Abstract
After more than 10 years of the Global Program to Eliminate Lymphatic Filariasis (GPELF) in Brazil, advances have been seen, but the endemic disease persists as a public health problem. The aim of this study was to describe the spatial distribution of lymphatic filariasis in the municipality of Jaboatão dos Guararapes, Pernambuco, Brazil. An epidemiological survey was conducted in the municipality, and positive filariasis cases identified in this survey were georeferenced in point form, using the GPS. A kernel intensity estimator was applied to identify clusters with greater intensity of cases. We examined 23 673 individuals and 323 individuals with microfilaremia were identified, representing a mean prevalence rate of 1.4%. Around 88% of the districts surveyed presented cases of filarial infection, with prevalences of 0–5.6%. The male population was more affected by the infection, with 63.8% of the cases (P<0.005). Positive cases were found in all age groups examined. The kernel intensity estimator identified the areas of greatest intensity and least intensity of filarial infection cases. The case distribution was heterogeneous across the municipality. The kernel estimator identified spatial clusters of cases, thus indicating locations with greater intensity of transmission. The main advantage of this type of analysis lies in its ability to rapidly and easily show areas with the highest concentration of cases, thereby contributing towards planning, monitoring, and surveillance of filariasis elimination actions. Incorporation of geoprocessing and spatial analysis techniques constitutes an important tool for use within the GPELF.
Keywords: Epidemiology, Filariasis, Geographic information systems, Spatial analysis, Health surveillance
Introduction
The neglected tropical diseases consist of a group of infections that affect around one billion individuals, i.e. one-sixth of the world’s population.1 Among the parasitoses in this group, lymphatic filariasis (LF) stands out because of its debilitating characteristics, the large number of individuals affected and, furthermore, the socioeconomic losses that it causes.2
LF is endemic in 81 countries and affects approximately 120 million individuals.2 In Brazil, it is caused solely by Wuchereria bancrofti. It is estimated that 1 500 000 people live in areas at risk of LF and that 49 000 people are infected.3 Currently, transmission occurs in seven municipalities in the state of Pernambuco.4
The World Health Organization (WHO) considers that LF is a potentially eradicable disease. For this reason, the Global Program to Eliminate Lymphatic Filariasis (GPELF) was created. Its target is to eradicate LF from the world by the year 2020.5 Following the proposal from WHO, Brazil became a cosignatory of GPELF, with the aims of interrupting filariasis transmission in endemic areas, through drug treatment and vector control, and providing comprehensive care for individuals with the disease.6
After more than 10 years of GPELF in Brazil, advances have been seen. These include eradication of the historical focus in the city of Belém, state of Pará and the recent interruption of transmission in the city of Maceió, state of Alagoas.6,7 Nonetheless, despite these advances, the endemic disease persists as a public health problem in three municipalities in the metropolitan region of Recife, State Pernambuco in Brazil: Recife, Jaboatão dos Guararapes, and Olinda.4,8
To plan the actions for controlling and eliminating this parasitosis, mapping of the distribution of the infection is a fundamental component. Hence, the aim of the present study was to describe the spatial distribution of LF in the municipality of Jaboatão dos Guararapes.
Materials and Methods
Study area
An epidemiological survey was conducted in the municipality of Jaboatão dos Guararapes, state of Pernambuco, Brazil, between 2000 and 2002. This municipality covers an area of 256 073 km2 and is divided into 27 districts and 492 census tracts. The resident population was 581 556 inhabitants (98% in the urban area), according to the 2000 demographic census.9 Two districts (eight census tracts) were excluded from this study because of lack of information.
Parasitological survey
The population data for this study were based on a sample from the total of 111 666 permanent private households in the municipality. The sample size was calculated by estimating a rate of positive findings of 6% among the households in the survey area and taking the acceptable error to be 5% for each 95% confidence interval.10 Through this, a sample of 5915 households was obtained.
Fourteen permanent private homes were registered in each of the 484 census tracts, taking into consideration possible refusals to participate in the survey. These households were selected from the census tract maps. The tracts were manipulated by defining their quadrants and applying straight lines along the diagonals (second and third quadrants), thus joining the opposite quadrants. After locating the midpoint in each quadrant (second and third), two street addresses were selected, and seven households were investigated in each street, thus totaling 14 households per census tract. A total of 28 612 people were registered to participate in the survey, but 4939 of them (17.26%) did not undergo the test.
