Abstract
Objectives
To assess the quality and safety of having community health workers (CHWs) in rural Zambia use rapid diagnostic tests (RDTs) and provide integrated management of malaria and pneumonia.
Design/methods
In the context of a cluster-randomized controlled trial of two models for community-based management of malaria and/or non-severe pneumonia in children under 5 years old, CHWs in the intervention arm were trained to use RDTs, follow a simple algorithm for classification and treat malaria with artemether–lumefantrine (AL) and pneumonia with amoxicillin. CHW records were reviewed to assess the ability of the CHWs to appropriately classify and treat malaria and pneumonia, and account for supplies. Patients were also followed up to assess treatment safety.
Results
During the 12-month study, the CHWs evaluated 1017 children with fever and/or fast/difficult breathing and performed 975 RDTs. Malaria and/or pneumonia were appropriately classified 94–100% of the time. Treatment based on disease classification was correct in 94–100% of episodes. Supply management was excellent with over 98% of RDTs, amoxicillin, and AL properly accounted for. The use of RDTs, amoxicillin, and AL was associated with few minor adverse events. Most febrile children (90%) with negative RDT results recovered after being treated with an antipyretic alone.
Conclusions
Volunteer CHWs in rural Zambia are capable of providing integrated management of malaria and pneumonia to children safely and at high quality.
Keywords: Malaria, Pneumonia, Children, Rapid diagnostic test, Community health worker, Amoxicillin, Artemether–umefantrine, Zambia
Introduction
Pneumonia and malaria are two leading causes of morbidity and mortality of children under 5 years old worldwide.1 Young children in rural sub-Saharan Africa are at increased risk of developing severe illness or dying from malaria and pneumonia due to limited access to adequate care.2 Increasing access to high-quality and safe treatments at the community level is one potential approach to help meet the Fourth Millennium Development Goal, reducing child mortality by two-thirds by 2015 compared to 1990 levels.3
Integrated community case management of common childhood illnesses by community health workers (CHWs) is an increasingly popular strategy to improve treatment delivery to children in developing countries. Although many countries have adopted policies and developed programs for pneumonia treatment4 and some programs have used CHWs to treat malaria,5,6 few programs have utilized CHWs to deliver integrated treatment for both diseases.7 Given the simplicity of rapid diagnostic tests (RDTs), and their potential to reduce the costs and development of resistance associated with widespread use of artemisinin-based combination therapy, interest in using RDTs for routine malaria case management, including by CHWs, has rapidly increased.8–10 Several studies in Asia and Africa have shown that with minimal training and job aids, CHWs can perform and interpret RDTs.5,6,11,12 However, little is known regarding the quality of care and safety of CHW use of RDTs in the integrated community-based management of malaria and pneumonia. Concerns have been raised regarding withholding antimalarials in young children with fever who are RDT test-negative for malaria13 because of the limited evidence of the safety of this approach.14
To inform the debate regarding optimal modes of community-based care by CHWs,7 we conducted a cluster-randomized, controlled trial to evaluate the feasibility and effectiveness of integrated management of malaria and pneumonia by CHWs in rural Zambia.15 This paper further describes the ability of the CHWs in the intervention arm to provide high-quality and safe integrated management of malaria and pneumonia in young children using RDTs, artemether–lumefantrine (AL), and amoxicillin. Quality was measured by how well the CHWs adhered to the evidence-based treatment algorithm and dosing tables, while safety was assessed by the presence of adverse effects associated with the drugs, finger prick for RDT, and withholding AL in children with negative RDTs. We also provide important information on CHWs’ capacity to manage the supplies needed for integrated community case management.
Patients and Methods
Study design and site
The Zambia Integrated Management of Malaria and Pneumonia Study was a cluster-randomized, controlled trial that compared two modes of diagnosis and treatment of febrile illness at the community level by CHWs.15 This study was conducted from December 2007 to November 2008 in the Chikankata Mission Hospital (CMH) catchment area in Siavonga and Mazabuka Districts in Southern Province, Zambia. CHWs in the intervention arm were trained to use RDTs, treat RDT-positive cases with AL, treat non-severe pneumonia with amoxicillin, and refer children with severe disease to the nearest health facility. By contrast, CHWs in the control arm treated all febrile children with AL and referred those with any form of pneumonia to the nearest health facility.
Study procedures
CHW baseline data collection
Before the study-related training began, each CHW was interviewed to collect baseline data on demographic characteristics, past training, responsibilities, supervision, supplies, situations requiring referrals, and receipt of payments or incentives.
