Abstract
We report here for the first time the in vitro effects of (1S,2R,4S)-1,7,7-trimethyl-bicyclo[2.2.1]heptan-2-yl-3′,4′,5′-trimethoxy benzoate (1) and (1S,2R,4S)-1,7,7-trimethyl-bicyclo[2.2.1]heptan-2-yl benzoate (2) on the growth and ultrastructure of Trypanosoma cruzi. These two synthetic compounds exerted an antiproliferative effect on the epimastigote forms of the parasite. The ICs50/72h of two synthetic L-bornyl benzoates, 1 and 2, was 10.1 and 12.8 μg/ml, respectively. Both compounds were more selective against epimastigotes than HEp-2 cells. Ultrastructural analysis revealed intense cytoplasmic vacuolization and the appearance of cytoplasmic materials surrounded by membranes. The treatment of peritoneal macrophages with compounds 1 and 2 caused a significant decrease in the number of T. cruzi-infected cells. L-Bornyl benzoate derivatives may serve as a potential source for the development of more effective and safer chemotherapeutic agents against T. cruzi infections.
Keywords: Antimicrobial activity, L-Bornyl benzoate, Trypanosoma cruzi
Introduction
Trypanosoma cruzi, a heteroxenic trypanosomatid, is the causative agent of Chagas’ disease,1 which currently affects more than 10 million people in Latin America.2 Natural transmission to humans occurs through contact with excreta containing metacyclic trypomastigotes from hematophagous reduviid insect of the subfamily Triatominae. The establishment of vector-control programs have led to total or partial interruption of domestic triatomine (such as Triatoma infestans and Rhodnius prolixus) transmission in several countries in Latin America.3 However, the presence of sylvatic triatomine bugs infected with T. cruzi associated with anthropogenic disturbances (such as deforestation and human invasion of triatomine sylvatic foci), may establish the peridomestic and domestic cycles of the parasite. This adaptation process represents a constant T. cruzi infection risk and the expansion of Chagas’ disease incidence.4 Furthermore, the parasite can also be transmitted by blood transfusion and organ transplantation, and by the congenital and oral route, which are the main mechanisms of transmission of Chagas’ disease in non-endemic areas.5
Chagas’ disease is characterized by large clinical manifestation spectra ranging from the absence of infection symptoms to severe chronic disease, involving cardiovascular or gastrointestinal pathologies.6 One of the greatest Chagas’ disease problems is associated with unsatisfactory efficacy of the available therapeutic drugs nifurtimox and benznidazole. These drugs induce serious collateral effects and show limited efficacy during the chronic phase.7 On the other hand, the existence of strains naturally resistant to both nifurtimox and benznidazole contributes to treatment failure.8,9 Therefore, this scenario indicates that new, safer, and less expensive drugs are urgently needed.
Despite the economic and social importance of Chagas’ disease, until this moment there have not been any introductory perspectives by pharmaceutical industries, about new compounds or vaccines against T. cruzi. In this context, several researchers have shown the antimicrobial activity of natural compounds obtained from medicinal plants against T. cruzi, with lower toxicity to the mammalian cells, which represents a great potential for the development of new drugs suitable for Chagas’ disease treatment.10
Borneol is a monoterpene commonly isolated from plants of different genera.11–13 Borneol can also be produced by the reduction of camphor, which is used in traditional medicine for analgesia and anesthesia. In addition, this compound inhibits rat bone resorption 14, by inhibiting the formation of osteoclasts 15, and causes microtubule depolymerization.16 Several terpenoid compounds have demonstrated antiprotozoal activity against T. cruzi.17–22
Many aromatic compounds containing three vicinal methoxyl groups on benzene ring, which exhibit biological activity, are found in nature.23 Moreover, in the last decades, several amines, amides, and esters derived from 3,4,5-trimethoxybenzoic acid were synthesized, such as trimazosin an antihypertensive compound,24 trimethoprim that present antibacterial effect,25 and podophyllotoxin which are related to antiviral action.26
In the aim of associate the biological activity with the presence of trimethoxybenzoic group in the structure of terpenes which practically do not exhibit collateral effects, the bornyl-3,4,5-trimetoxybenzoate was synthesized by the reaction of 3,4,5-trimethoxybenzoic acid derived from syringyl constituents of eucalyptus tar, a renewable biomass source. Then, this work presents the antimicrobial activity of the synthetic L-borneol derivatives: (1S,2R,4S)-1,7,7-trimethyl-bicyclo[2.2.1]heptan-2-yl-3′,4′,5′-trimethoxybenzoate (1) and (1S,2R,4S)-1,7,7-trimethyl-bicyclo[2.2.1]heptan-2-yl benzoate (2) (Fig. 1), against T. cruzi. The ultrastructural changes in epimastigotes and the effect on T. cruzi interaction with murine macrophage cells were also examined.
