Abstract
In a wide array of kidney diseases, type 1 angiotensin (AT1) receptors are present on the immune cells that infiltrate the renal interstitium. Here, we examined the actions of AT1 receptors on macrophages in progressive renal fibrosis and found that macrophage-specific AT1 receptor deficiency exacerbates kidney fibrosis induced by unilateral ureteral obstruction (UUO). Macrophages isolated from obstructed kidneys of mice lacking AT1 receptors solely on macrophages had heightened expression of proinflammatory M1 cytokines, including IL-1. Evaluation of isolated AT1 receptor–deficient macrophages confirmed the propensity of these cells to produce exaggerated levels of M1 cytokines, which led to more severe renal epithelial cell damage via IL-1 receptor activation in coculture compared with WT macrophages. A murine kidney crosstransplantation concomitant with UUO model revealed that augmentation of renal fibrosis instigated by AT1 receptor–deficient macrophages is mediated by IL-1 receptor stimulation in the kidney. This study indicates that a key role of AT1 receptors on macrophages is to protect the kidney from fibrosis by limiting activation of IL-1 receptors in the kidney.
Introduction
Chronic kidney disease (CKD) constitutes a major public health concern. Hematopoietic cells infiltrate the injured kidney and modulate the progression of immune-mediated glomerulonephritides and even “nonimmune” forms of kidney damage related to hypertension and ischemia-reperfusion (1–3). Among BM-derived cell lineages, macrophages play a prominent role in regulating kidney fibrosis (4), and the extent of fibrosis predicts organ failure in chronic diseases of the kidney and other tissues (5).
After infiltrating the kidney, macrophages can promote inflammatory injury or direct tissue repair depending on their phenotype, summarized in a dichotomous “M1 versus M2” paradigm (6). In this vernacular, proinflammatory M1 macrophages induce damage by secreting cytokines such as TNF-α and IL-1β, whereas reparative M2 macrophages secrete the decoy IL-1 receptor (IL-1R2) and other immunosuppressive mediators. However, the mechanisms through which key drivers of kidney fibrosis contribute to macrophage polarization require elucidation.
As the principal effector molecule of the renin-angiotensin system (RAS), Ang II is a fundamental driver of renal fibrosis (7). Accordingly, treatment with type 1 angiotensin (AT1) receptor blockers has achieved considerable success in managing CKD patients (8). Ang II provokes accumulation of macrophages in the kidney (9), and macrophages express all components of the RAS including AT1 receptors (10, 11). However, previous experiments have not determined the functions of AT1 receptors specifically on macrophages in vivo.
In the current studies, we therefore generated “Macro KO” mice lacking AT1 receptors solely on myeloid cells (LysM-Cre+ Agtr1aflox/flox mice; Supplemental Paragraph 1 and Supplemental Figures 1–4; supplemental material available online with this article; doi: 10.1172/JCI61368DS1) to determine whether AT1 receptor activation on macrophages exacerbates CKD by promoting a proinflammatory M1 phenotype. We employed the unilateral ureteral obstruction–induced (UUO-induced) kidney fibrosis model of CKD because UUO stimulates the RAS and macrophages are critical to the pathogenesis of renal fibrosis (12, 13). However, we found that AT1 receptors on macrophages protect the kidney from fibrosis and suppress M1 cytokine production.
Results and Discussion
AT1A receptor deficiency on macrophages exacerbates kidney fibrosis.
