Abstract
In the summer of 2011, an outbreak of Mycobacterium tuberculosis infection was suspected, and widely publicized, to have occurred in a maternity ward of an Italian University Hospital based on a case of tuberculosis in a nurse and another case in a newborn. More than 1300 newborns in the Hospital were surveyed for the occurrence of latent TB by the use of interferon-gamma released assays, which was positive in 118 newborns, all negative at the tuberculin skin test. We present here several theoretical arguments and literature data suggesting caution in interpreting the interferon-gamma released assays positivity alone as indication of latent TB infection in newborns.
Keywords: Latent tuberculosis, Interferon-gamma release assays, Tuberculin skin test, Newborns, Trogocytosis, Chimerism
The Event
In the summer of 2011, the occurrence of a large outbreak of Mycobacterium tuberculosis (MTB) transmission was suspected in the maternity ward of a large Italian University Hospital. The suspected index case was a smear-positive nurse with clinically and microbiologically confirmed diagnosis of pulmonary tuberculosis (TB). Noteworthy, a case of pulmonary and extrapulmonary (spleen) TB in a four-month-old infant was suspected to be linked to the index case.1
Immediately after TB diagnosis, a protocol for epidemiological investigation and adoption of preventive measures was established by an ad hoc task force of hospital infection specialists and public health officers. Extensive contact investigations in newborns for latent TB infection (LTBI) were initially made by the use of Quantiferon TB Gold in-tube (QTF-IT) rather than the tuberculin skin test (TST, Mantoux reaction) for the reasons detailed below. Nevertheless, a group of the children initially tested with QTF-IT were also subsequently tested with TST at a later stage of the surveillance program. Overall, a total of 1340 newborns were tested and 118 (9%) were positive to QTF-IT while all resulted TST-negative. With the exclusion of the mentioned infant who was identified with active TB, no other case of TB has been diagnosed so far (For a full and accurate description of the event, see Ref. 1).
This event received a great deal of attention by the Italian health officials and the media which, more or less consciously, fostered the concept that an extensive outbreak of MTB infection had indeed occurred at this Hospital, representing the first outbreak of this extension in an Italian Hospital and perhaps elsewhere. Consequences of this event, included severe parental and public distress, the need for increased funding for screening and surveillance and last but not least harsh public controversies about the real significance of tests used for identifying newborns with latent TB and, consequently, over the need and extension of TB prophylaxis
Discussion
The decision to use QTF-IT in the screening for MTB infection was taken because of a supposed lower sensitivity of TST, a lower accuracy of TST in low and middle income countries in children and also because testing by QTF-IT did not require a second visit as for TST.1 The absence of precise estimates of QTF-IT sensitivity and specificity in neonates and infants (<2 years old children) in current literature is of serious concern, and actually implies inability of accepting a single, isolated positive QTF-IT result as indicative of LTBI in this age setting. In one of the very few studies in this area that was conducted in a relatively large population of young children in South Africa,2 TST and QTF-IT assays demonstrated an excellent correlation (94%). According to this study the sensitivity and specificity of both assays was comparable, namely 38 and 81% for QTF-IT and 35 and 84% for TST. By even accounting for the large differences in the age, social environment, local prevalence of MTB infection and other factors that could potentially affect QTF-IT versus TST testing, to our knowledge there is no reported case in the literature showing such a substantial discrepancy between Quantiferon-based assays and TST as the one noticed in this event (i.e. 100% QTF-IT positivity versus 0% positivity by TST).
