been shown to have a role in male mammalian fertility,92 and chaperones, which can facilitate protein folding and sperm-egg interactions.93 Cysteine-rich secretory proteins (CRISPs) that in ascidians are involved in gamete interactions94 are instead more frequently observed in D. melanogaster than in mosquito species. Lipases are also more abundant in D. melanogaster than in mosquitoes; in Drosophila these enzymes are transferred to females during mating,95 influence egg-laying behavior and possibly receptivity to remating,96 and provide energy to sperm.97 Contrary to Drosophila and Aedes, in Anopheles, no lectins have been identified to date in the MAGs. Lectins are postulated to play a role in sperm-oocyte recognition.98
Finally, it is notable that proteins that participate in oxidation/reduction (redox) processes are more abundant in mosquitoes compared to D. melanogaster. In the fruitfly, many MAG-expressed redox proteins are prolyl 4-hydrohylases that are involved in the hydroxylation of collagen, whose function may be needed to ensure the integrity and functionality of the extracellular matrix, possibly necessary for the activity of the MAGs.99 In An. gambiae MAGs, the majority of identified redox proteins are oxidases. Among these, a number are involved in the synthesis of ecdysteroid hormones, which are transferred to females during mating70 and that control egg production after a blood meal.100 By contrast, in Ae. aegypti, MAG-specific redox proteins are mostly dehydrogenases involved in energetic metabolism, and subunits of the ATP synthase protein complex, which might supply the energetic requirement for protein synthesis in this secretory glands.42,43 A Rab3-like protein appears instead to be specific for An. gambiae MAGs. Rabs are proteins that regulate membrane trafficking and in particular Rab3 is associated with secretory vesicles.101
Although functional classes of seminal proteins are conserved across the species, the MAG-expression of individual genes rarely is. As shown in Table 2, among the 121 genes expressed in the An. gambiae MAGs, 109 and 71 have putative annotated D. melanogaster and Ae. aegypti orthologues, respectively. However, by comparing the tissue expression of orthologues in the three species, it is clear that only a small number of these are expressed in the MAGs of more than one species. Only 17% of the An. gambiae genes have orthologues in either D. melanogaster and/or Ae. aegypti that are expressed in the MAGs. Only two genes are expressed in the MAGs of all three species: they encode for a protein disulphide isomerase, which may promote protein folding, and a CRISP protein. A total of 16 genes are expressed in the MAGs of both An. gambiae and D. melanogaster but not Ae. aegypti, comprising predicted pro-hormonal peptides, antimicrobial peptides, protease inhibitors, and proteases. Putative orthologues of many Drosophila accessory gland peptides were identified in An. gambiae: among these is the putative An. gambiae orthologue of sex peptide. A further two genes are expressed in the MAGs of both An. gambiae and Ae. aegypti, but not D. melanogaster: a fibrinogen and a visgun-like peptide, whose functions in reproduction are unknown. The diversity of the factors synthetized in the MAGs may highlight different reproductive roles for these male reproductive tissues among insects, stressing the need for a detailed analysis of the reproductive molecular machinery in each species.
An. gambiae MAG Secretions are Coagulated to Form a Mating Plug
Unlike most mosquito species, An. gambiae (and its close relatives) transfers its seminal secretions as a gelatinous mating plug, which becomes coagulated during copulation. Many insects produce mating plugs with different functions. In the lepidopteran Cressida cressida, males transfer an external plug termed a sphragis that blocks the female copulatory opening, physically preventing remating by other males.102 In the hymenopteran Bombus terrestris, a linoleic fatty acid present in the mating plug renders females refractory to further insemination for life.103 In Diptera, there are examples of mating plugs that prevent remating, such as in the dung fly Coproica vagans,104 while in D. hibisci, the mating plug is associated with female loss of sexual receptivity and correct sperm storage.105,106 In D. melanogaster, mating plug function and composition have been well characterized. The plug is divided into anterior and posterior regions: the posterior part is composed of male ejaculatory bulb proteins (PEB-me, PEBII, and PEBIII) and is formed in the female reproductive tract 3 minutes after the start of mating but before sperm transfer.107 Acquisition of short-term refractoriness is associated with PEBII as shown in knockdown studies.108 The anterior region of the plug is formed after the transfer of sperm, and it is needed to prevent sperm backflow from the storage organs and is composed of MAG proteins such as Acp36DE.109 Acp36DE binds to sperm and enters the sperm storage organs,110,111 where it enhances the rate of sperm accumulation.112
Among mosquitoes mating plugs are only found in anophelines.113 The role of the plug in reproduction has been controversial for decades and various hypotheses ranged from a physical barrier against re-insemination or sperm loss to a vestigial trait with no function.31,114–116 Recently, it has been shown that in An. gambiae, mating plug transfer is crucial for correct sperm storage by the female after mating.68 Through RNAi-mediated knockdown of a MAG-specific plug-forming transglutaminase (TGase), Rogers et al.74 were able to show that females mated to males who failed to form and transfer the plug could not store sperm in their spermatheca, uncovering a crucial role of this feature in the reproduction of An. gambiae. This MAG-specific TGase coagulates seminal secretions by cross-linking other MAG secreted proteins, primarily Plugin, a glutamine-rich protein that is highly abundant in the mating plug.68 This mechanism is remarkably similar to semen coagulation in mammals.117 While An. gambiae has three TGases, of which only one is active in the MAGs, the genomes of the culicines Ae. aegypti and C. quinquefasciatus contain only two TGase and none shows activity in the male glands, consistent with the inability of Aedes and Culex mosquitoes to produce a mating plug.