Figure 1.
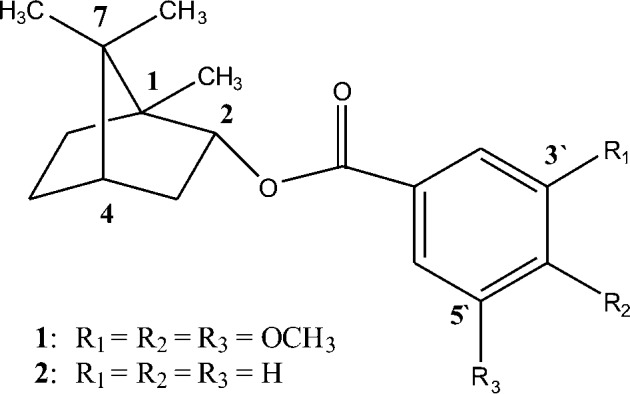
Chemical structure of (1S,2R,4S)-1,7,7-trimethyl-bicyclo[2.2.1]heptan-2-yl-3′,4′,5′-trimethoxy benzoate (1) and (1S,2R,4S)-1,7,7-trimethyl-bicyclo[2.2.1]heptan-2-yl benzoate (2).
Materials and Methods
Preparation of L-bornyl benzoates
L-bornyl benzoates were synthesized by the reaction of 1.0 mM carboxylic acid (3,4,5-trimethoxybenzoic acid or benzoic acid) with 1.2 mM thionyl chloride (SOCl2) under reflux (2 hours), to produce the acyl chloride.27 After SOCl2 excess elimination by distillation, each acyl derivative was then submitted to reaction with (1S,2R,4S)-1,7,7-trimethyl-bicyclo[2.2.1]heptan-2-ol (L-borneol) to obtain the correspondent esters (1S,2R,4S)-1,7,7-trimethyl-bicyclo[2.2.1]heptan-2-yl-3′,4′,5′-trimethoxy-benzoate (1) and (1S,2R,4S)-1,7,7-trimethyl-bicyclo[2.2.1]heptan-2-yl benzoate (2) (Fig. 1).28,29 The synthesis reactions were performed at the Núcleo de Pesquisas de Plantas Medicinais (NEPLAM), Departamento de Química, ICEx, of the Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil. NMR spectra were obtained on a Bruker DRX 400 Advance spectrometer at 400 or 100 MHz, at 300 K and equipped with an inverse detection 5 mm multinuclear head 1H/13C (90° pulse widths of 6.44 μs and 7.50 μs for 1H and 13C, respectively). Samples (10 mg) of compounds 1 and 2 were previously dissolved in 0.5 ml CDCl3 and transferred to a 5 mm o.d. tube. TMS was used as internal standard. One-dimensional 1H and 13C NMR spectra were acquired under standard conditions. 1H NMR spectra were obtained using a sweep width of 11 990.41 Hz over 65 536 data points and multiplied by an exponential factor corresponding to a 0.30 Hz line broadening prior to Fourier transformation. 13C spectra were obtained using a sweep width of 31.847 Hz. Data processing was carried out on the SGI workstation using Bruker (DRX 400) micro-programs and the XWIN-NMR 3.1 version program for Windows XP. Analyses by direct infusion electrospray ionization was performed using a mass spectrometer (IT-TOF; Shimadzu, Tokyo, Japan) with high resolution and mass accuracy (<5 ppm) capabilities. The following conditions were employed: ESI ionization in the positive mode at +4.5 KV, and nebulizer gas at 1.5 l min−1; curved desorption line interface at 200°C and drying gas at 100 KPa; and an octapole ion accumulation time of 100 ms. Mass spectra were obtained in the full scan mode within the 50–500 Da range. Stock solutions (10.0 mg/ml) of compound 1 or 2 were prepared in water containing 10% DMSO (v/v). These solutions were sterilized by filtration (0.22 μm; Millipore, Brazil) and added aseptically only once to growth medium at determined concentrations. The DMSO final concentration in the assays did not exceed 1%
Microorganisms
The T. cruzi Dm28c strain30 was maintained by weekly transfers in liver infusion tryptose medium31 supplemented with 10% fetal bovine serum at 28°C. Log-phase epimastigote forms were obtained from a 4-day incubation culture. Metacyclic trypomastigotes were obtained after in vitro differentiation under chemically defined conditions, as previously described by Contreras et al.32
Antitrypanosomal activity
Epimastigotes of the T. cruzi Dm28c strain in logarithmic growth phase (5×105 cells) were added to 18×180 mm screw-capped tubes containing 2 ml of growth medium with 5.0–100.0 μg/ml 1 or 2. The cultures were incubated at 28°C, and after 72 hours, cell growth was estimated by direct counting in a hemocytometer (Improved Double Neubauer). Tubes containing medium alone or medium plus 1% DMSO served as growth and sterility controls. The results were expressed as IC50/72h (50% inhibitory concentrations after 72 hours of incubation). Benznidazole (Roche Pharmaceuticals) was used as reference drug and its stock solution was prepared in 10% DMSO as described above.