The biological sampling consisted of drawing approximately 50 μl of capillary blood, using disposable syringes, between 23:00 and 01:00 hours. Thick smears were prepared from these samples.11 After hemoglobin removal, the smears were stained with eosin and counterstained using Giemsa. Quality control on the slide readings were carried out on 100% of the positive cases and 30% of the negative cases.12
Data analysis
The data collected were stored and analyzed using version 6.04d of the EpiInfo. Statistical comparisons were made using Student’s t-test, whenever appropriate. A P value of 0.05 was considered indicative of a statistically significant difference.
To determine the relative effect of explanatory variables on the prevalence of microfilaremia was fitted a generalized linear model with binomial logistic link function, using the variables: basis of home occupation (proportion of households that are not owner-occupiers or living in rented or assigned property); number of people in the household (proportion of households consisting of 10 people or more); inadequate water supply (proportion of households with water supply from wells or springs only in the yard of the property, not piped, and other forms of water supply); inadequate sewage disposal (proportion of households with sewage disposal into a rudimentary cesspit, ditch or gutter, or into a river, a lake or the sea, and without a bathroom); inadequate garbage collection (proportion of households in which garbage is burned, buried, dumped on vacant land or in rivers, or other destinations); schooling level (proportion of heads of households with not more than 1 year of schooling) and income level (proportion of heads of households with an income of half to one minimum monthly salary).
To classify the areas according to the microfilaremia prevalence rates, the criteria suggested by WHO were used. In these, rates lower than 5% defined areas of low prevalence; rates between 5 and 9% corresponded to the intermediate (medium) level; and rates greater than 10% were deemed to represent high prevalence.13
The positive cases of filariasis that were identified in the parasitological survey were georeferenced in point form by using a global positioning system (Garmin eTrex Summit) tracking device. A kernel intensity estimator was used to identify clusters of greater intensity of cases. This is an exploratory analysis method for spatial data that makes it possible to view exposed localities and identify different degrees of intensity within a given area.14 Kernel estimation involves a technique in which a symmetrical surface is overlain over the points and, based on a mathematical function, the distances from the points to a reference position are assessed and then the values for all the surfaces for this reference point are summed. This procedure is repeated for all positions.14
Ethical clearance
Biological samples were collected only after the informed consent statement had been signed. All the subjects who were found to present microfilaremia were treated with diethylcarbamazine.12
This study was approved by the Ethics Committee of the Aggeu Magalhães Research Center, Oswaldo Cruz Foundation, Recife, Pernambuco (CAAE no. 0034.0.095.000-07).
Results
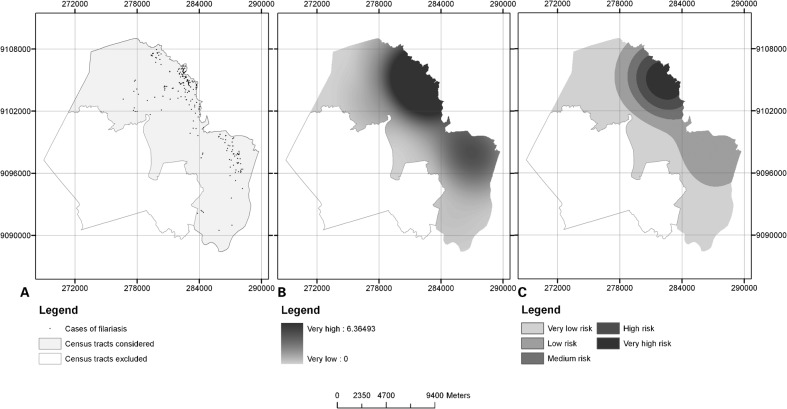
Out of 6507 households that were investigated, 255 had one or more individuals with microfilaremia forming part of the household. Figure 1A presents the distribution of the individuals with microfilaremia according to the districts in which they lived. Positive individuals were identified in 88.0% (22/25) of the districts, with prevalence rates ranging from 0.1 to 5.6%.