The An. gambiae plug is digested in the female atrium during the first 24–36 hours post-mating, possibly by female proteases,68,118 and this processing may produce factors that affect female post-copulatory behavior. This might explain the frequently observed inability of MAG transplantation or extract injections to induce oviposition and sexual refractoriness in An. gambiae, in contrast to what is observed in culicines.<</p>
Conclusions and Outlook
To date, the most effective strategies for the control of An. gambiae mosquitoes rely on the use of insecticides through indoor residual sprays and long-lasting insecticide treated bednets.119 In many regions where these tools are used, the size of vector populations is decreasing significantly, contributing to reducing malaria transmission and therefore placing these regions in the Malaria Elimination Group.120 However, in much of sub-Saharan Africa, the use of insecticides is not sufficient to stop the spread of disease. Furthermore, the origin and spread of insecticide resistance in vector populations is reducing the effectiveness of insecticide-based strategies.121 In this scenario, the study of the processes shaping the biology and physiology of An. gambiae mosquitoes and other disease vectors brings new promise to the generation of novel ideas and to the identification of targets for the manipulation of the mosquito vectorial capacity. Recent studies reviewed here have identified the factors produced and secreted by the MAGs in several species. However, the functions of the majority of these factors remain unknown.
Characterizing the effects of MAG proteins and of the female genes that they target can provide a gateway to understanding the genetic modulation of mosquito reproduction. This knowledge would benefit the development of novel control programs based on the genetic modification of the vector at two different but complementary levels. On the one hand, it may help generate males with increased mating competitiveness, crucial for a successful deployment of SIT and related strategies. Laboratory reared mosquitoes generally show extremely low mating success in competition with their wild counterparts (reviewed in Ref. 122). There is growing evidence that the MAGs are associated with male reproductive success across insects. In some species, including mosquitoes,123 depletion of the MAGs may result in infertility long before males exhaust their supplies of sperm. Moreover, seminal fluid availability can contribute to male motivation to mate in the first place,124 and a lack of accessory gland material as a result of sexual immaturity or exhaustion is often associated with low mating rates.125 In laboratory-reared anophelines, male mating rate reaches a maximum approximately 3–7 days after eclosion12,126–128 which corresponds to the period of time required to synthesize MAG secretions (72–100 hours).129 Improving the diets of adult male mosquitoes may be a simple way to improve mating competitiveness by promoting the rapid maturation and final size of the MAGs. For instance, supplementing the pre-release diets of males with protein or juvenile hormone analogues has been shown to dramatically increase the mating competitiveness of sterile males in several fruit fly species (reviewed in Ref. 130).
On the other hand, the study of reproductive biology will identify male genes important for fertility that could be targeted to induce genetic sterility in males for release, while genes responsible for female fertility could be disrupted in homing endonuclease mediated population depletion.131 Understanding the mode of action of MAG proteins may also allow the development of novel chemosterilants. Synthetic compounds could be developed that mimic the behavior-modulating effects of MAG proteins, or prevent the function of factors essential for fertility. Inducing the post-mating response, particularly the inhibition of remating, in virgin females would provide an excellent addition to the vector control arsenal. Novel chemosterilants would provide a second line of defense when used in combination with traditional insecticides used as indoor residual sprays or insecticide treated bednets, as resistant mosquitoes that escape insecticide action would be rendered sterile, preventing the spread of resistance genes. Moreover, as many MAG genes evolve rapidly132 and are therefore highly divergent between closely related species, it may be possible to develop chemosterilants that would precisely target only the desired vector. One example might be the MAG-specific plug forming transglutaminase identified by Rogers et al.68 whose function is important for ensuring correct storage of sperm by the female and which has no direct orthologue in aedine or culicine mosquitoes. A molecule that specifically inhibits this TGase, but not other related enzymes, could provide a specific mechanism for reducing the fertility of anopheline mosquitoes. Although speculative at present, these strategies are potentially highly rewarding. The full feasibility of such measures will only become clear once we have an improved understanding of the multiple functions of the MAGs.
Acknowledgments
The authors wish to thank members of the Catteruccia laboratory for their critical reading of the manuscript and Daniela Di Lascio for drawing Fig. 1. FC has been sponsored on research related to this topic by an MRC Career Development Award (agreement ID: 78415, file no. G0600062), by the European Research Council FP7 ERC Starting Grant project ‘Anorep’ (grant ID: 260897) and by the EC FP7 Collaborative Project 223601 ‘Malvecblok’.