CHW training
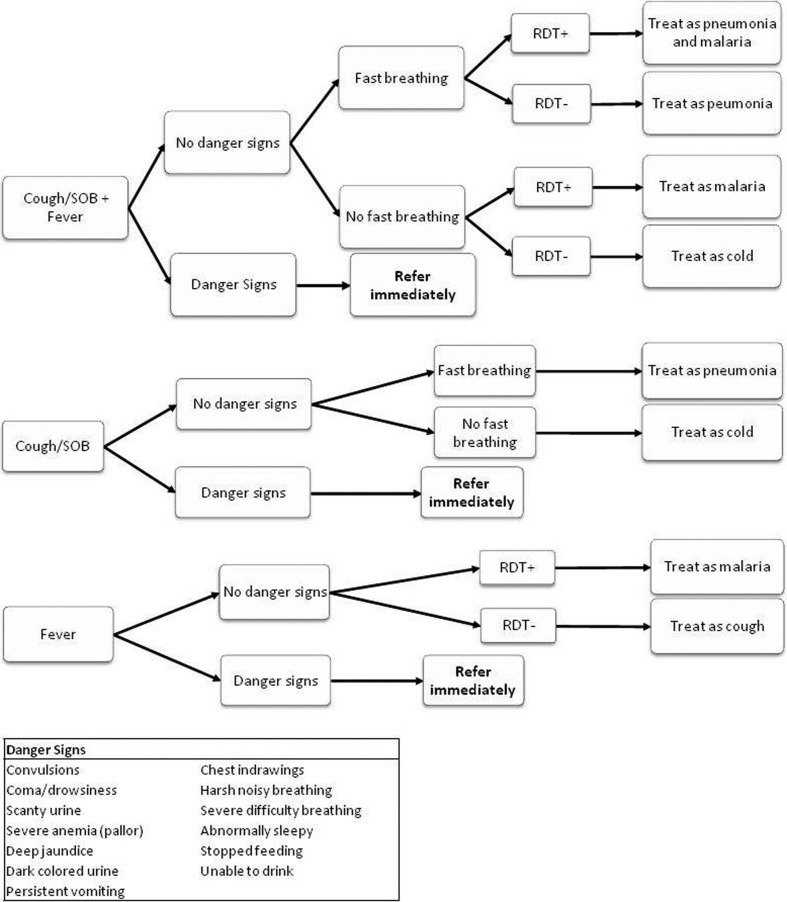
The CHWs participated in a 5-day training session at CMH during which they were taught to classify and treat febrile children with malaria and/or pneumonia according to a simple algorithm (Fig. 1). The details of the training have been described.15 The first step in the algorithm was for the CHW to identify children with danger signs (e.g. convulsions, coma/drowsiness, scanty urine output, pallor suggestive of severe anaemia, deep jaundice, dark coloured urine, persistent vomiting, chest wall in-drawing, harsh noisy breathing, severe difficulty breathing, abnormally sleepy, not feeding, and inability to drink) and refer them to the nearest rural health centre. Training also covered guidelines for the administration of AL and amoxicillin based on child weight or age.16,17 CHWs were shown how to manage and document drug and supply stocks, maintain a modified patient register, and complete data collection forms. The CHWs received an additional half-day of RDT training focused on using the ICT Malaria Pf RDT (ICT Diagnostics, Cape Town, South Africa). The training emphasized treating only RDT-positive cases with AL and giving paracetamol (100 mg) to RDT-negative children. Infection control measures such as maintenance of aseptic technique, proper biohazardous waste disposal, and avoidance of lancet injuries were also taught. CHWs were given a highly pictorial job aide detailing performance and interpretation of RDTs and disposal of biohazardous waste.
Figure 1.
Integrated classification and treatment algorithm for children aged from 6 months to 5 years presenting with fever and/or cough/difficulty breathing.
One month after initial training, the training team completed a follow-up skills assessment to ensure that CHWs retained necessary skills. After 6 months, all study CHWs completed a 2-day refresher course at CMH.
To support and supervise CHWs during the study and manage referrals by CHWs, rural health centre staff members without prior integrated management of childhood illness training were given full integrated management of childhood illness training and how to conduct performance assessment of CHWs and review CHW registers.