Interaction with macrophages
The murine peritoneal cells were prepared from BALB/c mice obtained from the breeding colonies of the animal facility of the Centro de Ciências Biológicas at Universidade Estadual de Londrina, Londrina, Paraná, Brazil. The experimental protocol was approved by the Ethics in Animal Experimentation Committee of the Universidade Estadual de Londrina (CEEA no. 05/11). Mice were injected intraperitoneally with 1.5 ml of 3% thioglycolate, and peritoneal cells were collected 4 days later by injecting 10 ml of cold sterile 50 mM sodium phosphate buffer, pH 7.4, containing 0.15 M NaCl (PBS). The cells obtained were centrifuged at 500g for 10 minutes at 4°C and resuspended in RPMI 1640 medium (Invitrogen-Gibco, USA) supplemented with 10% heat-inactivated fetal bovine serum (Invitrogen-Gibco), 50 μg/ml gentamicin, 100 IU/ml penicillin, 100 μg/ml streptomycin, and 2 mM glutamine. Peritoneal cells were counted and added to a 24-well culture plate (Techno Plastic Products, Switzerland) at a density of 1×106 cells/well. After incubation for 4 hours, non-adherent cells were removed by washing with sterile PBS. The plates were incubated for another 18 hours and metacyclic trypomastigotes at a protozooan to macrophage ratio of 10∶1 were added to the monolayer culture and the plate was incubated for 4 hours. Afterwards, the plates were washed three times with sterile PBS to remove the extracellular parasites. The fresh culture medium alone (control) or containing the IC50/72h of compound 1 or 2 (against epimastigotes) was added and the plates were incubated for a maximum period of 48 hours. The coverslips of controls and tests were fixed with methanol (Merck, Brazil), stained with Giemsa and permanently prepared in ERV-Mount resin. The percentage of infected host cells and the mean number amastigotes/200 cells were determined by direct counting with a light microscope.
Cytotoxicity assay
HEp-2 cells (human larynx carcinoma, ATCC, CCL-23) were cultured in Dulbecco’s modified Eagle’s medium (Invitrogen-Gibco) supplemented with 10% (v/v) heat-inactivated fetal bovine serum, 2 mM L-glutamine, 100 IU/ml penicillin, 100 μg/ml streptomycin, and 2.5 μg/ml amphotericin B, in 5% CO2 at 37°C. The cells were grown in a 96-well culture plate (Techno Plastic Products) at a density of 1×105 cells/well for 24 hours. At confluence, non-adherent cells were removed by washing with sterile PBS. The medium containing different concentrations of 1 or 2 was added to each well containing the cells, and the plates were incubated for 72 hours. Cell viability was determined by the MTT [dimethylthiazol diphenyl tetrazolium bromide (Sigma Chemical Co., USA)] method according to the manufacturer’s recommendation. The concentration of the compounds needed to reduce the number of viable cells up to 50% by regression analysis corresponded to the 50% cytotoxic concentration (CC50) and the selectivity index (SI) was calculated using the equation: SI = CC50/IC50.