References
- 1.Sinden RE. Malaria, sexual development and transmission: retrospect and prospect. Parasitology. 2009;136:1427–34. doi: 10.1017/S0031182009990667. [DOI] [PubMed] [Google Scholar]
- 2.Tripet F, Touré TY, Dolo G, Lanzaro GC. Frequency of multiple inseminations in field-collected Anopheles gambiae females revealed by DNA analysis of transferred sperm. Am J Trop Med Hyg. 2003;68:1–5. [PubMed] [Google Scholar]
- 3.Clements AN. London: Chapman and Hall; 1992. The biology of mosquitoes: sensory reception and behaviour. [Google Scholar]
- 4.Knipling EF. Possibilities of insect control or eradication through the use of sexually sterile males. J Econ Entomol. 1955;48:459–62. [Google Scholar]
- 5.Benedict MQ, Robinson AS. The first releases of transgenic mosquitoes: an argument for the sterile insect technique. Trends Parasitol. 2003;19:349–55. doi: 10.1016/s1471-4922(03)00144-2. [DOI] [PubMed] [Google Scholar]
- 6.Panicker KN, Rajagopalan PK. Field observations on the swarming & the mating behaviour of Anopheles subpictus Grassi 1899. Indian J Med Res. 1984;80:60–2. [PubMed] [Google Scholar]
- 7.Charlwood JD, Pinto J, Sousa CA, Madsen H, Ferreira C, do Rosario VE. The swarming and mating behaviour of Anopheles gambiae s.s. (Diptera: Culicidae) from Sao Tome Island. J Vector Ecol. 2002;27:178–83. [PubMed] [Google Scholar]
- 8.Yuval B, Bouskila A. Temporal dynamics of mating and predation in mosquito swarms. Oecologia. 1993;95:65–9. doi: 10.1007/BF00649508. [DOI] [PubMed] [Google Scholar]
- 9.Charlwood JD, Jones MDR. Mating in the mosquito, Anopheles gambiae sl. Physiol Entomol. 1980;5:315–20. [Google Scholar]
- 10.Charlwood JD, Thompson R, Madsen H. Observations on the swarming and mating behaviour of Anopheles funestus from southern Mozambique. Malar J. 2003;2:2. doi: 10.1186/1475-2875-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reisen WK, Sakai RK, Baker RH, Azra K, Niaz S. Anopheles culicifacies: observations on population ecology and reproductive behavior [Mosquito, Pakistan]. Mosq News. 1982;42:93–101. [Google Scholar]
- 12.Howell PI, Knols BGJ. Male mating biology. Malar J. 2009;8(Suppl 2):S8. doi: 10.1186/1475-2875-8-S2-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reisen WK, Knop NF, Peloquin JJ. Swarming and mating behavior of laboratory and field strains of Culex tarsalis (Diptera: Culicidae). Ann Entomol Soc Am. 1985;78:667–73. [Google Scholar]
- 14.Yuval B. The vertebrate host as mating encounter site for its ectoparasites: ecological and evolutionary considerations. Bull Soc Vector Ecol. 1994;19:115–20. [Google Scholar]
- 15.Smith SM, Gadawski RM. Swarming and mating in Aedes provocans (Diptera: Culicidae). Great Lakes Entomol. 1994;27:175–84. [Google Scholar]
- 16.Gibson G. Swarming behaviour of the mosquito Culex pipiens quinquefasciatus: a quantitative analysis. Physiol Entomol. 1985;10:283–96. [Google Scholar]
- 17.Nielsen ET, Bell RT, Haeger JS. Swarming and mating in mosquitoes. Ann Entomol Soc Am. 1960;1:71–95. [Google Scholar]
- 18.Hartberg WK. Observations on the mating behaviour of Aedes aegypti in nature. Bull World Health Organ. 1971;45:847–50. [PMC free article] [PubMed] [Google Scholar]
- 19.Brogdon WG. Measurement of flight tone differences between female Aedes aegypti and A. albopictus (Diptera: Culicidae). J Med Entomol. 1994;31:700–3. doi: 10.1093/jmedent/31.5.700. [DOI] [PubMed] [Google Scholar]
- 20.Brogdon WG. Measurement of flight tone differentiates among members of the Anopheles gambiae species complex (Diptera: Culicidae). J Med Entomol. 1998;35:681–4. doi: 10.1093/jmedent/35.5.681. [DOI] [PubMed] [Google Scholar]
- 21.Pennetier C, Warren B, Dabirè KR, Russell IJ, Gibson G. ‘Singing on the wing’ as a mechanism for species recognition in the malarial mosquito Anopheles gambiae. Curr Biol. 2010;20:131–6. doi: 10.1016/j.cub.2009.11.040. [DOI] [PubMed] [Google Scholar]
- 22.Cator LJ, Arthur BJ, Harrington LC, Hoy RR. Harmonic convergence in the love songs of the dengue vector mosquito. Science. 2009;323:1077–9. doi: 10.1126/science.1166541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diabate A, Dao A, Yaro AS, Adamou A, Gonzalez R, Manoukis NC, et al. Spatial swarm segregation and reproductive isolation between the molecular forms of Anopheles gambiae. Proc R Soc Biol Sci. 2009;276:4215–22. doi: 10.1098/rspb.2009.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diabate A, Dabire RK, Kengne P, Brengues C, Baldet T, Ouari A, et al. Mixed swarms of the molecular M and S forms of Anopheles gambiae (Diptera: Culicidae) in sympatric area from Burkina Faso. J Med Entomol. 2006;43:480–3. doi: 10.1603/0022-2585(2006)43[480:msotmm]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 25.Gwadz RW, Craig GB, Jr, Hickey WA. Female sexual behavior as the mechanism rendering Aedes aegypti refractory to insemination. Biol Bull. 1971;140:201–14. doi: 10.2307/1540069. [DOI] [PubMed] [Google Scholar]