Enrolment and data collection
All children between 6 months and 5 years presenting to CHWs with complaints of fever, difficulty breathing, or cough were enrolled unless they demonstrated danger signs, i.e. signs of severe disease, in which case they were referred to the nearest rural health centre. The CHW asked the child’s caregiver about specific complaints, when the signs of infection appeared, and medication history. They did a basic examination including counting respiratory rate (RR) and measuring temperature and weight. A RDT was performed, following which the CHW classified the child as having malaria, malaria and pneumonia, pneumonia, or RDT-negative fever. RR of ⩾50 breaths per minute in children <12 months and ⩾40 breaths per minute in children ⩾12 months was considered elevated and suggestive of pneumonia. Children were classified with malaria, if the RDT result was positive and the RR was normal; with malaria and pneumonia, if the RDT result was positive and the RR was increased; with pneumonia, if the RDT result was negative and the RR was high; or with RDT-negative fever, if the RDT result was negative and RR was normal.
Children classified with malaria who weighed 5–15 kg or were 6 months to 3 years old were treated with six tablets of AL (20 mg artemether/120 mg lumefantrine; Coartem®, Novartis or Lumet; Cipla Ltd, Mumbai, India), including an initial CHW-administered dose, followed by caregiver-administered doses 8 hours later and then twice daily for 2 days. Children weighing >15 kg or aged >3 years to 5 years were administered two tablets twice a day for 3 days according to the same schedule. For pneumonia treatment, one half tablet of amoxicillin (250 mg) (Sparsh Bio-tech Pvt. Ltd, Jamnagar, India) was administered three times a day for 5 days to infants weighing 4–9 kg or aged 6–11 months. Children aged 12 months to 5 years received a full tablet of amoxicillin three times daily for 5 days. Children classified as RDT-negative fever were treated with 100 mg of panadol (acetaminophen) three times daily for 2–3 days.
The CHWs maintained a register where all patients seen were documented. In addition, the CHWs completed a baseline form for each child enrolled. The information documented on the baseline form included the presence or absence of fever, cough, difficult breathing, fast breathing, diarrhoea, vomiting, and convulsions, and when the first symptom of the illness started. The findings of the basic examination including axillary temperature, weight, and RR, as well as the RDT results, illness classification, and treatment prescribed. The CHWs also maintained a stock and dispensing record, which included a detailed record of drugs received, drugs dispensed daily, and a monthly reconciliation of drugs dispensed and issued.
CHWs visited rural health centres monthly for a performance assessment and to help with clinic work. The health centre manager reviewed the CHW’s records of consultations, referrals, illness classification, medications provided, and drug supply documentation. CHWs were observed attending to patients and performing RDTs. The health centre manager documented and kept a monthly record of the performance of all the CHWs affiliated with the centre. The record summarized the performance of the CHWs as ‘satisfactory’ if (1) illness classification was correct, (2) medications provided were correct and (3) they followed the steps in perfoming RTDs. If any of these three actions was not performed correctly the performace was adjudged ‘unsatisfactory’. Performance assessments aimed to ensure that CHWs maintained the skills required to manage patients via study treatment algorithms. The health centre manager provided any CHW with a ‘not satisfactory’ performance with remedial instruction.
Trained data collectors visited each CHW every other day to collect baseline forms which were used to assess the CHW’s adherence to the treatment algorithm and guidelines. The data collectors visited the child’s caregiver once on days 5–7 after the CHW visit, assessed the outcome of the child’s treatment, and asked about any symptoms that suggested an adverse reaction from the RDT or use of the prescribed medication. The data collectors also visited each CHW monthly and collected data on number of patients seen including number of children (6 months to 5 years), number of children who had presented with fever or cough/difficult breathing/fast breathing, and whether they were classified as having malaria, pneumonia, both or neither. The monthly data also captured information on the drugs (AL, amoxicillin) that were received and used; RDTs that were received and used; and whether there was a documented self-finger prick during the course of performing the RDT. In addition, the data collectors visited the health centres and collected reports on the performance assessment of the CHWs carried out by the health centre managers.
Data entry and analysis
A data manager oversaw all data collection and double entry of data by staff into CS Pro (US Census Bureau, Washington, DC, USA). Descriptive statistics were performed with SAS v. 9.1.3 (SAS Institute, Cary, NC, USA). For categorical variables, we calculated percentages, means, and interquartile range for continuous variables.
Ethical clearance
Ethical approval was obtained from Boston University’s Institutional Review Board and the University of Zambia Research Ethics Committee. Informed consent was obtained from both CHWs and caregivers.