Several additional considerations seem to be appropriate to this case. First, it is surprising that no direct correlation could be shown between QTF-IT positivity rate and month of birth, as would be expected in the scenario of an increasing infectiousness of the index case by time. Second, there was concurrence between potential exposure and birth. The possible exposure timeframe was therefore limited to a couple of days maximum in most children.1 Henceforth, the likelihood/risk of infection under this setting would be estimated to be very low. In fact, this is supported by previous work showing that in closely similar events, no or very few infants were infected).3–6 A third aspect that is surprising and not in agreement with the possibility of an ongoing sequential transmission from a single source (nurse) to newborns in a nursery is the observation that there was one single case of TB disease among exposed newborns, compared to an estimated rate of infections of 9% occurring based on QFT-IT alone. Indeed, a somewhat higher rate of disease occurrence would be expected in newborns with immature immune system. Finally, in a recent meta-analysis conducted on studies enrolling 26 680 participants, which also included children, demonstrated that in seven out of eleven studies the positivity of interferon-gamma released assays was lower than TST positivity.7
Thus, based on the above considerations, the diagnosis of LTBI in newborns and infants who tested positive for QTF-IT and negative for TST would be neither warranted nor justified, and may derive from pitfalls and unperceived sources of error associated with the use of an indirect diagnostic technique in this age setting. The use of QFT-IT for the diagnosis of latent TB infection in newborns/infants necessarily implies that interferon-gamma (IFN-gamma) production by peripheral blood mononuclear cells (PBMCs) revealed by the QTF-IT is generated by MTB antigen-specific CD4+ or CD8+ T cells of infants. We wondered whether there is any other possible explanation of the event, i.e. the relatively high proportion of infants testing QTF-IT-positive and TST-negative. Namely, is there any possible production of IFN-gamma by other cells of the newborn/infant, or by non-MTB antigen-specific cells or even by non-infant, but rather maternal MTB antigen-specific CD4 or CD8 cells?
The Hypothesis
Starting from the above observations, we propose a hypothetical different interpretation of the event which extends to the potential everyday use of QFT-IT testing in newborns and infants. Our hypothesis is essentially based on the following cornerstones: (1) IFN-gamma detected by QTF-IT in newborns may be released by cells of the immune system other than the MTB-specific CD4 or CD8 T effector or memory cells; (2) these cells can not only be of newborn, but also be of maternal origin. In both cases, the QTF-IT assay results cannot be interpreted by themselves as sufficient to diagnose latent TB infection.
The hypothesis below discusses potential different aspects which alone or in combination could fully or largely justify the event.
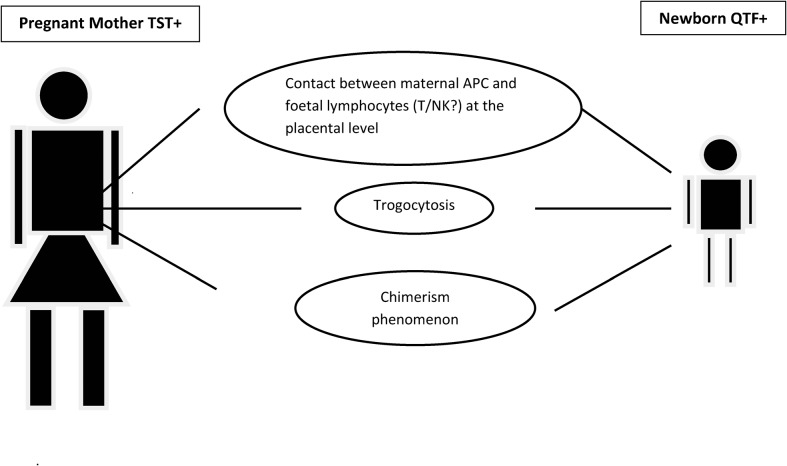
A first aspect to consider is that foetal lymphocytes get in contact with maternal endothelial cells of the antigen-presenting cell system at placental level (Fig. 1). If maternal antigen-presenting cell system carries mycobacterial antigens due to MTB infection they may receive a specific immunological imprinting. A relevant phenomenon here is the so-called ‘trogocytosis’, i.e. a process whereby lymphocytes [B, T, and natural killer (NK) cells] conjugated to antigen-presenting cells extract surface molecules from these cells and express them on their own surface. If newborn (CD4 or, CD8 lymphocytes incorporate MTB-recognizing T-cell receptor, they could produce IFN-gamma following exposure to dominant mycobacterial antigens.
Figure 1.
A schematic illustration of the different biological phenomena of mother-newborn immune interaction which can theoretically affect newborn immune responses to microbial antigens.