- 26.Craig GB. Mosquitoes: female monogamy induced by male accessory gland substance. Science. 1967;156:1499–501. doi: 10.1126/science.156.3781.1499. [DOI] [PubMed] [Google Scholar]
- 27.Yuval B, Fritz GN. Multiple mating in female mosquitoes-evidence from a field population of Anopheles freeborni (Diptera: Culicidae). Bull Entomol Res. 1994;84:137–9. [Google Scholar]
- 28.Scarpassa VM, Tadei WP, Kerr WE. Biology of Amazonian anopheline mosquitoes XVI. Evidence of multiple insemination (polyandry) in Anopheles nuneztovari Gabaldon. Braz J Genet. 1940;1:51–64. [Google Scholar]
- 29.Baimai V, Green CA. Monandry (monogamy) in natural populations of anopheline mosquitoes. J Am Mosq Control Assoc. 1987;3:481–4. [PubMed] [Google Scholar]
- 30.Clements AN. London: Chapman & Hall; 1992. The biology of mosquitoes. Vol. 1. Development, nutrition and reproduction. [Google Scholar]
- 31.Giglioli ME, Mason GF. The mating plug in anopheline mosquitoes. Proc R Entomol Soc Lond. 1966;41:123–9. [Google Scholar]
- 32.Gillott C. Male accessory gland secretions: modulators of female reproductive physiology and behavior. Annu Rev Entomol. 2003;48:163–84. doi: 10.1146/annurev.ento.48.091801.112657. [DOI] [PubMed] [Google Scholar]
- 33.Chapman T, Davies SJ. Functions and analysis of the seminal fluid proteins of male Drosophila melanogaster fruit flies. Peptides. 2004;25:1477–90. doi: 10.1016/j.peptides.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 34.Ram KR, Wolfner MF. Seminal influences: Drosophila Acps and the molecular interplay between males and females during reproduction. Integr Comp Biol. 2007;47:427–45. doi: 10.1093/icb/icm046. [DOI] [PubMed] [Google Scholar]
- 35.Avila FW, Sirot LK, LaFlamme BA, Rubinstein CD, Wolfner MF. Insect seminal fluid proteins: identification and function. Annu Rev Entomol. 2011;56:21–40. doi: 10.1146/annurev-ento-120709-144823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Young ADM, Downe AER. Male accessory gland substances and the control of sexual receptivity in female Culex tarsalis. Physiol Entomol. 1987;12:233–9. [Google Scholar]
- 37.Leahy MG, Craig GB. Accessory gland substance as a stimulant for oviposition in Aedes aegypti and A. albopictus. Mosq News. 1965;25:448–52. [Google Scholar]
- 38.Fuchs MS, Hiss EA. The partial purification and separation of the protein components of matrone from Aedes aegypti. J Insect Physiol. 1970;16:931–9. doi: 10.1016/0022-1910(70)90223-4. [DOI] [PubMed] [Google Scholar]
- 39.Fuchs MS, Craig GB, Jr, Hiss EA. The biochemical basis of female monogamy in mosquitoes. I. Extraction of the active principle from Aedes aegypti. Life Sci. 1968;7:835–9. doi: 10.1016/0024-3205(68)90114-8. [DOI] [PubMed] [Google Scholar]
- 40.Fuchs MS, Craig GB Jr, Despommier DD. The protein nature of the substance inducing female monogamy in Aedes aegypti. J Insect Physiol. 1969;15:701–9. [Google Scholar]
- 41.Hiss EA, Fuchs MS. The effect of matrone on oviposition in the mosquito, Aedes aegypti. J Insect Physiol. 1972;18:2217–27. doi: 10.1016/0022-1910(72)90250-8. [DOI] [PubMed] [Google Scholar]
- 42.Sirot LK, Poulson RL, Caitlin McKenna M, Girnary H, Wolfner MF, Harrington LC. Identity and transfer of male reproductive gland proteins of the dengue vector mosquito, Aedes aegypti: potential tools for control of female feeding and reproduction. Insect Biochem Mol Biol. 2008;38:176–89. doi: 10.1016/j.ibmb.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sirot LK, Hardstone MC, Helinski MEH, Ribeiro JMC, Kimura M, Deewatthanawong P, et al. Towards a semen proteome of the dengue vector mosquito: protein identification and potential functions. PLoS Negl Trop Dis. 2011;5:e989. doi: 10.1371/journal.pntd.0000989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leahy SMG. Non-specificity of the male factor enhancing egg-laying in Diptera. J Insect Physiol. 1967;13:1283–92. [Google Scholar]
- 45.Ramalingam S, Craig GB., Jr Functions of the male accessory gland secretions of Aedes mosquitoes (Diptera: Culicidae): transplantation studies. Can Entomol. 1976;108:955–60. [Google Scholar]
- 46.Yeh C, Klowden MJ. Effects of male accessory gland substances on the pre-oviposition behaviour of Aedes aegypti mosquitoes. J Insect Physiol. 1990;36:799–803. [Google Scholar]
- 47.Davidson G, Paterson HE, Coluzzi M, Mason GF, Micks DW.The Anopheles gambiae complex Wright J W, Pal R.ed editorsGenetics of insect vectors of disease. Amsterdam: Elsevier1967. p.211–50. [Google Scholar]
- 48.Bryan JH. Results of consecutive matings of female Anopheles gambiae species B with fertile and sterile males. Nature. 1968;218:489. doi: 10.1038/218489a0. [DOI] [PubMed] [Google Scholar]