Results
Baseline CHW characteristics
Baseline characteristics of CHWs are shown in Table 1. Most CHWs were men and had completed secondary school education. They were experienced, having practiced for an average of 7.0 years, though nearly three-quarters worked half-time or less as CHWs. Besides the provision of care for minor illnesses to community members, most CHWs were involved in disease prevention, health education, community sensitization, and provision of outreach services. Nearly all CHWs carried out home visits (17/18, 94%), primarily for patients with chronic disease, tuberculosis, and severe illness, usually on the day following the CHW’s initial evaluation.
Table 1. Baseline characteristics of community health workers (CHWs).
| Characteristic | N = 18% (range) | ||
| Mean age (years) | 41.5 (32–48) | ||
| Gender (male) | 83.3 | ||
| Marital status | |||
| Single | 11.1 | ||
| Married | 88.9 | ||
| Education (highest level attained) | |||
| Primary | 27.8 | ||
| Secondary | 72.2 | ||
| Proportion time spent on CHW work | |||
| Quarter | 5.6 | ||
| Half | 66.7 | ||
| Three-quarters | 22.2 | ||
| Full | 5.6 | ||
| Mean years as practicing CHW | 7.0 (3–19) | ||
| Source of initial CHW training | |||
| DHMT | 11.1 | ||
| CMH | 55.6 | ||
| Other NGO | 33.3 | ||
| Mean number of refresher courses | 3 (1–7) | ||
| Last refresher course attended | |||
| Less than 1 year ago | 55.6 | ||
| 1–2 years ago | 5.6 | ||
| More than 2 years ago | 22.2 | ||
| No refresher course | 16.7 | ||
| Mean RHC visits in past 12 months | 2 (0–6) | ||
| Most recent RHC supervisory visit | |||
| Less than 6 months | 44.4 | ||
| 6–12 months | 11.1 | ||
| No supervisory visit in past 12 months | 44.4 | ||
| Mean CMH staff visits in past 12 months | 0 | ||
Note: DHMT, District Health Management Team; RHC, Rural Health Centre; CMH, Chikankata Mission Hospital.
CHWs received medical kits from the DHMT through the nearest health centre (11/18, 61%) or CMH (7/18, 39%). The baseline survey revealed inadequate distribution of supplies to CHWs, as 72.2% of intervention CHWs had not received a CHW kit in the 6 months before study initiation. Slightly less than one-half of the CHWs had a supervisory visit in the last 6 months and one-half had attended a refresher course in the year before the intervention’s initiation. One-half owned a bicycle, which typically had been (7/9, 78%) donated by a non-governmental organization. Few CHWs received payment in kind (3/18, 17%), cash (1/18, 6%), or both (1/18, 6%) for their services; one-half of these payments were provided once a year. Most CHWs indicated that they were highly satisfied (5/18, 37%) or satisfied 11/18, 61%) with their CHW jobs.
Quality of illness classification
RDTs were performed for 95.9% (975/1017) of children who were enrolled. RDTs were not done for 42 children because they presented in the evening when there was insufficient ambient light for the CHW to perform and interpret the RDT results (6) or they did not report fever (36). Two of the 36 children, who did not report fever, had a temperature >37.5°C. RR was not counted for 1.2% (12/1017) of all children enrolled. CHWs followed the classification algorithm well (Table 2). CHWs had some difficulty in correctly classifying children who presented with both fever and cough/difficulty breathing. About 6% of children who were RDT-negative with rapid RR were incorrectly classified. All other conditions were correctly classified with 99–100% accuracy. Two children classified as having malaria were RDT-negative.
Table 2. Appropriateness of illness classification by community health workers based on rapid diagnostic tests (RDTs) and respiratory rate assessment.
| RDT results or clinical feature* | Expected (correct) classification | Appropriate CHW classification, N (%) |
| RDT-positive | Malaria or both malaria and pneumonia | 270/271 (99.6%) |
| RDT-negative | Pneumonia or RDT-negative fever | 702/704 (99.7%) |
| Presence of fast breathing | Pneumonia or both malaria and pneumonia | 362/378 (98.3%) |
| Absence of fast breathing | Malaria or RDT-negative fever | 627/627 (100%) |
| RDT-positive and presence of fast breathing | Both malaria and pneumonia | 100/103 (97.1%) |
| RDT-positive and absence of fast breathing | Malaria | 162/162 (100%) |
| RDT-negative and presence of fast breathing | Pneumonia | 223/239 (93.3%) |
| RDT-negative and absence of fast breathing | RDT-negative fever | 460/460 (100%) |
Note: RDT, rapid diagnostic test; CHW, community health worker.
*RDT was done for 975 of 1017 children; RR counted for 1005 of 1017; and both RDT done and RR counted in 964 of 1017.