Figure 1.
Microfilaraemia distribution (A); density of the cases (B); strata of density of the cases (C) Jaboatão dos Guararapes, Pernambuco, Brazil.
In the households investigated, 23 673 individuals and 323 cases of microfilaremia were identified, with a mean prevalence rate for the municipality of 1.4%, the interval prevalence was 1.22–1.52% with confidence interval of 95%. Among the positive individuals, 117 (36.2%) were female and 206 (63.8%) were male, with prevalence rates of 0.9 and 2.0% respectively (P<0.05) (Table 1).
Table 1. Prevalence of microfilaremia according to age group and sex in Jaboatão dos Guararapes, Pernambuco, Brazil.
| Female | Male | Total | ||||||||
| Age group in years | No. examined | No. microfilaraemic | Prevalence | No. examined | No. microfilaraemic | Prevalence | Odds ratio | Χ2 | P | |
| 1–9 | 1854 | 5 | 0.3 | 1806 | 6 | 0.3 | 3660 | 1.23 | … | 0.96 |
| 10–19 | 2862 | 48 | 1.7 | 2679 | 49 | 1.8 | 5541 | 1.09 | 0.1 | 0.74 |
| 20–29 | 2396 | 25 | 1.0 | 1815 | 68 | 3.7 | 4211 | 3.59 | 32.11 | 0.00 |
| 30–39 | 2218 | 16 | 0.7 | 1414 | 41 | 2.9 | 3632 | 4.02 | 24.22 | 0.00 |
| 40–49 | 1774 | 10 | 0.6 | 1115 | 16 | 1.4 | 2889 | 2.55 | 4.79 | 0.02 |
| 50–59 | 1089 | 7 | 0.6 | 732 | 12 | 1.6 | 1821 | 2.55 | 3.22 | 0.07 |
| 60–99 | 1171 | 6 | 0.5 | 696 | 13 | 1.9 | 1867 | 3.65 | 6.5 | 0.01 |
| Unknown | 22 | … | … | 30 | 1 | 3.3 | 52 | … | … | … |
| Total | 13 386 | 117 | 0.9 | 10 287 | 206 | 2.0 | 23673 | 2.28 | 52.08 | … |
Individuals presenting microfilaremia were found in all the age groups studied (Table 1). The mean age among the individuals with microfilaremia was 29.6 years (ranging from 6 to 82 years). Analysis on the distribution of the individuals with microfilaremia according to sex and age group showed that among females, the highest prevalence rate was observed in the age group between 10 and 19 years, while among males, it was in the group from 20 to 29 years. In general, the highest prevalence was among the younger population between 20 and 39 years of age (Table 1).
The logistic regression model demonstrated that income of between half and one minimum monthly salary for heads of households, water supply only in one room of the home, water supply only in the yard of the property, other nonstandard methods of water supply and the presence of 10 or more people in the household increased the prevalence of microfilaremia by 0.34, 0.15, 0.48, 0.23, and 5.53%, respectively.
Out of all the individuals investigated in the survey, 741 reported some type of clinical manifestation. Among the 323 positive cases, only 12 reported signs or symptoms of filariasis. Among these individuals with complaints, six presented hydrocele and six had erysipelas.
Through the kernel intensity method, the areas of greatest intensity and lowest intensity of cases of filarial infection were identified (Fig. 1B and C). It was observed that the cases were distributed heterogeneously across the municipality.
Discussion
The first epidemiological survey in the municipality of Jaboatão dos Guararapes was conducted in the 1950s. It identified the presence of positive cases of filarial infection, but these were allochthonous cases coming from Recife.15 At that time, LF was not considered to be a public health problem, and surveillance actions were not implemented. Thus, transmission in this municipality was allowed to become established. The results from the present survey show that LF is now endemic in this municipality, given that positive cases were found in 88.0% of the districts. By applying the WHO (1988) classification to the districts in which positive individuals were detected, it could be seen that 17 districts were in the low prevalence stratum, while two (Floriano and Cavaleiro) were in the intermediate prevalence stratum. Therefore, these last two districts need to be prioritized by GPELF for mass treatment.