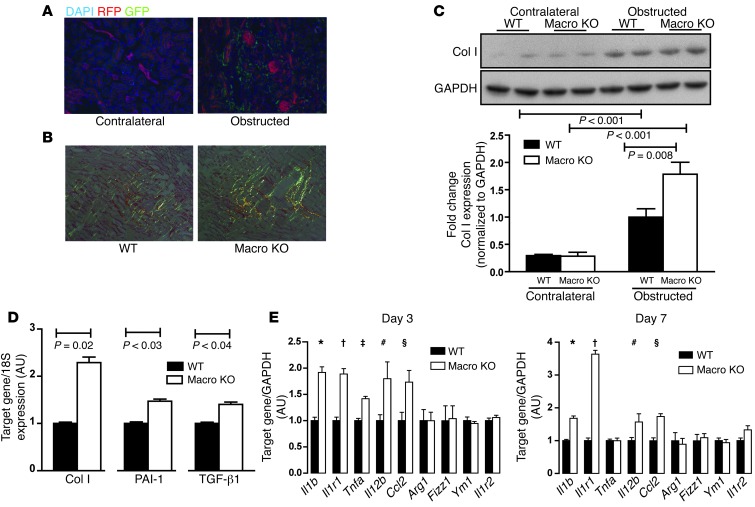
To examine infiltration of LysM-expressing macrophages in the kidney during renal fibrosis, we subjected LysM-Cre+ mT/mG reporter mice to UUO. By day 7 of UUO, robust accumulation of GFP+ macrophages was detected in the interstitial areas of obstructed kidneys, but not the contralateral, unobstructed kidneys (Figure 1A), confirming that LysM-Cre–mediated gene excision affects macrophages infiltrating the diseased kidney. To evaluate the actions of AT1 receptors on macrophages that infiltrate the kidney undergoing fibrosis, we harvested kidneys from mice with macrophage-specific deletion of Agtr1a (LysM-Cre+ Agtr1aflox/flox, herein referred to as Macro KO) and WT mice 7 days following UUO. Obstructed kidneys from Macro KO mice contained 64% more interstitial fibrosis (Figure 1B and Supplemental Figure 5), 80% more collagen-producing myofibroblasts (Supplemental Figure 6), and 80% more collagen I (Col I) protein (Figure 1C) than in WT mice. Moreover, at day 7 after UUO, mRNA expressions of Col I, PAI-1, and TGF-β1 were enhanced by 129%, 47%, and 40%, respectively, in Macro KO kidneys compared with WTs (Figure 1D). Additional experiments further confirmed our results (Supplemental Paragraph 2 and Supplemental Figures 7 and 8). These findings contrast starkly with studies in which global AT1A receptor deficiency ameliorates kidney fibrosis (14), but align with reports of exaggerated tissue damage in AT1A receptor–deficient BM chimeras (15, 16). Collectively, our data indicate that activation of AT1 receptors on infiltrating macrophages protects the kidney from progressive fibrosis.
Figure 1. AT1A receptor deficiency on macrophages exacerbates kidney fibrosis induced by UUO.
(A) Representative kidney sections from LysM-Cre+ mT/mG mice 7 days after UUO with contralateral unobstructed kidney on left and obstructed kidney on right. (B) Polarized images of representative sections from obstructed WT and Macro KO kidneys stained with picrosirius red for collagen fibrils at day 7 after UUO. Original magnification, ×20. (C) Western blot for Col I in whole kidney at day 7 after UUO. (D) mRNA expression of ColI, Pai1, and Tgfb1 in obstructed kidney at day 7 after UUO. (E) Gene expression of M1 markers Il1b, Il1r1, Tnfa, Il12b, and Ccl2 and M2 markers Arg1, Fizz1, Ym1, and Il1r2 in obstructed kidneys at 3 and 7 days after UUO. At day 3, *P < 0.02 vs. WT, †P = 0.02 vs. WT, ‡P = 0.01 vs. WT, #P = 0.03, §P < 0.02. At day 7, *P < 0.03 vs. WT, †P = 0.02 vs. WT, #P = 0.05, §P < 0.001.
The heterogeneity of macrophage polarization plays a critical role in the pathogenesis of kidney damage and fibrosis (6). In simplified terms, the balance between proinflammatory M1 and reparative M2 macrophages determines the local composition of cytokine reservoirs that orchestrate disruptions in renal architecture following an insult. Therefore, on days 3 and 7 after UUO, we profiled the renal expression of M1 and M2 markers, including M1 cytokines IL-1β and TNF-α, which have been implicated in the progression of renal fibrosis (Figure 1E). Obstructed Macro KO kidneys had significantly higher expression of M1 markers, including IL-1β, IL-1R1, TNF-α, IL-12p40, and CCL2, compared with obstructed WT kidneys 3 days after UUO, whereas expression of M2 markers Arg-1, FIZZ-1, YM-1, and IL-1R2 was virtually identical between cohorts. A similar pattern was noted at day 7 after UUO (Figure 1E), suggesting that activation of AT1 receptors on macrophages represses M1 cytokine expression during kidney fibrosis.