Quality of prescription of treatment
CHWs correctly prescribed treatment based on their illness classification in 94–100% of children (Table 3) or based on RDT test results and presence or absence of fast breathing in 93–99.6% of the children (Table 4). They adhered to the treatment algorithm in 99.6% of negative RDT cases (701/704). Among children classified with both malaria and pneumonia, one child received sulphadoxine–pyrimethamine (SP) in addition to AL and amoxicillin; another child incorrectly received AL only; and five received amoxicillin alone. One child who received amoxicillin alone also received SP. For children who had positive RDTs and tachypnoea, seven were incorrectly treated: three received AL only and four received amoxicillin alone. All 21 children classified with RDT-negative fever who were not appropriately treated received amoxicillin. Of 1017 children seen with fever and/or cough/difficult breathing, five received SP and one was given cotrimoxazole.
Table 3. Appropriate prescription (treatment) based on RDT results and presence of fast breathing.
| RDT results/fast breathing | Correct treatment | Appropriate treatment (%) |
| RDT-positive | AL | 267/271 (98.5%) |
| RDT-negative | No AL | 701/704 (99.6%) |
| RDT-positive and fast breathing | AL and amoxicillin | 96/103 (93.2%) |
| RDT-negative and fast breathing | Amoxicillin | 235/239 (98.3%) |
| Fast breathing | Amoxicillin | 371/378 (98.1%) |
| No fast breathing | No amoxicillin | 618/627 (98.6%) |
| RDT-negative and no fast breathing | No AL and no amoxicillin | 452/460 (98.3%) |
Table 4. Appropriate prescription (treatment) provided based on CHW diagnosis.
| Classification | Correct treatment | Appropriate treatment (%) |
| All malaria | AL | 267/272 (98.2%) |
| Malaria only | AL | 170/170 (100%) |
| All pneumonia | Amoxicillin | 358/362 (98.9%) |
| Pneumonia only | Amoxicillin | 257/260 (98.8%) |
| Malaria and pneumonia | AL and amoxicillin | 96/102 (94.1%) |
| RDT-negative fever | Analgesics or no treatment | 464/485 (95.7%) |
Note: AL, artemether–lumefantrine.
CHW performance review and supply/medication management
After training, instructor assessment indicated that all CHWs demonstrated competence in performing and interpreting RDTs, assessing RR, and following treatment algorithms. Monthly performance reviews at the rural health facility showed that the CHWs generally were able to correctly perform RDTs, classify illness, and prescribe treatment. Performance assessment scores ranged from a low of 84% in March 2008 to 100% in May–October with a mean score of 97%. Scores declined slightly in March–April and again in November 2008 (92%), 5–6 months after the initial training and refresher trainings.
Reviews of supply records showed that accountability was high. For both AL and amoxicillin, comparisons between supplies provided to CHWs and those remaining in their possession were 99.6%. For AL, 15 CHWs recorded 100% of all drugs used; for amoxicillin, 13 CHWs recorded 100% of drugs used. Similarly, monthly reviews showed full accounting for 98.9% of RDTs.
Safety
Adverse effects of study drugs and RDTs
AL and amoxicillin were associated with few adverse events. Days 5–7 follow-up data were available for 93.6% (248/267) of children prescribed AL and 95.5% (363/380) for amoxicillin. The most commonly reported side effects were loss of appetite (7.2%), diarrhoea (2.4%), vomiting (2.0%), rash (1.2%), and dizziness (1.2%) for AL and loss of appetite (5.8%), diarrhoea (1.7%), vomiting (1.4%), dizziness (1.4%), and rash (1.2%) for amoxicillin. Among the 96.3% (939/973) RDT-tested children available at the follow-up visit, minor side effects associated with the RDT skin prick described by caregivers were prolonged bleeding (bleeding longer than caregiver’s expectation) (1.5%), bruises (0.4%), and local skin infection (0.2%). One CHW accidentally pricked himself; no CHWs reported accidental blood exposure while performing RDTs.
Outcomes for children with negative RDTs
The majority of febrile children who had a negative RDT recovered on antipyretic (paracetamol) alone (631/704, 89.6%). Only 58 children (8.2%) remained ill (caregiver complained that the child was still unwell, febrile, or had temperature ⩾37.5°C) at the time of the days 5–7 home visit, but none had sought alternate care after visiting the CHW. Another 13 children recovered after seeking additional care; two remained ill although they sought additional care. Two children (2/1017, 0.2%) died during the course of the study between the time that they were evaluated by the CHW and the home visit by the study data collector. One was a 36-month-old girl with fever, diarrhoea, temperature 37.7°C, and RR 36 breaths/min. Since her RDT was negative, she was appropriately classified as RDT-negative fever and given antipyretics. The other was a 9-month-old female with fever, cough, difficult breathing, vomiting, diarrhoea, temperature 38°C, and RR 58 breaths/min. Her RDT was negative and she was appropriately classified as pneumonia and given amoxicillin. Since verbal autopsies were not performed, we could not determine the cause of death in these two children.