Explanatory models for the endemicity of LF in the municipality need to take into consideration the complexity of social phenomena and their relationship with the infectious cycle. Socioeconomic conditions associated with insufficiency or lack of basic sanitation, inadequacy of housing and cultural and educational factors are factors that favor proliferation of the vector mosquito (Culex quinquefasciatus).16
In this municipality, 65.4% of the districts presented inadequate sewage disposal systems.16 The deficient coverage of the sewage system is depicted by the presence of rudimentary septic cesspits and drains, along with excrement left out in the open air. These significantly favor proliferation of the vector mosquito. A study conducted by Bonfim et al.,16 showed that 73.0% of the cases of microfilaremia were located in the areas of worst socioenvironmental conditions, thus signifying that the risk of occurrences of filariasis in such areas was 4.86 times greater than in areas with better conditions.
In the present study, 323 individuals with microfilaremia were identified (1.4%), distributed in 255 households. Family-based surveys conducted in India17 and in Brazil18 suggested that the environment within households is an important factor in maintaining transmission. These studies indicated that family aggregation and the presence of naturally infected vectors within the household environment were conditioning factors for new cases of parasitosis to arise.
Analysis on the distribution of filariasis according to sex and age group showed that the prevalence among men was greater than the prevalence among women, in the general population (P<0.05). These data were similar to what was seen in previous studies in Brazil,19 Haiti20 and Samoa21 which also found higher prevalence among males.
The stratification according to age group made it possible to see the same profile among individuals aged 20–39 years, i.e. within this age group, men had a greater chance of contracting filarial infection than did women. The age group from 1 to 9 years stood out as the group with the lowest prevalence, while the highest prevalence was among individuals aged 20–29 years. This pattern resembled what was described by Bradin,22 Maciel et al.19 and Bonfim et al.10 in investigations in areas endemic for LF areas. These authors demonstrated that the pediatric population presented relatively low microfilaremia rates, while the rates were higher among young individuals of productive age.
From an epidemiological point of view, special attention needs to be paid towards investigating individuals aged over 65 years. Such individuals play an important role in maintaining the infection, given that mass treatment actions do not consider these populations.18 In the present study, 10 infected individuals were identified in this age group, thus indicating the need to implement a policy for supervising these individuals, within GPELF.
Among the clinical complaints reported, it was observed that 741 (3.1%) of the individuals investigated mentioned one or more signs of filariasis. However, among these, only 12 individuals presented microfilaremia. Investigations conducted in endemic areas have noted that most of the microfilaremic individuals are asymptomatic.10 A survey carried out in the Philippines found that 14% of the individuals with microfilaremia presented some type of chronic manifestation. Hydrocele was observed in men aged 16–80 years and seven cases with lymph scrotum, aged 26–64 years were identified on outpatients from Brazil.23 It is estimated that around 15% of the individuals infected will develop a clinical form of the filariasis.24
The kernel intensity estimator identified spatial clusters of cases, thus indicating localities with greater concentration of cases. It therefore contributes towards planning, monitoring and surveillance of actions for eradicating LF. Studies conducted in Africa25,26 and India27 characterized spatial analysis as a tool for planning GPELF actions. Analysis on the spatial distribution of individuals with microfilaremia indicated that there was greater concentration of cases in areas bordering the city of Recife, an area that historically has been endemic,15,19,28 and bordering the municipality of Cabo de Santo Agostinho, an area showing recent endemicity.29
These data demonstrate the complexity of the process of controlling and eradicating this urban endemic disease. They also indicate the need to investigate new areas, particularly those that border localities that are considered endemic for the disease, since so far, there are very few studies characterizing the limits between endemic areas and areas free from LF. There is no consensus regarding the dynamics of investigating endemic areas and their surrounds. The main advantage of this type of analysis is that it enables rapid and easy viewing of areas with greater concentration of cases. It therefore incorporation of geoprocessing techniques constitutes and important tool for use within GPELF.
Acknowledgments
We would like to thank the drivers and health agents who participated of this study. Also we would like to thank Mr João Quaresma and Mr José Costa for their helpful work at the epidemiological survey. Without the logistical support of Health Secretary of Jaboatão dos Guararapes, this study would not have been possible.
