Abstract
A 2-year-old boy presented with seizures and two parenchymal brain ring-enhancing lesions. Diagnosis of neurocysticercosis was confirmed by positive serology and response to albendazole therapy. The patients's mother was a Taenia solium carrier, who had most likely infected the child through the fecal-oral route. Household contacts should always be investigated in children with neurocysticercosis. Proper identification and treatment of Taenia solium carriers will reduce the risk of further spread of the disease.
Keywords: Cysticercosis, Neurocysticercosis, Taenia solium
A 2-year-old previously healthy boy was admitted for recent onset of recurrent seizures. Some episodes started as clonic movements in the left leg while others started in the right side of the body. In some episodes, there was secondary generalization with loss of consciousness and tonic-clonic involuntary movements involving all limbs. On admission, the patient was alert and oriented, and neurological examination showed a mild left paresis and a left Babinski sign. MRI showed two small ring-enhancing lesions surrounded by edema, symmetrically located in both parietal lobes (Fig. 1A). A serum immunoblot test for the detection of anticysticercal antibodies was strongly positive. The patient was started on phenytoin therapy, and the left paresis resolved on the following day. We then started a one-week course of albendazole at 15 mg/kg/day. Two weeks after the end of the trial, a new MRI showed reduction in the size of the left-sided lesion, and marked reduction in the size of the edema surrounding the right-sided lesion (Fig. 1B). He was discharged, and repeated MRI performed after three months, showed almost complete resolution of the right lesion (Fig. 1C). The patient remained seizure-free, on phenytoin therapy, for 13 months of further follow-up. We requested serial stool examinations in all family members, including the mother and two elder brothers (aged five and seven years), and the patient’s mother was positive for taenia eggs. None of the family members had history of seizures or any other neurological symptom. Upon inquiry the mother disclosed that she had passed a long and thin parasite with feces some months ago. She was treated with praziquantel, 10 mg/kg as a single dose.
Figure 1.
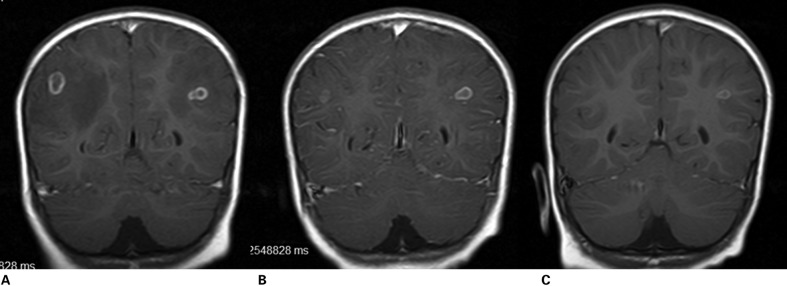
Contrast-enhanced MRI showing left and right parietal lobe cysticerci in acute encephalitic phase (A). Two weeks (B) and three months (C) after albendazole, lesions vanished as the result of therapy.
Neurocysticercosis, a parasitic disease caused by infection with the larval stage of Taenia solium, is the most common cause of acquired epilepsy in the developing world. Mass immigration of people from endemic to non-endemic areas has recently increased the prevalence of neurocysticercosis in developed countries as well.1 The immune system (particularly in children and young women) may actively react when parasites enter the nervous system, and such immune reaction may cause degenerative changes in the parasite along with inflammatory changes in the surrounding brain parenchyma. This causes the ‘acute encephalitic phase of neurocysticercosis’, which appears on neuroimaging studies as ring-enhancing lesions surrounded by edema.2 This form of neurocysticercosis may pose a diagnostic challenge, since other infectious or non-infectious diseases may present with seizures and similar neuroimaging findings. In these cases, proper interpretation of current diagnostic criteria is of value to avoid the practice of unnecessary invasive procedures.3
Cysticercosis is not acquired by eating pork meat. Instead, humans become infected after ingesting T. solium eggs from contaminated food or, more often, by the fecal–oral route through a household contact harboring the adult parasite in the intestine.4 In general, children with neurocysticercosis may be more likely than adults to have acquired their infection at home because of their limited exposure to other environments. This phenomena has not only been reported from endemic areas, but from developed nations, where autochthonous children have developed neurocysticercosis after being infected by a family member or a babysitter recently coming from endemic areas, who had enter that cysticercosis-free area carrying an adult tape worm in their intestine.5 Unfortunately, compulsory search of taenia carriers around infected children is not performed, an omission that may cause further spread of the disease.
References
- 1.Garcia HH, Del Brutto OH. Neurocysticercosis: updated concepts about an old disease. Lancet Neurol. 2005;4:653–61. doi: 10.1016/S1474-4422(05)70194-0. [DOI] [PubMed] [Google Scholar]
- 2.Del Brutto OH. Single parenchymal brain cysticercus in the acute encephalitic phase: definition of a distinct form of neurocysticercosis with a benign prognosis. J Neurol Neurosurg Psychiatry. 1995;58:247–9. doi: 10.1136/jnnp.58.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Del Brutto OH, Rajshekhar V, White AC Jr, Tsang VC, Nash TE, Takayanagui OM, et al. Proposed diagnostic criteria for neurocysticercosis. Neurology. 2001;57:177–83. doi: 10.1212/wnl.57.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gonzalez AE, Lopez-Urbina T, Tsang B, Gavidia C, Garcia HH, Silva ME, et al. Transmission dynamics of Taenia solium and potential for pig-to-pig transmission. Parasitol Int. 2006;55:S131–5. doi: 10.1016/j.parint.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 5.Asnis D, Kazakov J, Toronjadze T, Bern C, Garcia HH, McAuliffe I, et al. Neurocysticercosis in the infant of a pregnant mother with a tapeworm. Am J Trop Med Hyg. 2009;81:449–51. [PubMed] [Google Scholar]