Transmission electron microscopy
Epimastigotes of T. cruzi Dm28c treated with IC50/72h of 1 or 2 were fixed for 2 hours at room temperature with 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer, pH 7.2. Post-fixation was carried out in 1% osmium tetroxide in cacodylate buffer containing 0.8% potassium ferrocyanide and 5 mM CaCl2 for 1 hour at room temperature. The cells were dehydrated in acetone and embedded in Epon resin. Ultrathin sections were stained with uranyl acetate and lead citrate, and examined with a Zeiss EM900 electron microscope.
Statistical analysis
The results were evaluated by the Tukey–Kramer test using the software Statistic for Windows, version 6.0 (Statsoft, Inc., Oklahoma City, OK, USA). P values less than 0.05 indicated a significant difference.
RESULTS
Structural identification of compounds
Compound 1: Colorless oil, C20H2805, FW = 348.433 g mol−1. 13C NMR (400 MHz, CDCl3): 13.66 (C-10), 18.95 (C-8), 19.74 (C-9), 27.49 (C-6), 28,12 (C-5), 36.94 (C-3), 45.02 (C-4), 47.89 (C-7), 49.14 (C-1), 56.26 (C-3′-OCH3, C-5′-OCH3), 60.92 (C-4′-OCH3), 80.69 (C-2), 106.88 (C-2′, C-6′), 125.99 (C-1′), 142.25 (C-4′), 152.97 (C-3′, C-5′), 166.40 (C = O). MS Data (I.E., 70 eV) main fragments: m/z [assignment]: 348 [molecular cation radical, M+]; 212 [3,4,5-trimethoxybenzoic acid cation radical, (M-136)+]; 195 [acyl cation, base peak, (M-153)+]. CHN analysis: C = 68.70%; H = 8.47%; N = 0.12%.
Compound 2: Colorless oil, C17H2202, FW = 258.322 g mol−1. 13C NMR (400 MHz, CDCl3): 13.60 (C-10), 18.92 (C-8), 19.72 (C-9), 27.41 (C-6), 28.10 (C-5), 36.92 (C-3), 45.01 (C-4), 47.88 (C-7), 49.10 (C-1), 80.52 (C-2), 129.50 (C-2′, C-6′), 130.92 (C-1′), 132.72 (C-4′), 128.31 (C-3′, C-5′), 166.80 (C = O). MS Data (I.E., 70 eV) main fragments: m/z [assignment]: 258 [molecular cation radical, M+]; 122 [3,4,5-trimethoxybenzoic acid cation radical, (M-136)+]; 105 [acyl cation, base peak, (M-153)+]. CHN analysis: C = 78.79%; H = 8.31%; N = 0.08%.
Antitrypanosomal activity and cytotoxicity to mammalian cells
This study demonstrates for the first time the biological activity of L-bornyl benzoates 1 and 2 against epimastigote forms of T. cruzi Dm28c. The in vitro effects of compounds 1 and 2 against the epimastigote forms are shown in Fig. 2A. A dose-dependent antiprotozoal effect was found for both compounds. The L-bornyl benzoate 1 at 100 μg/ml inhibited more than 98% of parasite growth after 72 hours of incubation. At the same concentration, compound 2 completely inhibited epimastigote proliferation. The ICs50/72h calculated for 1 and 2 were 10.1 and 12.8 μg/ml, respectively. The presence of DMSO in the culture did not interfere with the parasite growth pattern. The IC50/72h of benznidazole for T. cruzi Dm28c strain was 2.5 μg/ml.
Figure 2.
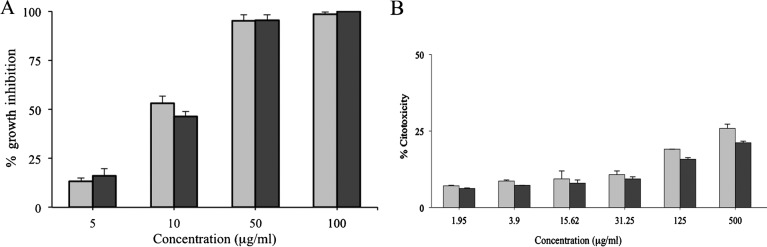
Effect of L-bornyl benzoates compounds 1 (gray bar) and 2 (black bar) on growth of epimastigotes of Trypanosoma cruzi Dm28c (A) and cytotoxicity to mammalian cells (B). The cells were cultured for 72 hours in the presence of indicated concentrations. The results are the average of two experiments in duplicate and expressed as percentage of growth inhibition or cytotoxicity in relation to untreated cells.