Discussion
Recognizing the potential contribution of CHWs to the Millennium Development Goal for child survival,18 we evaluated the ability of CHWs to provide high-quality, safe integrated care for malaria and pneumonia in two rural districts in Zambia. CHWs were able to correctly classify children using a simple algorithm, to provide appropriate treatment, and to manage both diagnostic and drug supplies. We found that integrated management of malaria and pneumonia using RDTs could be provided safely at the community level. Notably, only 10.4% of children with a history of fever and a negative RDT result did not recover by days 5–7 after receiving an antipyretic alone. Based on maternal reports, there were few medication side effects and only minor, transient adverse events associated with CHWs’ use of RDTs. CHWs were able to handle RDTs without significant risks to themselves or their patients.
Cross-sectional and randomized controlled trials have demonstrated that health-care workers at primary health facilities often do not adhere to the results of RDTs.9,19,20 In many African countries, a clinical dogma persists that blood smear-negative results should be treated as probable malaria.21,22 CHWs in our study showed a greater ability to follow guidelines and to honour RDT results than facility-based health care workers. Our findings conform with several recent studies which have demonstrated the ability of CHWs to correctly perform RDTs11 and appropriately prescribe antimalarials for RDT-negative patients.6 However, a recent study in the Sudan found that community volunteers prescribed artemisinin-based combination therapy in 30% of subjects with fever and a negative RDT result,5 indicating that this challenge persists.
Along with studies conducted in Benin, Tanzania, Uganda, and Zanzibar,14,23–25 our study provides further evidence that febrile RDT-negative children can be managed safely without antimalarial therapy. However, in contrast to our study, where, based on the CHW classification of illness, few children were given antibiotics, more than 90% of febrile children in the Tanzanian study were prescribed antibiotics.23 These findings provide additional confirmation that the WHO’s new guidelines for malaria treatment26 which recommend treatment based on a positive diagnostic test for all patients, including children under 5 years old, can be safely implemented at the community level in malaria-endemic areas of sub-Saharan Africa.
We believe that several aspects of the training and logistics in the context of our study strengthened the ability of the CHWs to appropriately perform RDTs and adhere to test results. First, CHWs were trained to provide an antipyretic to children with negative RDT results, an approach that likely contributed to clinical improvement of the child and satisfaction on the part of their caregiver, because something was done for the child. Second, the simplicity of the treatment algorithm, along with the explicitness of the treatment chart, provided clear guidance to CHWs. Finally, the attention to supply chain management, high quality of training and attention to supervision strengthened the capacity of the CHWs to provide integrated community case management.
This study was designed to be closely integrated into Zambia’s existing health-care system. For example, the government of Zambia procured all study medications and supplies (e.g. RDTs). This drug and supply distribution functioned relatively smoothly. However, we needed to develop an effective approach to distribute supplies from DHMTs to the CHWs, suggesting that this type of program may require drug distribution strengthening at lower levels of the health system. The success of supply management in the present study was also likely due to the transparency provided by well-organized recordkeeping by CHWs and the engagement of study supervisors to ensure adequate supplies. To strengthen stock management, daily registers and periodic reconciliation of stocks could be performed to assess commodity use and ensure that none have passed their expiration dates. Based on our review of the CHW registers, we found that they were able to carefully manage supplies (RDTs) and medications (AL, amoxicillin) with no evidence of inappropriate use.