References
- WHO Neglected tropical diseases, hidden successes, emerging opportunities. A future free of neglected tropical diseases. Geneva: World Health Organization 2006[Cited 2010 Apr 14; ](52 p)Available from: http://whqlibdoc.who.int/hq/2006/WHO_CDS_NTD_2006.2_eng.pdf [Google Scholar]
- Perera M, Whitehead M, Molyneux D, Weerasooriya M, Gunatilleke G. Neglected patients with a neglected diseases? A qualitative study of lymphatic filariasis. PLoS-NTD. 2007;1:e317. doi: 10.1371/journal.pntd.0000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Health. Relatório da reunião de avaliação do programa de controle da filariose linfática no Brasil. Centro Nacional de Epidemiologia, Brasília-DF; 2000 [Google Scholar]
- Ministry of Health. Gerência Técnica do Programa de Eliminação da Filariose/Coordenação de Doenças Transmissíveis por Vetores. Síntese Epidemiológica da Filariose, Brasília-DF. 2006 [Google Scholar]
- Global Alliance to Eliminate Lymphatic Filariasis (GAELF) Fifth Meeting of the Global Alliance to Eliminate Lymphatic Filariasis, Arusha Tanzania. 2008. 10 years of progress towards elimination: success in a changing policy environment. p. 122 p. [Google Scholar]
- Ministry of Health. O Programa de eliminação da filariose bancroftiana nas Américas. Centro Nacional de Epidemiologia. Bolet Epidemiol. 1997;1(6):12. [Google Scholar]
- Freitas H, Vieira JB, Braun R, Medeiros Z, Rocha EMM, Aguiar-Santos AM, et al. Workshop to evaluate the epidemiologic situation of lymphatic filariasis in the Municipality of Belém, Pará, Northern Brazil. Rev Bras Malariol Doencas Trop. 20082212–16. [DOI] [PubMed] [Google Scholar]
- Medeiros Z, Bonfim C, Alves A, Oliveira C, Netto MJE, Aguiar-Santos AM. The epidemiological delimitation of lympatic filariasis in an endemic area of Brazil, 41 years after the first recorded case. Ann Trop Med Parasitol. 2008;102:6–11. doi: 10.1179/136485908X311821. [DOI] [PubMed] [Google Scholar]
- Instituto Brasileiro de Geografia e Estatística (IBGE). 2001. Censos Demográficos. [Cited 2009 Oct 30]. Available from: http://www.ibge.gov.br/censo. [Google Scholar]
- Bonfim C, Lessa F, Oliveira C, Evangelista MJ, Santo ME, Meireles E, et al. The occurrence and distribution of lymphatic filariasis in Greater Metropolitan Recife: the case of an endemic area in Jaboatão dos Guararapes, Pernambuco, Brazil. Cad Saude Publica. 2003;5:1497–505. doi: 10.1590/s0102-311x2003000500028. [DOI] [PubMed] [Google Scholar]
- Dreyer G, Pimentel A, Medeiros Z, Béliz F, Moura I, Coutinho A, et al. Studies on the periodicity and intravascular distribution of Wuchereria bancrofti microfilariae impaired samples of capillary and venous blood from Recife, Brazil. Trop Med Int Health. 1996;2:264–72. doi: 10.1111/j.1365-3156.1996.tb00037.x. [DOI] [PubMed] [Google Scholar]
- WHO. Lymphatic filariasis infection and diseases. Control strategies: report of a consultative meeting at the University Sains Malaysia Penang, Malaysia; Geneva: World Health Organization. 1994 [Google Scholar]
- WHO. Lucha contra la filasiasis linfática. Manual para personal sanitario. Geneva: World Health Organization. 1988 [Google Scholar]
- Bailey TC. Spatialanalysis and GIC. Taylor, Francis, Reino Unido. 1995 [Google Scholar]
- Dobbin JuniorJE, Cruz AE. Rev Bras Malariol Doencas Trop. Vol. 19. Portuguese; 1967. Inquérito de filariose em alguns municípios do litoral-mata de Pernambuco. pp. 45–51. [PubMed] [Google Scholar]