The effects of 1 and 2 on the intracellular amastigotes were determined by treating peritoneal macrophage cells with both compounds for 48 hours. As compared to the control cells, a significant decrease in the percentage of macrophage-infected and 1 (10.1 μg/ml) or 2 (12.8 μg/ml)-treated cells was observed. At the concentrations tested, there was 52.3% and 64.5% inhibition of macrophage infection by T. cruzi. No significant difference was observed, between the two compounds, in the number of amastigotes per infected and treated and untreated cell (data not shown). In addition, no morphological alterations were observed in treated and infected macrophages.
The determination of the cytotoxicity of 1 and 2 against HEp-2 cells after 72 hours of incubation is shown in Fig. 2B. It was not possible to determine the CC50/72h of both compounds since with the highest concentration of 1 and 2 tested (500 μg/ml) around 74.3% and 78.9% of the cells were viable, respectively. Then, the SIs of 1 (>49.5) and 2 (>39.1) indicate that these two L-bornyl benzoate derivatives are more selective against the T. cruzi epimastigotes than the mammalian cells.
Ultrastructural changes induced by bornyl benzoates
The epimastigotes treated with 1 or 2 at the respective IC50/72h were analyzed by transmission electron microscopy. The results showed multiple cytoplasmic vacuoles and the presence of myelin-like structures (Fig. 3). Some of the vacuoles in the cells treated with both compounds appeared to contain cytoplasmic components (Fig. 3b and e, asterisk). Interestingly, a cellular aggregation with possible parasites membrane fusion was only observed after 1 treatment (Fig. 3b and c, black arrow).
Figure 3.
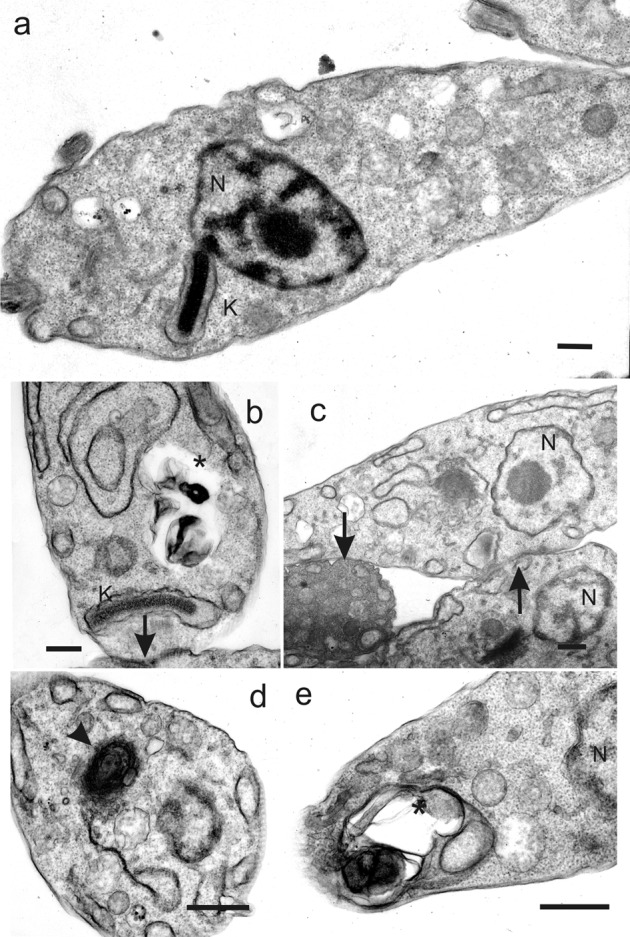
Effect of compounds 1 (b–c) and 2</emph> (d–e) on epimastigote forms of Trypanosoma cruzi after 72-hour treatment, as observed by transmission electron microscopy. (a) Untreated cell showing the normal morphology. (b–c) Treated cell with 10.1 μg/ml of compound 1. Note vacuoles containing cytoplasmic material (asterisk), and membrane aggregation (arrow). (d–e) Treated cell with 12.8 μg/ml of compound 2. Note the myelin-like figure inside of citoplasm (arrowhead) and vacuoles containing cytoplasmic material (asterisk). Scale bar = 1 μm. N, nucleus; K, kinetoplast.