AT1A receptors on macrophages limit their expression of M1 cytokines.
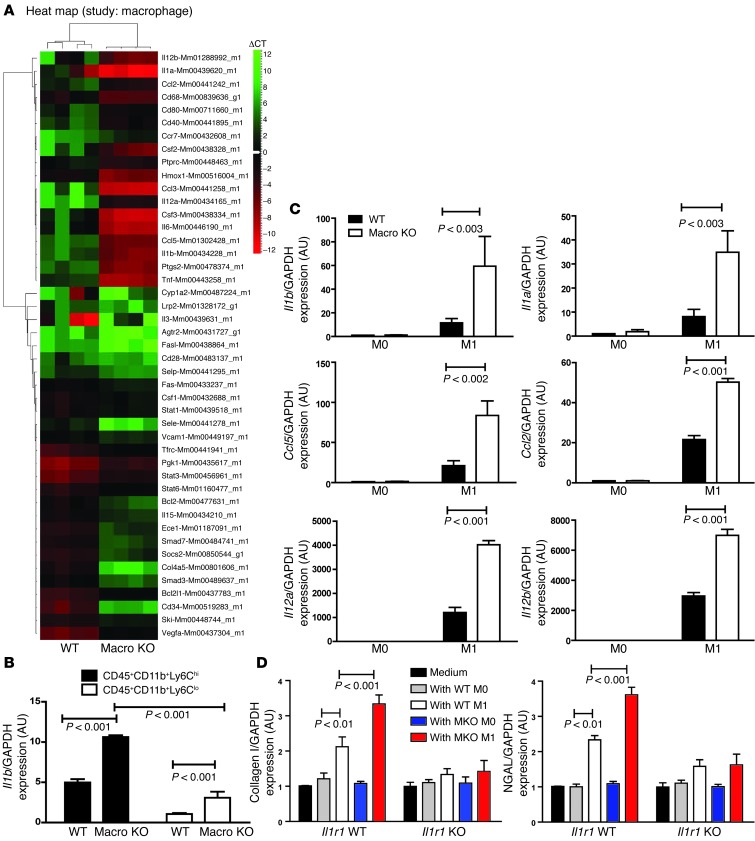
We next examined the effects of macrophage AT1 receptor activation on the phenotype of infiltrating macrophages at day 7 after UUO by histologic staining (Supplemental Paragraph 3 and Supplemental Figures 9 and 10) and, more precisely, by isolating CD45+CD11b+Ly6Chi and CD45+CD11b+Ly6Clo macrophages directly from the obstructed kidney (Supplemental Figure 11). Based on reports that CD45+CD11b+Ly6Chi macrophages promote tissue damage and fibrosis (4, 17), TaqMan low-density array (TLDA) card–based microarray was performed to survey gene expression for inflammatory mediators and signaling molecules in the Ly6Chi-activated macrophages (Figure 2A and Supplemental Table 1). Among the upregulated genes, IL-1β but not TNF-α showed enhanced expression in Macro KO kidneys at both days 3 and 7 of UUO (Figure 1E). Moreover, by real-time PCR, Ly6Chi and Ly6Clo macrophages from the Macro KO kidneys had higher IL-1β expression levels than found in WT controls (Figure 2B). These data indicate that AT1A receptor activation constrains infiltrating macrophage activation by suppressing key inflammatory cytokines including IL-1 during kidney fibrosis.
Figure 2. AT1A receptor activation on macrophages suppresses their M1 proinflammatory polarization.
(A) Heat map showing hierarchical cluster analysis of ΔCT values of CD11b+Ly6Chi macrophages isolated from obstructed WT and Macro KO kidneys. (B) mRNA expression of IL-1β measured by real-time RT-PCR in Ly6Chi and Ly6Clo macrophages isolated from obstructed kidneys. (C) mRNA levels of proinflammatory cytokines Il1b, Il1a, Ccl5, Ccl2, Il12a, and Il12b in WT and Macro KO peritoneal macrophages following M1 stimulation. (D) ColI and Ngal gene expression in WT (Il1r1 WT) or IL-1R–deficient (Il1r1 KO) renal tubular epithelial cells cocultured with M0- or M1-activated macrophages from WT or Macro KO (MKO) animals.