- 49.Klowden MJ. Sexual receptivity in Anopheles gambiae mosquitoes: absence of control by male accessory gland substances. J Insect Physiol. 2001;47:661–6. doi: 10.1016/s0022-1910(00)00127-x. [DOI] [PubMed] [Google Scholar]
- 50.Shutt B, Stables L, Aboagye-Antwi F, Moran J, Tripet F. Male accessory gland proteins induce female monogamy in anopheline mosquitoes. Med Vet Entomol. 2010;24:91–4. doi: 10.1111/j.1365-2915.2009.00849.x. [DOI] [PubMed] [Google Scholar]
- 51.Klowden MJ. Switchover to the mated state by spermathecal activation in female Anopheles gambiae mosquitoes. J Insect Physiol. 2006;52:679–84. doi: 10.1016/j.jinsphys.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 52.Gomulski L. Polyandry in nulliparous Anopheles gambiae mosquitoes (Diptera: Culicidae). Bull Entomol Res. 1990;80:393–6. [Google Scholar]
- 53.Thailayil J, Magnusson K, Godfray HCJ, Crisanti A, Catteruccia F. Spermless males elicit large-scale female responses to mating in the malaria mosquito Anopheles gambiae. Proc Natl Acad Sci USA. 2011;108:13677–81. doi: 10.1073/pnas.1104738108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Riehle MA, Garczynski SF, Crim JW, Hill CA, Brown MR. Neuropeptides and peptide hormones in Anopheles gambiae. Science. 2002;298:172. doi: 10.1126/science.1076827. [DOI] [PubMed] [Google Scholar]
- 55.Rogers DW, Whitten M, Thailayil J, Soichot J, Levashina EA, Catteruccia F. Molecular and cellular components of the mating machinery in Anopheles gambiae females. Proc Natl Acad Sci USA. 2008;105:19390–5. doi: 10.1073/pnas.0809723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen PS, Stumm-Zollinger E, Aigaki T, Balmer J, Bienz M, Bohlen P. A male accessory gland peptide that regulates reproductive behavior of female D. melanogaster. Cell. 1988;54:291–8. doi: 10.1016/0092-8674(88)90192-4. [DOI] [PubMed] [Google Scholar]
- 57.Gromko MH, Newport MEA, Kortier MG. Sperm dependence of female receptivity to remating in Drosophila melanogaster. Evolution. 1984;38:1273–82. doi: 10.1111/j.1558-5646.1984.tb05649.x. [DOI] [PubMed] [Google Scholar]
- 58.Manning A. The control of sexual receptivity in female Drosophila. Anim Behav. 1967;15:239–50. doi: 10.1016/0003-3472(67)90006-1. [DOI] [PubMed] [Google Scholar]
- 59.Manning A. A sperm factor affecting the receptivity of Drosophila melanogaster females. Nature. 1962;194:252–3. [Google Scholar]
- 60.Peng J, Chen S, Busser S, Liu H, Honegger T, Kubli E. Gradual release of sperm bound sex-peptide controls female postmating behavior in Drosophila. Curr Biol. 2005;15:207–13. doi: 10.1016/j.cub.2005.01.034. [DOI] [PubMed] [Google Scholar]
- 61.Liu H, Kubli E. Sex-peptide is the molecular basis of the sperm effect in Drosophila melanogaster. Proc Natl Acad Sci USA. 2003;100:9929–33. doi: 10.1073/pnas.1631700100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chapman T, Bangham J, Vinti G, Seifried B, Lung O, Wolfner MF, et al. The sex peptide of Drosophila melanogaster: female post-mating responses analyzed by using RNA interference. Proc Natl Acad Sci USA. 2003;100:9923–8. doi: 10.1073/pnas.1631635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gwadz RW. Neuro-hormonal regulation of sexual receptivity in female Aedes aegypti. J Insect Physiol. 1972;18:259–66. doi: 10.1016/0022-1910(72)90126-6. [DOI] [PubMed] [Google Scholar]
- 64.Bryan JH. Biological sciences: further studies on consecutive matings in the Anopheles gambiae complex. Nature. 1972;239:519–20. doi: 10.1038/239519a0. [DOI] [PubMed] [Google Scholar]
- 65.Hartmann R, Loher W. Control mechanisms of the behavior ‘secondary defense’ in the grasshopper Gomphocerus rufus L. (Gomphocerinae: Orthoptera). J Comp Physiol A. 1996;178:329–36. doi: 10.1007/BF00193971. [DOI] [PubMed] [Google Scholar]
- 66.Hartmann R, Loher W. Post-mating effects in the grasshopper, Gomphocerus rufus L. mediated by the spermatheca. J Comp Physiol A. 1999;184:325–32. [Google Scholar]
- 67.Schnakenberg SL, Matias WR, Siegal ML. Sperm-storage defects and live birth in Drosophila females lacking spermathecal secretory cells. PLoS Biol. 2011;9:e1001192. doi: 10.1371/journal.pbio.1001192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rogers DW, Baldini F, Battaglia F, Panico M, Dell A, Morris HR, et al. Transglutaminase-mediated semen coagulation controls sperm storage in the malaria mosquito. PLoS Biol. 2009;7:e1000272. doi: 10.1371/journal.pbio.1000272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dottorini T, Nicolaides L, Ranson H, Rogers DW, Crisanti A, Catteruccia F. A genome-wide analysis in Anopheles gambiae mosquitoes reveals 46 male accessory gland genes, possible modulators of female behavior. Proc Natl Acad Sci USA. 2007;104:16215–20. doi: 10.1073/pnas.0703904104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pondeville E, Maria A, Jacques JC, Bourgouin C, Dauphin-Villemant C. Anopheles gambiae males produce and transfer the vitellogenic steroid hormone 20-hydroxyecdysone to females during mating. Proc Natl Acad Sci USA. 2008;105:19631–6. doi: 10.1073/pnas.0809264105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Baker D, Nolan T, Fischer B, Pinder A, Crisanti A, Russell S. A comprehensive gene expression atlas of sex-and tissue-specificity in the malaria vector, Anopheles gambiae. BMC Genomics. 2011;12:296. doi: 10.1186/1471-2164-12-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Aigaki T, Fleischmann I, Chen PS, Kubli E. Ectopic expression of sex peptide alters reproductive behavior of female D. melanogaster. Neuron. 1991;7:557–63. doi: 10.1016/0896-6273(91)90368-a. [DOI] [PubMed] [Google Scholar]