There were several study limitations. First, the assessment of the ability of CHWs to appropriately classify illness and treat was based on a review of their documentation and not actual observations of their evaluations of sick children. However, their capacity to correctly classify and treat children evaluated by the health centre manager at the monthly performance assessment was consistent with review of documents. Second, the study data collectors saw children on only one occasion 5–7 days after the CHW evaluation. Consequently, interim events (which would have helped to clarify why two children died) and subsequent outcomes were not determined. Thus, we lack longer-term follow-up data for the 10.4% of RDT-negative children who had not fully recovered by the time of the data collector visit. We also did not perform verbal autopsies so the cause of death could not be elucidated. Third, blood smears were not performed at the time of the initial evaluation or the days 5–7 follow-up visit, so we were unable to evaluate the effectiveness of the antimalarial therapy provided. We also do not have longer follow-up on the small group of RDT-negative children (8.2%) who had not clinically improved at the time of the days 5–7 visit. The caregivers for these children were advised to take them to the local rural health centre for evaluation, but due to our study design, final outcomes for this group are not available. Fourth, children referred for severe disease were not followed, so we lack outcome data for this group, including whether they complied with the recommended referral. Fifth, we did not assess the ability of CHWs to differentiate children with signs of severe disease from those with non-severe illnesses that they themselves treated. Finally, we did not track the dose of AL or amoxicillin prescribed and were unable to determine whether age- or weight-appropriate dosing was performed.
Conclusions
In summary, we have demonstrated that with effective training, a steady supply chain, and adequate supervision, CHWs are able to appropriately classify and treat malaria and pneumonia at the community level, and that this can be done safely even when children with RDT-negative febrile syndromes are provided only antipyretics. Training and supervision should make sure that CHWs are able to appropriately classify children with symptoms and signs suggestive of both malaria and pneumonia.
Acknowledgments
We thank the caregivers and their children who took part in the trial, and the staff at the rural health centres and Chikankata Mission Hospital. We would also like to acknowledge support from the Siavonga and Mazabuka District Health Management Teams. The study was funded by the Child and Family Applied Research Project at Boston University by means of a cooperative agreement (GHS-A-00-03-00020-00) with the United States Agency for International Development (USAID) and the President’s Malaria Initiative. The opinions expressed herein are those of the authors and do not necessarily reflect the views of USAID.
References
- 1.Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, Bassani DG, et al. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375:1969–87. doi: 10.1016/S0140-6736(10)60549-1. [DOI] [PubMed] [Google Scholar]
- 2.Kinney MV, Kerber KJ, Black RE, Cohen B, Nkrumah F, Coovadia H, et al. Sub-Saharan Africa’s mothers, newborns, and children: where and why do they die? PLoS Med. 2010;7:e1000294. doi: 10.1371/journal.pmed.1000294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.United Nations. The millenium development goals report 2009. New York: United Nations. 2009 [Google Scholar]
- 4.Marsh DR, Gilroy KE, van de Weert R, Wansi E, Qazi S. Community case management of pneumonia: at a tipping point? Bull World Health Org. 2008;86:381–9. doi: 10.2471/BLT.07.048462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elmardi KA, Malik EM, Abdelgadir T, Ali SH, Elsyed AH, Mudather MA, et al. Feasibility and acceptability of home-based management of malaria strategy adapted to Sudan’s conditions using artemisinin-based combination therapy and rapid diagnostic test. Malar J. 2009;8:39. doi: 10.1186/1475-2875-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yasuoka J, Poudel KC, Poudel-Tandukar K, Nguon C, Ly P, Socheat D, et al. Assessing the quality of service of village malaria workers to strengthen community-based malaria control in Cambodia. Malar J. 2010;9:109. doi: 10.1186/1475-2875-9-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winch PJ, Gilroy KE, Wolfheim C, Starbuck ES, Young MW, Walker LD, et al. Intervention models for the management of children with signs of pneumonia or malaria by community health workers. Health Policy Plan. 2005;20:199–212. doi: 10.1093/heapol/czi027. [DOI] [PubMed] [Google Scholar]