- Bonfim C, Netto MJ, Pedroza D, Portugal JL, Medeiros Z. A socioenvironmental composite índex as a tool for identifying urban areas at risk of lymphatic filariasis. Trop Med Int Health. 2009;14:877–84. doi: 10.1111/j.1365-3156.2009.02317.x. [DOI] [PubMed] [Google Scholar]
- Vanamail P, Ramaiah KD, Krishnamoorthy K, Pani SP, Das PK. Distribuition of microfilaria carriers and clinical cases of bancroftian filariasis in relation to family size in an urban situation. Trop Biomed. 1992;9:91–8. [Google Scholar]
- Rocha A, Marcondes M, Nunes JRV, Miranda T, Veiga J, Araujo P, Tenorio W, Aguiar-Santos A. Programa de controle e eliminação da filariose linfática: uma parceria da Secretaria de Saúde de Olinda-PE, Brasil, com o Serviço de Referência Nacional em Filariose. Rev Pat Trop. 2010;39:233–49. [Google Scholar]
- Maciel MA, Marzochi KB, Silva EC, Rocha A, Furtado AF. Comparative studies on endemic areas of bancroftian filariasis in Greater Recife, Brazil. Cads Saúde Pública. 1994;10:301–9. [PubMed] [Google Scholar]
- Rochars MV, Milord DM, Jean YS, Désarmeaux AM, Dorvil JJ, Lafontant JG, et al. Geographic distribuition of lymphatic filariasis in Haiti. Am J Trop Med Hyg. 2004;5:598–601. [PubMed] [Google Scholar]
- McCarthy DD, Fitzgerald N. Habit, habitat and hyperfilariation in the epidemiology of filariasis in Western Samoa. Trans R Soc Trop Med Hyg. 1956;50:58–65. doi: 10.1016/0035-9203(56)90009-8. [DOI] [PubMed] [Google Scholar]
- Bradin L. Sex differentials in susceptibility to lymphatic filariasis and implications for maternal child immunity. Epidemiol Infect. 1990;105:335–53. doi: 10.1017/s0950268800047932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguiar-Santos AM, Leal-Cruz M, Netto MJ, Carrera A, Lima G, Rocha A. Lymph scrotum: an unusual urological presentation of lymphatic filariasis. A case series study. Rev Inst Med Trop Sao Paulo. 2009;4:79–83. doi: 10.1590/s0036-46652009000400001. [DOI] [PubMed] [Google Scholar]
- Dreyer G, Norões J.Filariose bancroftiana. Lucena V G, et al.editorsCondutas em Clínica Médica. Recife: Editora Universitária; 1997. p339–421. [Google Scholar]
- Gyapong JO, Kyelem D, Kleinschmidt I, Agbo K, Ahouandogbo F, Gaba J, et al. The use spatial analysis in mapping the distribution of bancroftian filariasis in four West African countries. Ann Trop Med Parasitol. 2002;96:695–705. doi: 10.1179/000349802125001735. [DOI] [PubMed] [Google Scholar]
- Lindsay SW, Thomas CJ. Mapping and estimating the population at risk from lymphatic filariasis in Africa. Trans R Soc Trop Med Hyg. 2000;94:37–45. doi: 10.1016/s0035-9203(00)90431-0. [DOI] [PubMed] [Google Scholar]
- Sabesan S, Raju HKK, Srividya A, Das PK.Delimitation of lymphatic filariasis transmission risk areas: a geo-environmental approach. Filaria J. 2006[Cited 2009 Nov 14; ]Available from: http://www.filariajournal.com/content/pdf/1475-2883-5-12.pdf> [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros Z, Dreyer G, Andrade LD, Pires ML, Mendes J, Pimentel R. Wuchereria bancrofti microfilarial density of autochthonous cases and natural Culex infectivity rates in Northeast Brazil. J Trop Med Hyg. 1992;95:214–7. [PubMed] [Google Scholar]
- Medeiros Z, Alves A, Brito JÁ, Borba L, Santos Z, Costa JP, et al. The present situation regarding lymphatic filariasis in Cabo de Santo Agostinho, Pernambuco, Northeast Brazil. Rev Inst Med Trop Sao Paulo. 2006;48:263–7. doi: 10.1590/s0036-46652006000500005. [DOI] [PubMed] [Google Scholar]