A second relevant aspect is related to the so-called ‘chimerism’ phenomenon. It is established that cells of maternal origin, including T cells, may be present for some time in the infant bloodstream. If parts of these cells are MTB-antigen specific due to maternal latent TB infection, they will produce IFN-gamma upon specific antigenic challenge which can be revealed by a highly sensitive assay as QFT-IT. Of note, both phenomena above may persist for a limited and variable length of time in infants but they are probably present and functional in most of the newborns. In this case a positive QFT-IT test would be a ‘true positive’ testing of maternal, not of newborn/infant, PBMC thus implying that latent TB is active in the mother, not in the offspring. A third relevant aspect is that the IFN-gamma measured by the QTF-IT test may well be produced by other effector cells which do not necessarily belong to the T-cell lineage and are non-MTB antigen specific. A major candidate in this respect would be NK cells.8
Although in the absence of specific studies it is impossible to quantify the potential contribution to the event by any of the three factors above, the overall hypothesis is supported by a number of literature evidences, as detailed below, ad suggests that any positive QTF-IT in infants/newborns should actually include information on ‘which’ and ‘whose’ cells actually are responsible for IFN-gamma production.
The placenta hosts cells belonging to the maternal immune system (macrophages, dendritic cells, NK cells and B and T lymphocytes); it is therefore plausible that the placenta represents and important site for trafficking between maternal and foetal immune systems.8,9–11
Naive cells from the cord blood produce IFN-gamma, IL-5, IL-13 (at low levels) if stimulated by PPD in vitro.12 Similarly, low levels of IFN-gamma, IL-10 and IL-5 are produced by cells from the cord blood after stimulation with BCG in vitro.13 In addition, IFN-gamma, IL-10, IL-12, and low levels of IL-13 and IL-5 but no IL-4 were produced by cord blood mononuclear cells after stimulation with BCG. Intracellular staining showed that IL-10 and IL-12 were produced by monocytes and that IFN-gamma was produced by NK cells but not by CD4(+) or CD8(+) T cells as in the adult immune response.14 Although the cord blood is a mix of foetal and maternal cells and does not reproduce a uniquely foetal response, the data warrant consideration also in view of the relative proportion of NK cells and T cells in the infant blood (with NK cells equaling CD8+T cells), of the direct binding ability of MTB to NK cells in vitro,15,16 of the specific recognition of NK cells of MTB antigens17 and of the demonstration that ESAT-6 induces IFN production by bovine NK cells.18 Overall, interpretation of the results according to these reports and data points to a potentially relevant involvement of NK cells as IFN-gamma producing cells in newborn/infant PBMC assayed with QFT-IF.
The IFN-gamma response to BCG and MTB antigens is increased in HIV negative un-infected and un-exposed newborns compared to newborns from HIV positive mothers.19 This, in our opinion, confirms that the immune memory of the newborn can be affected in utero by the asset of maternal antigens.
In a murine model a higher IFN-gamma response can be obtained from neonatal lymphocytes stimulated in vitro in the offspring of mothers exposed to mycobacterial antigens compared to the offspring of unexposed mothers.20 Neonatal vaccination of the offspring of exposed mothers elicited greater protection from experimental challenge compared to controls. This effect has been put in association with the transfer of antigens administered to the pregnant mother to placental and foetal tissues. This observation might be consistent with the fact that pregnant women who have latent TB infection transfer mycobacterial antigens to the newborn in utero through the placental barrier, or that trogocytosis or chimerism are active in these cases.
Offspring of mothers infected with mumps or toxoplasmosis during pregnancy develop antigen specific memory T cells.21 However, it is described1 that in the TB setting the mothers were not acutely infected during pregnancy, though they might have had latent infection.
Interestingly, a study conducted in 10 mother/newborn pairs in Ethiopia demonstrated a full consistency between lymphocyte responses to Mycobacterium leprae antigens in the mother and the newborn at birth (i.e. when the mother showed sensitization, the newborn did too).22 At that time this phenomenon was attributed to the passage of a soluble lymphocytic factor from the mother to the newborn through the placenta. Since maternal chimerism, NK cells, or trogocytosis were not yet known at that time, a revisitation of those results would rather point to a common mechanism for mycobacterial reactivity in children born to infected mothers, which would entice one or more of the mechanisms discussed above.