To explore in vitro the role of the macrophage AT1A receptor in regulating macrophage activation, we cultured Macro KO and WT peritoneal macrophages and subjected them to M1- or M2-polarizing stimuli or vehicle (M0). M1 stimulation increased mRNA expression of M1 markers in both groups, confirming M1 polarization (Figure 2C). However, M1 macrophages lacking the AT1A receptor showed exaggerated expression levels for each marker. These findings together with M2 polarization studies (Supplemental Paragraph 4 and Supplemental Figures 12 and 13) suggest that AT1A receptor activation during M1 differentiation constrains M1 cytokine production without altering the macrophage’s susceptibility to M2 differentiation.
Enhanced IL-1 production by AT1 receptor–deficient macrophages drives renal cell injury in coculture.
Proinflammatory cytokines including IL-1β have been implicated in renal tubular cell (RTC) damage (18). We therefore hypothesized that exaggerated production of inflammatory cytokines by AT1A receptor–deficient macrophages could alter gene expression programs for injury within the kidney tubular cell. Testing this hypothesis in vitro, we measured expression of Col I and kidney injury marker neutrophil gelatinase–associated lipocalin (NGAL) in RTCs following coculture with M0- or M1-conditioned macrophages from our experimental animals (Figure 2D). At 6 hours, M1 macrophages from Macro WT or KO mice, but not M0 macrophages, upregulated Col I and Ngal mRNA expression in RTCs. Thus, cytokines from activated macrophages can mediate damage to kidney tubular cells without direct contact. Moreover, compared with WT M1 macrophages, M1 macrophages lacking AT1A receptors induced an even more profound increase in RTC expression of Col I and NGAL (Figure 2D). To explore whether AT1 receptor–deficient macrophages mediate this exaggerated tubular cell damage through enhanced IL-1 production acting on the renal cell IL-1 receptor (IL-1R1), we repeated our coculture experiments with Il1r1-deficient (KO) RTCs (Figure 2D). In Il1r1 KO RTCs, coincubation with M1-conditioned macrophages from WT or Macro KO animals induced severely blunted and equivalent increases in Col I and NGAL expression. Finally, recombinant IL-1β induced robust expression of Col I and NGAL in WT but not Il1r1 KO RTCs (Supplemental Figure 14). Thus, activation of the IL-1R on kidney cells by IL-1 secreted from activated macrophages triggers RTC damage. We therefore posited that AT1 receptor activation on infiltrating macrophages limits their IL-1 production, which in turn suppresses gene expression programs for injury in kidney parenchymal cells by preventing renal IL-1R stimulation.
The kidney crosstransplant UUO model.