Discussion
The traditional use of plant materials for treatment of microbial infections is common since ancient times. The chemical structure and biological activity of the natural products, mainly those related to T. cruzi treatment, have been gradually studied by many researchers.10 Furthermore, natural products have served as building blocks or scaffolds in the synthesis of active analogues. The synthesis of more active molecules under laboratory conditions is involved in combinatorial strategies in order to obtain a potent antimicrobial derivative without collateral effects to the host and with low cost of large-scale production.33
In this work, the in vitro effects of two synthetic L-bornyl benzoates on growth and ultrastructure of epimastigote forms of Trypanosoma cruzi are reported for the first time. Both compounds exerted an antiproliferative effect on the epimastigote forms and were more selective against epimastigotes than HEp2 cells. In addition, 1 and 2 caused a decrease in the number of T. cruzi-infected macrophages. Both compounds may affect host cell invasion by the parasite, but the number of amastigotes inside the treated cells was not significantly different compared to the untreated cells. However, this possibility requires further investigation.
Terpenoids comprise a large class of natural products that are used as commercial flavor and fragrance compounds and as drugs for different disease treatments. The biological activities of terpenoids obtained from plants have been extensively reported in the literature including the antimicrobial effect against T. cruzi.17–22 Among monoterpenes, Kiuchi et al.17 showed the inhibitory activity of four monoterpene 1- or 2-hydroperoxides against epimastigotes and trypomastigotes of T. cruzi. The substitution of the hydroperoxide radicals by hydrogen abolished these effects. On the other hand, Saeidnia et al.20 showed that two monoterpene-10-O-beta-D-glucopyranosides were inactive against epimastigote forms. However, no reports of the antitrypanosomal activity of the monoterpene borneol or derivatives was described previously.
The L-bornyl benzoates 1 and 2 induced intense cytoplasmic vacuolization, while the presence of myelin-like structures was also observed by electron microscopy. Intense vacuolization has been demonstrated in epimastigotes treated with 5-epi-icetexone, a diterpene compound with antitrypanosomal activity.22 Interestingly, the presence of epimastigote aggregates was observed only after 1 treatment, suggesting the occurrence of plasma membrane fusion. The mechanisms by which both compounds affect parasite growth are unclear. The presence of a methoxy group at the 3′, 4′, and 5′ benzoyl ring position makes 1 more suitable for phase I and phase II metabolic reactions. These conditions may explain the difference observed in compound 1-treated epimastigotes in relation to its effect on the plasma membrane.
The appearance of cytoplasmic materials surrounded by membranes may suggest the presence of autophagic vacuoles, which is one of the first pieces of ultrastructural evidence of autophagic cell death phenotype.34 This phenotype can be observed in epimastigotes of T. cruzi treated with naphthoimidazoles derived from beta-lapachone.35
In conclusion, the results of this study showed for the first time an inhibitory effect of synthetic L-bornyl benzoate derivatives 1 and 2 against T. cruzi, with low toxicity to host cells. Further studies are warranted to understand the mode of action of these two compounds and to confirm their efficacy in the treatment of Chagas’ disease.
Acknowledgments
This study was supported by grants from DECIT/SCTIE/MS and MCT by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Programa de Núcleos de Excelência (PRONEX/Fundação Araucária), and Programa de Pós-Graduação em Microbiologia da Universidade Estadual de Londrina. We thank Dr A. Leyva for reading this manuscript. This work is part of the MSc dissertation of P. R. C. Corrêa.