Conclusion
Our hypothesis could be at least in part validated or rejected by simple, though often ethically and logistically difficult, experiments: (1) testing by QTF-IT and TST the mothers of the 118 neonates potentially exposed to the index case and resulting QTF-IT positive; (2) by retesting by QTF-IT the subjects at a distance of time; (3) by evaluating subset reactivity (T and NK cells) to QFT-IT in children (newborns/infants) born to TST-negative mothers. Our hypothesis warrants that, in the absence of exposure to MTB, at least the majority of those mothers are TST (and QTF-IT) positive and that the QTF-IT is expected to become negative in these infants.
In a nutshell, we wish here to draw attention to the high risk of misinterpretation of the results of excessively sensitive immunological assays to assess LTBI in children, particularly in newborns and young infants. Several alternative explanations exist in newborns and young children to support that IFN-gamma production detected by those assays may not be generated by MTB antigen-specific T cells of the newborn, consequently invalidating the assumption of LTBI in these subjects. Ideally, before assuming the diagnosis of latent TB infection with all consequent public health actions, any QFT-IT positive result from newborn/infant should be accompanied either by a concomitant validated assay such as TST or by proof of absence of contaminating maternal cells/maternal antigenic material and of other IFN-gamma producing cells. Studies on these aspects, as well as on the true predictive value for TB of the available diagnostic tools (which are uniquely based on host immune response without evidence for pathogen activity7) should be strongly encouraged and implemented by public health authorities. This would greatly help avoid uncertainties on data interpretations regarding both diagnosis of latent infection and actual risk of developing TB.
References
- 1.Borgia P, Cambieri A, Chini F, Coltella L, Delogu G, Di Rosa E, et al. Supected transmission of tuberculosis in a maternity ward from a smear-positive nurse: preliminary results of clinical evaluations and testing of neonates potentially exposed, Rome, Italy 1 January to 28 July 2011. Euro Surveill. 2011;16(40) doi: 10.2807/ese.16.40.19984-en. [DOI] [PubMed] [Google Scholar]
- 2.Moyo S, Isaacs F, Gelderbloem S, Verver S, Hawkridge AJ, Hatherill M, et al. Tuberculin skin test and Quantiferon assay in young children investigated for tuberculosis in South Africa. Int J Tuberc Lung Dis. 2011;15(9):1176–81. doi: 10.5588/ijtld.10.0770. [DOI] [PubMed] [Google Scholar]
- 3.Light H, Saidleman M, Sutherland JM. Management of newborns after nursery exposure to tuberculosis. Am Rev Resp Dis. 1974;109(4):415–9. doi: 10.1164/arrd.1974.109.4.415. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC) Mycobacterium tuberculosis transmission in a newborn nursery and maternity ward, New York city, 2003. MMWR. 2005;54:1280–3. [PubMed] [Google Scholar]
- 5.Nania JJ, Skinner J, Wilkerson K, Warkentin JV, Thayer V, Swift M, et al. Exposure to pulmonary tuberculosis in a neonatal intensive care unit: unique aspects of contact investigations and management of hospitalized patients. Infect Control Hosp Epidemiol. 2007;28:661–5. doi: 10.1086/517975. [DOI] [PubMed] [Google Scholar]
- 6.Lighter J, Rigaud M, Eduardo R, Peng CH, Pollack H. Latent TB diagnosis in children by using the Quantiferon TO gold in-tube test. Pediatrics. 2009;123:30–7. doi: 10.1542/peds.2007-3618. [DOI] [PubMed] [Google Scholar]
- 7.Rangoka MX, Wilkinson KA, Glynn JR, Ling D, Menzies D, Mwansa-Kambafwile J, et al. Predictive value of interferon-gamma release assay for incident active tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:45–55. doi: 10.1016/S1473-3099(11)70210-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marcenaro E, Ferranti B, Falco M, Moretta L, Moretta A. Human NK cells directly recognize Mycobacterium bovis via TLR2 and acquire the ability to kill monocyte-derived DC. Int Immunol. 2008;20:1155–67. doi: 10.1093/intimm/dxn073. [DOI] [PubMed] [Google Scholar]