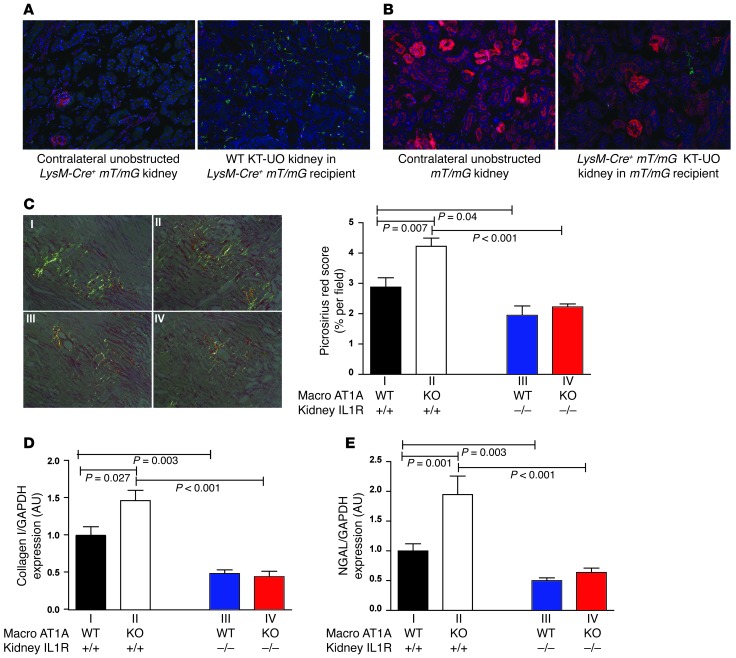
Testing this possibility in vivo requires a system that can separate the contribution of AT1 receptor activation on macrophages from the downstream effects of renal IL-1R activation during UUO-mediated renal fibrosis. We therefore developed a kidney transplantation–ureteral obstruction (KT-UO) model (Supplemental Paragraph 5 and Supplemental Figure 15). To examine the infiltration of macrophages from the kidney recipient into the donor KT-UO kidney, we transplanted a nonfluorescent WT kidney into a LysM-Cre+mT/mG recipient mouse in which green GFP signals present in the KT-UO kidney represented macrophages from the kidney recipient, and red fluorescent protein (RFP) signals represented other BM-derived cells from the recipient not of the myeloid lineage. Seven days following KT-UO, robust GFP signals that mark infiltrating macrophages were evident in the KT-UO kidneys along with a far less prominent infiltrating population of red-fluorescing nonmyeloid cells (Figure 3A). Thus, the vast majority of BM-derived cells infiltrating the donor kidney in the KT-UO model are macrophages. In contrast, the native unobstructed kidney in the LysM-Cre+mT/mG recipient contained only a rare GFP fluorescing macrophage (Figure 3A). Next, we transplanted a kidney from a LysM-Cre+mT/mG animal into a LysM-Cre–mT/mG recipient whose native unobstructed kidney fluoresces red due to the absence of Cre-mediated RFP excision (Figure 3B). Here, GFP signals in the KT-UO kidney represented macrophages resident in the transplanted kidney that could constitute an alternative source of inflammatory cytokines. However, only sparse GFP signals were detected in LysM-Cre+mT/mG KT-UO kidney sections (Figure 3B). Thus, the overwhelming majority of macrophages present in the KT-UO kidney arrive from the circulation of the kidney recipient.
Figure 3. Activation of the kidney IL-1R mediates exaggerated renal damage induced by AT1A receptor–deficient macrophages.
(A) Right panel shows section of a WT kidney transplanted into a LysM-Cre+ mT/mG recipient and subjected to ureteral obstruction (KT-UO). Green fluorescence marks infiltrating LysM+ myeloid cells from recipient. Left panel shows section of an unobstructed native contralateral kidney from the same LysM-Cre+ mT/mG recipient. (B) Right panel shows section of a LysM-Cre+ mT/mG kidney transplanted into a non-Cre mT/mG recipient and subjected to ureteral obstruction. Green fluorescence marks LysM+ macrophages resident in KT-UO kidney. Left panel shows section of an unobstructed native contralateral kidney from the same non-Cre mT/mG recipient. Blue fluorescence is a nuclear DAPI stain. Original magnification, ×20. (C–E) Use of KT-UO model to dissect the contribution of WT vs. AT1 receptor–deficient macrophages to kidney damage mediated through renal IL-1R activation. WT (Macro AT1A WT) or Macro KO (KO) recipients were transplanted with an IL-1R WT or KO kidney subjected to UUO (groups I–IV as in Supplemental Table 2; n = 6–7 per group). (C) Representative polarized images of obstructed kidneys stained with picrosirius red at day 7 KT-UO. Original magnification, ×20. Quantitation is shown on the right. Excessive fibrosis due to AT1 receptor deficiency on macrophages in Macro KO recipient is abrogated in IL-1R–deficient donor kidneys. mRNA expression of (D) Col I and (E) NGAL in obstructed kidneys 7 days after KT-UO.
Activation of the AT1 receptor on infiltrating macrophages ameliorates kidney fibrosis by limiting renal IL-1R stimulation.