References
- 1.Chagas C. Nova tripanosomiase humana. Estudo sobre a morfolojia e cyclo evolutivo do Schyzotrypanum cruzi n. gen., n. sp., ajente etiolojico de nova entidade morbida no homem. Mem Inst Oswaldo Cruz. 1909;1:159–218. [Google Scholar]
- 2.WHO . Geneva: WHO; 2010. Fact Sheet No.: 340; Chagas disease. Special Programme for Research and Training in Tropical Diseases, TDR. [Google Scholar]
- 3.Dias JC. Elimination of Chagas disease transmission: perspectives. Mem Inst Oswaldo Cruz. 2009;104:41–5. doi: 10.1590/s0074-02762009000900007. [DOI] [PubMed] [Google Scholar]
- 4.Coura JR, Borges-Pereira J. Chagas disease: 100 years after its discovery: a systemic review. Acta Trop. 2010;115:5–13. doi: 10.1016/j.actatropica.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Schmunis GA, Yadon ZE. Chagas disease: a Latin American health problem becoming a world health problem. Acta Trop. 2010;115:14–21. doi: 10.1016/j.actatropica.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Prata A. Clinical and epidemiological aspects of Chagas disease. Lancet Infect Dis. 2001;1:92–100. doi: 10.1016/S1473-3099(01)00065-2. [DOI] [PubMed] [Google Scholar]
- 7.Urbina JA. Specific chemotherapy of Chagas disease: relevance, current limitations and new approaches. Acta Trop. 2010;115:55–68. doi: 10.1016/j.actatropica.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 8.Filardi LS, Brener Z. Susceptibility and natural resistance of Trypanosoma cruzi strains to drugs used clinically in Chagas disease. Trans R Soc Trop Med Hyg. 1987;81:755–9. doi: 10.1016/0035-9203(87)90020-4. [DOI] [PubMed] [Google Scholar]
- 9.León-Pérez F, Gómez-Garcia L, Alejandre-Aguilar R, López R, Monteón VM. Mexican Trypanosoma cruzi isolates: in vitro susceptibility of epimastigotes to anti-Trypanosoma cruzi drugs and metacyclic forms to complement-mediated lysis. Vector Borne Zoonotic Dis. 2007;7:330–6. doi: 10.1089/vbz.2006.0604. [DOI] [PubMed] [Google Scholar]
- 10.Izumi E, Ueda-Nakamura T, Dias Filho BP, Veiga Júnior VF, Nakamura CV. Natural products and Chagas’ disease: a review of plant compounds studied for activity against Trypanosoma cruzi. Nat Prod Rep. 2011;28:809–23. doi: 10.1039/c0np00069h. [DOI] [PubMed] [Google Scholar]
- 11.Uzel A, Guvensen A, Cetin E. Chemical composition and antimicrobial activity of the essential oils of Anthemis xylopoda O. Schwarz from Turkey. J Ethnopharmacol. 2004;95:151–4. doi: 10.1016/j.jep.2004.06.034. [DOI] [PubMed] [Google Scholar]
- 12.Orav A. Arak E, Raal A. Phytochemical analysis of the essential oil of Achillea millefolium L. from various European countries. Nat Prod Res. 2006;20:1082–8. doi: 10.1080/14786410500510849. [DOI] [PubMed] [Google Scholar]
- 13.Wenqiang G, Shufen L, Ruixiang Y, Yanfeng H. Comparison of composition and antifungal activity of Artemisia argyi Lévl. et Vant inflorescence essential oil extracted by hydrodistillation and supercritical carbon dioxide. Nat Prod Res. 2006;20:992–8. doi: 10.1080/14786410600921599. [DOI] [PubMed] [Google Scholar]
- 14.Mühlbauer RC, Lozano A, Palacio S, Reinli A, Felix R. Common herbs, essential oils, and monoterpenes potently modulate bone metabolism. Bone. 2003;32:372–80. doi: 10.1016/s8756-3282(03)00027-9. [DOI] [PubMed] [Google Scholar]
- 15.Dolder S, Hofstetter W, Wetterwald A, Mühlbauer RC, Felix R. Effect of monoterpenes on the formation and activation of osteoclasts in vitro. J Bone Miner Res. 2006;21:647–55. doi: 10.1359/jbmr.060111. [DOI] [PubMed] [Google Scholar]
- 16.Klar U, Graf H, Schenk O, Röhr B, Schulz H. New synthetic inhibitors of microtubule depolymerization. Bioorg Med Chem Lett. 1998;8:1397–402. doi: 10.1016/s0960-894x(98)00226-1. [DOI] [PubMed] [Google Scholar]
- 17.Kiuchi F, Itano Y, Uchiyama N, Honda G, Tsubouchi A, Nakajima-Shimada J, et al. Monoterpene hydroperoxides with trypanocidal activity from Chenopodium ambrosioides. J Nat Prod. 2002;65:509–12. doi: 10.1021/np010445g. [DOI] [PubMed] [Google Scholar]