- 73.Soller M, Bownes M, Kubli E. Control of oocyte maturation in sexually mature Drosophila females. Dev Biol. 1999;208:337–51. doi: 10.1006/dbio.1999.9210. [DOI] [PubMed] [Google Scholar]
- 74.Soller M, Bownes M, Kubli E. Mating and sex peptide stimulate the accumulation of yolk in oocytes of Drosophila melanogaster. Eur J Biochem. 1997;243:732–8. doi: 10.1111/j.1432-1033.1997.00732.x. [DOI] [PubMed] [Google Scholar]
- 75.Wigby S, Chapman T. Sex peptide causes mating costs in female Drosophila melanogaster. Curr Biol. 2005;15:316–21. doi: 10.1016/j.cub.2005.01.051. [DOI] [PubMed] [Google Scholar]
- 76.Isaac RE, Li C, Leedale AE, Shirras AD. Drosophila male sex peptide inhibits siesta sleep and promotes locomotor activity in the post-mated female. Proc R Soc B Biol Sci. 2010;277:65–70. doi: 10.1098/rspb.2009.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Carvalho GB, Kapahi P, Anderson DJ, Benzer S. Allocrine modulation of feeding behavior by the sex peptide of Drosophila. Curr Biol. 2006;16:692–6. doi: 10.1016/j.cub.2006.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Peng J, Zipperlen P, Kubli E. Drosophila sex-peptide stimulates female innate immune system after mating via the Toll and Imd pathways. Curr Biol. 2005;15:1690–4. doi: 10.1016/j.cub.2005.08.048. [DOI] [PubMed] [Google Scholar]
- 79.Domanitskaya EV, Liu H, Chen S, Kubli E. The hydroxyproline motif of male sex peptide elicits the innate immune response in Drosophila females. FEBS J. 2007;274:5659–68. doi: 10.1111/j.1742-4658.2007.06088.x. [DOI] [PubMed] [Google Scholar]
- 80.Yang C, Rumpf S, Xiang Y, Gordon MD, Song W, Jan LY, et al. Control of the postmating behavioral switch in Drosophila females by internal sensory neurons. Neuron. 2009;61:519–26. doi: 10.1016/j.neuron.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hasemeyer M, Yapici N, Heberlein U, Dickson BJ. Sensory neurons in the Drosophila genital tract regulate female reproductive behavior. Neuron. 2009;61:511–8. doi: 10.1016/j.neuron.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 82.Yapici N, Kim YJ, Ribeiro C, Dickson BJ. A receptor that mediates the post-mating switch in Drosophila reproductive behaviour. Nature. 2008;451:33–7. doi: 10.1038/nature06483. [DOI] [PubMed] [Google Scholar]
- 83.Heifetz Y, Vandenberg LN, Cohn HI, Wolfner MF. Two cleavage products of the Drosophila accessory gland protein ovulin can independently induce ovulation. Proc Natl Acad Sci USA. 2005;102:743–8. doi: 10.1073/pnas.0407692102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fiumera AC, Dumont BL, Clark AG. Sperm competitive ability in Drosophila melanogaster associated with variation in male reproductive proteins. Genetics. 2005;169:243–57. doi: 10.1534/genetics.104.032870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Clark AG, Aguade M, Prout T, Harshman LG, Langley CH. Variation in sperm displacement and its association with accessory gland protein loci in Drosophila melanogaster. Genetics. 1995;139:189–201. doi: 10.1093/genetics/139.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mancini E, Baldini F, Tammaro F, Calzetta M, Serrao A, George P, et al. Molecular characterization and evolution of a gene family encoding male-specific reproductive proteins in the African malaria vector Anopheles gambiae. BMC Evol Biol. 2011;11:292. doi: 10.1186/1471-2148-11-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Naccarati C, Audsley N, Keen JN, Kim JH, Howell GJ, Kim YJ, et al. The host-seeking inhibitory peptide, Aea-HP-1, is made in the male accessory gland and transferred to the female during copulation. Peptides. 2012;34:150–7. doi: 10.1016/j.peptides.2011.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brown MR, Klowden MJ, Crim JW, Young L, Shrouder LA, Lea AO. Endogenous regulation of mosquito host-seeking behavior by a neuropeptide. J Insect Physiol. 1994;40:399–406. [Google Scholar]
- 89.Ravi Ram K, Sirot LK, Wolfner MF. Predicted seminal astacin-like protease is required for processing of reproductive proteins in Drosophila melanogaster. Proc Natl Acad Sci USA. 2006;103:18674–9. doi: 10.1073/pnas.0606228103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Park M, Wolfner MF. Male and female cooperate in the prohormone-like processing of a Drosophila melanogaster seminal fluid protein. Dev Biol. 1995;171:694–702. doi: 10.1006/dbio.1995.1315. [DOI] [PubMed] [Google Scholar]