- 8.D’Acremont V, Lengeler C, Mshinda H, Mtasiwa D, Tanner M, Genton B. Time to move from presumptive malaria treatment to laboratory-confirmed diagnosis and treatment in African children with fever. PLoS Med. 2009;6:e252. doi: 10.1371/journal.pmed.0050252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamer DH, Ndhlovu M, Zurovac D, Fox M, Yeboah-Antwi K, Chanda P, et al. Does improving coverage of parasitological diagnostic tests change malaria treatment practices? An operational cross-sectional study in Zambia. J Am Med Assoc. 2007;297:2227–31. [Google Scholar]
- 10.Hopkins H, Bebell L, Kambale W, Dokomajilar C, Rosenthal PJ, Dorsey G. Rapid diagnostic tests for malaria at sites of varying transmission intensity in Uganda. J Infect Dis. 2008;197:510–8. doi: 10.1086/526502. [DOI] [PubMed] [Google Scholar]
- 11.Harvey SA, Jennings L, Chinyama M, Masaninga F, Mulholland K, Bell D. Improving community health worker use of rapid diagnostic tests in Zambia: package instructions, job aid and job aid-plus-training. Malar J. 2008;7:160. doi: 10.1186/1475-2875-7-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mayxay M, Newton PN, Yeung S, Pongvongsa T, Phompida S, Phetsouvanh R, et al. short communication: an assessment of the use of malaria rapid tests by village health volunteers in rural Laos. Trop Med Int Health. 2004;9:325–9. doi: 10.1111/j.1365-3156.2004.01199.x. [DOI] [PubMed] [Google Scholar]
- 13.English M, Reyburn H, Goodman CA, Snow RW. Abandoning presumptive antimalarial treatment for febrile children aged less than five years — a case of running before we can walk? PLoS Med. 2009;6:e1000015. doi: 10.1371/journal.pmed.1000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Njama-Meya D, Clark TD, Nzarubara B, Staedke S, Kamya M, Dorsey G. Treatment of malaria restricted to laboratory confirmed cases: a prospective cohort study in Ugandan children. Malar J. 2007;6:7. doi: 10.1186/1475-2875-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yeboah-Antwi K, Pilangana P, MacLeod WB, Semrau K, Siazeele K, Kalesha P, et al. Community case management of fever due to malaria and pneumonia in children under five in Zambia: a cluster randomized controlled trial. PLoS Med. 2010;7:e1000340. doi: 10.1371/journal.pmed.1000340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ministry of Health, Zambia. Lusaka: Ministry of Health; 2007. Community health worker’s handbook: a reference manual for community health workers. 3rd edn. [Google Scholar]
- 17.USAID Quality Assurance Project. Bethesda, MD: University Research Corporation, LLC/Geneva: World Health Organization; 2008. How to use a rapid diagnostic test (RDT): a guide for training at a village and clinic level. [Google Scholar]
- 18.Haines A, Sanders D, Lehmann U, Rowe AK, Lawn JE, Jan S, et al. Achieving child survival goals: potential contribution of community health workers. Lancet. 2007;369:2121–31. doi: 10.1016/S0140-6736(07)60325-0. [DOI] [PubMed] [Google Scholar]
- 19.Bisoffi Z, Sirima BS, Angheben A, Lodesani C, Gobbi F, Tinto H, et al. Rapid malaria diagnostic tests vs. clinical management of malaria in rural Burkina Faso: safety and effect on clinical decisions. A randomized trial. Trop Med Int Health. 2009;14:1–8. doi: 10.1111/j.1365-3156.2009.02246.x. [DOI] [PubMed] [Google Scholar]
- 20.Reyburn H, Mbakilwa H, Mwangi R, Mwerinde O, Olomi R, Drakeley C, et al. Rapid diagnostic tests compared with malaria microscopy for guiding outpatient treatment of febrile illness in Tanzania: randomised trial. Br Med J. 2007;334:403. doi: 10.1136/bmj.39073.496829.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amexo M, Tolhurst R, Barnish G, Bates I. Malaria misdiagnosis: effects on the poor and vulnerable. Lancet. 2004;364:1896–8. doi: 10.1016/S0140-6736(04)17446-1. [DOI] [PubMed] [Google Scholar]
- 22.Zurovac D, Midia B, Ochola SA, English M, Snow RW. Microscopy and outpatient malaria case management among older children and adults in Kenya. Trop Med Int Health. 2006;11:432–40. doi: 10.1111/j.1365-3156.2006.01587.x. [DOI] [PubMed] [Google Scholar]
- 23.D’Acremont V, Malila A, Swai N, Tillya R, Kahama-Maro J, Lengeler C, et al. Withholding antimalarials in febrile children who have a negative result for a rapid diagnostic test. Clin Infect Dis. 2010;51:506–11. doi: 10.1086/655688. [DOI] [PubMed] [Google Scholar]
- 24.Faucher JF, Makoutode P, Abiou G, Béhéton T, Houzé P, Ouendo E, et al. Can treatment of malaria be restricted to parasitologically confirmed malaria? A school-based study in Benin in children with and without fever. Malar J. 2010;9:104. doi: 10.1186/1475-2875-9-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Msellem MI, Martensson A, Rotllant G, Bhattarai A, Stromberg J, Kahigwa E, et al. Influence of rapid malaria diagnostic tests on treatment and health outcome in fever patients, Zanzibar — a crossover validation study. PLoS Med. 2009;6:e1000070. doi: 10.1371/journal.pmed.1000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.World Health Organization. Guidelines for the treatment of malaria 2nd ed.Geneva: World Health Organization; 2010 [Google Scholar]