- 18.Uchiyama N, Matsunaga K, Kiuchi F, Honda G, Tsubouchi A, Nakajima-Shimada J, et al. Trypanocidal terpenoids from Laurus nobilis L. Chem Pharm Bull. 2002;50:1514–6. doi: 10.1248/cpb.50.1514. [DOI] [PubMed] [Google Scholar]
- 19.Araya JE, Neira I, da Silva S, Mortara RA, Manque P, Cordero E, et al. Diterpenoids from Azorella compacta (Umbelliferae) active on Trypanosoma cruzi. Mem Inst Oswaldo Cruz. 2003;98:413–8. doi: 10.1590/s0074-02762003000300022. [DOI] [PubMed] [Google Scholar]
- 20.Saeidnia S, Gohari AR, Uchiyama N, Ito M, Honda G, Kiuchi F. Two new monoterpene glycosides and trypanocidal terpenoids from Dracocephalum kotschyi. Chem Pharm Bull. 2004;52:1249–50. doi: 10.1248/cpb.52.1249. [DOI] [PubMed] [Google Scholar]
- 21.Leite JP, Oliveira AB, Lombardi JA, Filho JD, Chiari E. Trypanocidal activity of triterpenes from Arrabidaea triplinervia and derivatives. Biol Pharm Bull. 2006;29:2307–9. doi: 10.1248/bpb.29.2307. [DOI] [PubMed] [Google Scholar]
- 22.Sanchez AM, Jimenez-Ortiz V, Sartor T, Tonn CE, García EE, Nieto M, et al. A novel icetexane diterpene, 5-epi-icetexone from Salvia gilliessi is active against Trypanosoma cruzi. Acta Trop. 2006;98:118–24. doi: 10.1016/j.actatropica.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 23.Brossi A, Cordell GA.editorsThe alkaloids. San Diego, CA: Academic Press; 1992 [Google Scholar]
- 24.Taylor CR, Leader JP, Singleton W, Munster EW, Falkner FC, O’Neil JA. Profile of trimazosin: an effective and safe antihypertensive agent. Am Heart J. 1983;106:1269–81. doi: 10.1016/0002-8703(83)90188-6. [DOI] [PubMed] [Google Scholar]
- 25.Roth B, Strelitz JZ, Rauckman BS. 2,4-Diamino-5-benzylpyrimidines and analogues as antibacterial agents. 2. C-Alkylation of pyrimidines with Mannich bases and application to the synthesis of trimethoprim and analogues. J Med Chem. 1980;23:379–84. doi: 10.1021/jm00178a007. [DOI] [PubMed] [Google Scholar]
- 26.Eich E, Pertz H, Kaloga M, Schulz J, Fesen MR, Mazumder A, et al. (−)-Arctigenin as a lead structure for inhibitors of human immunodeficiency virus type-1 integrase. J Med Chem. 1996;39:86–95. doi: 10.1021/jm950387u. [DOI] [PubMed] [Google Scholar]
- 27.Barnes RP. Mesitoic acid and mesitoyl chloride. Org. Synth. Coll. 1955;3:553. [Google Scholar]
- 28.Meyers AI, Mihelich ED. The synthetic utility of 2-oxazolines. Angew Chem Int Ed Engl. 1976;15:270–81. [Google Scholar]
- 29.Reuman M, Meyers AI. The synthetic utility of oxazolines in aromatic-substitution. Tetrahedron. 1985;41:837–60. [Google Scholar]
- 30.Goldenberg S, Contreras VT, Salles JM, Bonaldo MC, Lima Franco MP, Linss J, et al. Facts and hypothesis on Trypanosoma cruzi differentiation. Mem Inst Oswaldo Cruz. 198479Suppl.:39–44. [Google Scholar]
- 31.Camargo EP. Growth and differentiation in Trypanosoma cruzi. I. Origin of metacyclic trypanosomes in liquid media. Rev Inst Med Trop São Paulo. 1964;6:93–100. [PubMed] [Google Scholar]
- 32.Contreras VT, Salles JM, Thomas N, Morel CM, Goldenberg S.In vitro differentiation of Trypanosoma cruzi under chemically defined conditions. Mol Biochem Parasitol. 198516315–27. [DOI] [PubMed] [Google Scholar]
- 33.Nielsen J. Combinatorial synthesis of natural products. Curr Opin Chem Biol. 2002;6:297–305. doi: 10.1016/s1367-5931(02)00330-7. [DOI] [PubMed] [Google Scholar]
- 34.Bursch W. Multiple cell death programs: Charon’s lifts to Hades. FEMS Yeast Res. 2004;5:101–10. doi: 10.1016/j.femsyr.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 35.Menna-Barreto RF, Corrêa JR, Cascabulho CM, Fernandes MC, Pinto AV, Soares MJ, et al. Naphthoimidazoles promote different death phenotypes in Trypanosoma cruzi. Parasitology. 2009;136:499–510. doi: 10.1017/S0031182009005745. [DOI] [PubMed] [Google Scholar]