- 91.LaFlamme BA, Ram KR, Wolfner MF. The Drosophila melanogaster seminal fluid protease ‘seminase’ regulates proteolytic and post-mating reproductive processes. PLoS Genetics. 2012;8:e1002435. doi: 10.1371/journal.pgen.1002435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Murer V, Spetz JF, Hengst U, Altrogge LM, De Agostini A, Monard D. Male fertility defects in mice lacking the serine protease inhibitor protease nexin-1. Proc Natl Acad Sci USA. 2001;98:3029–33. doi: 10.1073/pnas.051630698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Asquith KL, Harman AJ, McLaughlin EA, Nixon B, Aitken RJ. Localization and significance of molecular chaperones, heat shock protein 1, and tumor rejection antigen gp96 in the male reproductive tract and during capacitation and acrosome reaction. Biol Reprod. 2005;72:328–37. doi: 10.1095/biolreprod.104.034470. [DOI] [PubMed] [Google Scholar]
- 94.Yamaguchi A, Saito T, Yamada L, Taniguchi H, Harada Y, Sawada H. Identification and localization of the sperm CRISP family protein CiUrabin involved in gamete interaction in the ascidian Ciona intestinalis. Mol Reprod Dev. 2011;78:488–97. doi: 10.1002/mrd.21329. [DOI] [PubMed] [Google Scholar]
- 95.Smith GM, Rothwell K, Wood SL, Yeaman SJ, Bownes M. Specificity and localization of lipolytic activity in adult Drosophila melanogaster. Biochem J. 1994;304(Pt 3):775–9. doi: 10.1042/bj3040775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Meikle DB, Sheehan KB, Phillis DM, Richmond RC. Localization and longevity of seminal-fluid esterase 6 in mated female Drosophila melanogaster. J Insect Physiol. 1990;36:93–101. [Google Scholar]
- 97.Walker MJ, Rylett CM, Keen JN, Audsley N, Sajid M, Shirras AD, et al. Proteomic identification of Drosophila melanogaster male accessory gland proteins, including a pro-cathepsin and a soluble gamma-glutamyl transpeptidase. Proteome Sci. 2006;4:9. doi: 10.1186/1477-5956-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Clark GF. Molecular models for mouse sperm-oocyte binding. Glycobiology. 2011;21:3–5. doi: 10.1093/glycob/cwq159. [DOI] [PubMed] [Google Scholar]
- 99.Xu R, Boudreau A, Bissell MJ. Tissue architecture and function: dynamic reciprocity via extra-and intra-cellular matrices. Cancer Metastasis Rev. 2009;28:167–76. doi: 10.1007/s10555-008-9178-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bai H, Gelman DB, Palli SR. Mode of action of methoprene in affecting female reproduction in the African malaria mosquito, Anopheles gambiae. Pest Manag Sci. 2010;66:936–43. doi: 10.1002/ps.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fukuda M. Membrane traffic in the secretory pathway. Cell Mol Life Sci. 2008;65:2801–13. doi: 10.1007/s00018-008-8348-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Orr AG, Rutowski RL. The function of the sphragis in Cressida cressida (Fab.)(Lepidoptera, Papilionidae): a visual deterrent to copulation attempts. J Nat Hist. 1991;25:703–10. [Google Scholar]
- 103.Baer B, Morgan ED, Schmid-Hempel P. A nonspecific fatty acid within the bumblebee mating plug prevents females from remating. Proc Natl Acad Sci USA. 2001;98:3926–8. doi: 10.1073/pnas.061027998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lachmann AD. Sexual receptivity and post-emergence ovarian development in females of Coproica vagans (Diptera: Sphaeroceridae). Physiol Entomol. 1998;23:360–8. [Google Scholar]
- 105.Polak M, Wolf LL, Starmer WT, Barker JSF. Function of the mating plug in Drosophila hibisci Bock. Behav Ecol Sociobiol. 2001;49:196–205. [Google Scholar]
- 106.Polak M, Starmer WT, Barker JSF. A mating plug and male mate choice in Drosophila hibisci Bock. Anim Behav. 1998;56:919–26. doi: 10.1006/anbe.1998.0850. [DOI] [PubMed] [Google Scholar]
- 107.Lung O, Kuo L, Wolfner MF. Drosophila males transfer antibacterial proteins from their accessory gland and ejaculatory duct to their mates. J Insect Physiol. 2001;47:617–22. doi: 10.1016/s0022-1910(00)00151-7. [DOI] [PubMed] [Google Scholar]
- 108.Bretman A, Lawniczak MKN, Boone J, Chapman T. A mating plug protein reduces early female remating in Drosophila melanogaster. J Insect Physiol. 2009;56:107–13. doi: 10.1016/j.jinsphys.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 109.Lung O, Wolfner MF. Identification and characterization of the major Drosophila melanogaster mating plug protein. Insect Biochem Mol Biol. 2001;31:543–51. doi: 10.1016/s0965-1748(00)00154-5. [DOI] [PubMed] [Google Scholar]
- 110.Bertram MJ, Neubaum DM, Wolfner MF. Localization of the Drosophila male accessory gland protein Acp36DE in the mated female suggests a role in sperm storage. Insect Biochem Mol Biol. 1996;26:971–80. doi: 10.1016/s0965-1748(96)00064-1. [DOI] [PubMed] [Google Scholar]