- 9.Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol. 2004;4(7):553–64. doi: 10.1038/nri1394. [DOI] [PubMed] [Google Scholar]
- 10.Hunt JS. Stranger in a strange land. Immunol Rev. 2006;213:36–47. doi: 10.1111/j.1600-065X.2006.00436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takata K, Jameson SC. Naive T cell homeostasis: from awareness of space to a sense of place. Nat Rev Immunol. 2009;9(12):823–32. doi: 10.1038/nri2657. [DOI] [PubMed] [Google Scholar]
- 12.Ota MO, Vekemans J, Schlegel-Haueter SE, Fielding K, Sanneh M, Kidd M, et al. Influence of Mycobacterium bovis bacillus Calmette-Guerin on antibody and cytokine responses to human neonatal vaccination. J Immunol. 2002;168:919–25. doi: 10.4049/jimmunol.168.2.919. [DOI] [PubMed] [Google Scholar]
- 13.Hussy GD, Watkins ML, Goddard EA, Gottschalk S, Hughes EJ, Iloni K, et al. Neonatal mycobacterial specific cytotoxic T-lymphocyte and cytokine profiles in response to distinct BCG vaccination strategies. Immunology. 2002;105:314–24. doi: 10.1046/j.1365-2567.2002.01366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watkins ML, Semple PL, Abel B, Hanekom WA, Kaplan G, Ress SR. Exposure of cord blood to mycobacterium bovis BCG induces an innate response but not a T-cell cytokine response. Clin Vaccine Immunol. 2008;15(11):1666–73. doi: 10.1128/CVI.00202-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Esin S, Batoni G, Counoupas C, Stringaro A, Brancatisano FL, Colone M, et al. Direct binding of human NK cell natural cytotoxicity receptor NKp44 to the surfaces of mycobacteria and other bacteria. Infect. Immun. 2008;76:1719–27. doi: 10.1128/IAI.00870-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marcenaro E, Ferranti B, Falco M, Moretta L, Moretta A. Human NK cells directly recognize Mycobacterium bovis via TLR2 and acquire the ability to kill monocyte-derived DC. Int. Immunol. 2008;20:1155–67. doi: 10.1093/intimm/dxn073. [DOI] [PubMed] [Google Scholar]
- 17.Vankayalapati R, Wizel B, Weis SE, Safi H, Lakey DL, Mandelboim O, et al. The NKp46 receptor contributes to NK cell lysis of mononuclear phagocytes infected with an intracellular bacterium. J Immunol. 2002;168:3451–7. doi: 10.4049/jimmunol.168.7.3451. [DOI] [PubMed] [Google Scholar]
- 18.Olsen I, Boysen P, Kulberg S, Hope JC, Jungersen G, Storset AK. Bovine NK cells can produce gamma interferon in response to the secreted mycobacterial proteins ESAT-6 and MPP14 but not in response to MPB70. Infect Immun. 2005;73:5628–35. doi: 10.1128/IAI.73.9.5628-5635.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miles DJC, Gadama L, Gumbi A, Nyalo F, Makanani B, Heyderman RS. Human immunodeficiency virus (HIV) infection during pregnancy induces CD4 T-cell differentiation and modulates responses to Bacille Calmette-Guérin (BCG) vaccine in HIV-uninfected infants. Immunology. 2010;129:446–54. doi: 10.1111/j.1365-2567.2009.03186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rahman MJ. Influence of maternal gestational treatment with mycobacterial antigens on postnatal immunity in an experimental murine model. PLoS ONE. 2010;5:e9699. doi: 10.1371/journal.pone.0009699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hara T. Human V delta 2+ gamma delta T-cell tolerance to foreign antigens of Toxoplasma gondii. Proc Natl Acad Sci USA. 1996;93:5136. doi: 10.1073/pnas.93.10.5136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barnetson R. Evidence for a soluble lymphocyte factor in the transplacental transmission of T-lymphocyte responses to Mycobacterium leprae. Nature. 1976;260:150–1. doi: 10.1038/260150a0. [DOI] [PubMed] [Google Scholar]