- 111.Neubaum DM, Wolfner MF. Mated Drosophila melanogaster females require a seminal fluid protein, Acp36DE, to store sperm efficiently. Genetics. 1999;153:845–57. doi: 10.1093/genetics/153.2.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bloch Qazi MC, Wolfner MF. An early role for the Drosophila melanogaster male seminal protein Acp36DE in female sperm storage. J Exp Biol. 2003;206:3521–8. doi: 10.1242/jeb.00585. [DOI] [PubMed] [Google Scholar]
- 113.Yuval B. Mating systems of blood-feeding flies. Annu Rev Entomol. 2006;51:413–40. doi: 10.1146/annurev.ento.51.110104.151058. [DOI] [PubMed] [Google Scholar]
- 114.Parker GA. Sperm competition and its evolutionary consequences in the insects. Biol Rev. 1970;45:525–67. [Google Scholar]
- 115.Lum P. The reproductive system of some Florida mosquitoes. II. The male accessory glands and their role. Ann Entomol Soc Am. 1961;54:430–3. [Google Scholar]
- 116.Gerber GH. Evolution of the methods of spermatophore formation in pterygotan insects. Can Entomol. 1970;102:358–62. [Google Scholar]
- 117.Peter A, Lilja H, Lundwall Ö, Malm J. Semenogelin I and semenogelin II, the major gel-forming proteins in human semen, are substrates for transglutaminase. Eur J Biochem. 1998;252:216–21. doi: 10.1046/j.1432-1327.1998.2520216.x. [DOI] [PubMed] [Google Scholar]
- 118.Mancini E, Tammaro F, Baldini F, Via A, Raimondo D, George P, et al. Molecular evolution of a gene cluster of serine proteases expressed in the Anopheles gambiae female reproductive tract. BMC Evol Biol. 2011;11:72. doi: 10.1186/1471-2148-11-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Enayati A, Hemingway J. Malaria management: past, present, and future. Annu Rev Entomol. 2010;55:569–91. doi: 10.1146/annurev-ento-112408-085423. [DOI] [PubMed] [Google Scholar]
- 120.Anonymous A research agenda for malaria eradication: vector control. PLoS Med. 2011;8:e1000401. doi: 10.1371/journal.pmed.1000401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ranson H, Guessan R, Lines J, Moiroux N, Nkuni Z, Corbel V. Pyrethroid resistance in African anopheline mosquitoes: what are the implications for malaria control? Trends Parasitol. 2011;27:91–8. doi: 10.1016/j.pt.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 122.Ferguson HM, John B, Ng’habi K, Knols BGJ. Redressing the sex imbalance in knowledge of vector biology. Trends Ecol Evol. 2005;20:202–9. doi: 10.1016/j.tree.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 123.Foster WA, Lea AO. Renewable fecundity of male Aedes aegypti following replenishment of seminal vesicles and accessory glands. J Insect Physiol. 1975;21:1085–90. doi: 10.1016/0022-1910(75)90120-1. [DOI] [PubMed] [Google Scholar]
- 124.Reinhardt K, Naylor R, Siva-Jothy MT. Male mating rate is constrained by seminal fluid availability in bedbugs, Cimex lectularius. PloS One. 2011;6:e22082. doi: 10.1371/journal.pone.0022082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jones JC. A study on the fecundity of male Aedes aegypti. J Insect Physiol. 1973;19:435–9. doi: 10.1016/0022-1910(73)90118-2. [DOI] [PubMed] [Google Scholar]
- 126.Mahmood F, Reisen WK. Anopheles culicifacies: effects of age on the male reproductive system and mating ability of virgin adult mosquitoes. Med Vet Entomol. 1994;8:31–7. doi: 10.1111/j.1365-2915.1994.tb00380.x. [DOI] [PubMed] [Google Scholar]
- 127.Mahmood F, Reisen WK. Anopheles stephensi (Diptera: Culicidae): changes in male mating competence and reproductive system morphology associated with aging and mating. J Med Entomol. 1982;19:573–88. doi: 10.1093/jmedent/19.5.573. [DOI] [PubMed] [Google Scholar]
- 128.Oliva CF, Benedict MQ, Lempérière G, Gilles J. Laboratory selection for an accelerated mosquito sexual development rate. Malar J. 2011;10:135. doi: 10.1186/1475-2875-10-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chambers GM, Klowden MJ. Age of Anopheles gambiae Giles male mosquitoes at time of mating influences female oviposition. J Vector Ecol. 2001;26:196–201. [PubMed] [Google Scholar]
- 130.Pereira R, Yuval B, Liedo P, Teal PEA, Shelly TE, McInnis DO, et al. Improving sterile male performance in support of programmes integrating the sterile insect technique against fruit flies. J Appl Entomol. 2011 doi: 10.1111/j.1439–0418.2011.01664.x. [Google Scholar]
- 131.Deredec A, Godfray HCJ, Burt A. Requirements for effective malaria control with homing endonuclease genes. Proc Natl Acad Sci USA. 2011;108:E874–80. doi: 10.1073/pnas.1110717108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Begun DJ, Lindfors HA. Rapid evolution of genomic Acp complement in the melanogaster subgroup of Drosophila. Mol Biol Evol. 2005;22:2010–21. doi: 10.1093/molbev/msi201. [DOI] [PubMed] [Google Scholar]