After validating our model, we transplanted Il1r1 WT or Il1r1 KO kidneys into Macro WT or Macro KO animals (Supplemental Paragraph 6 and Supplemental Table 2) to quantify the contribution of macrophage-generated IL-1 from the recipient to IL-1R–mediated fibrosis in the donor kidney. Seven days after KT-UO, the transplanted kidney in WT mice exhibited a moderate level of fibrosis similar to that seen earlier in nontransplanted WT mice (Figure 3C and Figure 1B). The extent of fibrosis in the KT-UO kidney from Macro KO Il1r1 WT mice (Figure 3C) exceeded that in the WT KT-UO kidney by approximately 50%, comparable to the discrepancy seen in the nontransplanted WT and Macro KO groups (Supplemental Figure 5), confirming that AT1A receptor deficiency on macrophages in the recipient mice exacerbates the severity of kidney fibrosis in the KT-UO model. The abrogation of IL-1R signaling in the KT-UO kidney of the Macro WT Il1r1 KO mice reduced the level of kidney fibrosis by a third compared with the WT group, implicating the renal IL-1R as a key mediator of kidney fibrosis in our model. IL-1R deletion in the KT-UO kidneys of Macro KO Il1r1 KO mice decreased the extent of kidney fibrosis by half compared with their Macro KO Il1r1 WT counterparts lacking AT1A receptors on macrophages, but expressing IL-1Rs in the KT-UO kidney, and down to levels seen in the Macro WT Il1r1 KOs (Figure 3C). Col I protein content in the KT-UO kidney (Supplemental Figure 16) mirrored the levels of fibrosis across the groups. Thus, enhanced activation of the kidney IL-1R accounts for the exaggerated renal fibrosis that accrues from AT1 receptor deficiency on macrophages. As seen earlier in the nontransplanted groups, renal macrophage infiltration in the Macro KO Il1r1 WT group was numerically but not significantly higher than in the WT group (Supplemental Figure 17). Levels of renal macrophage accumulation in the 2 groups lacking IL-1Rs on the KT-UO kidney were similar to each other, but significantly reduced compared with their counterparts with Il1r1 WT KT-UO kidneys, highlighting a role for renal IL-1R signals to recruit macrophages into the kidney undergoing fibrosis. Finally, Col I and NGAL expression (Figure 3, D and E) in the KT-UO kidneys followed a pattern strikingly similar to that of fibrosis across the transplant groups and corroborated our coculture findings.
Summary.
These experiments establish a mechanism through which AT1 receptors on macrophages function to protect the kidney from progressive fibrosis. Activation of AT1 receptors on infiltrating macrophages suppresses their release of the proinflammatory cytokine IL-1 and thereby prevents stimulation of the kidney IL-1R. We also establish a new KT-UO model that, in conjunction with conditional gene targeting, can serve as a tool for elucidating interactions between hematopoietic and kidney parenchymal cells. The ability of AT1 receptor activation on macrophages to mitigate kidney damage has broad implications for the design of potent therapies to overcome the shortcomings of global angiotensin receptor blockade related not only to previously recognized side effects but also to patently detrimental effects of blocking angiotensin receptors on hematopoietic cells.
Methods
Detailed Supplemental Methods are available online.
Statistics.
Data are expressed as mean ± SEM and analyzed by 2-tailed unpaired t test or ANOVA according to statistical methods described in detail in the Supplemental Methods. P < 0.05 was considered significant.
Study approval.
Mice were bred and maintained in the Association for Assessment and Accreditation of Laboratory Animal Care–accredited animal facilities at the Durham Veterans’ Affairs Medical Center (DVAMC) per NIH guidelines. The animal studies were approved by the DVAMC Institutional Animal Care and Use Committee and conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Supplementary Material
Acknowledgments
This work was supported by funding from the Medical Research Service of the Veterans Administration, NIH grant DK087893-03, the Edna and Fred L. Mandel Center for Hypertension and Atherosclerosis Research, the Duke O’Brien Center for Kidney Research, and an American Heart Association postdoctoral fellowship. M.A. Sparks is funded by Career Development Award BXIK2BX002240 from the Department of Veterans Affairs, Biomedical Laboratory Research and Development Service. Some of the histologic analysis was carried out by the Animal Pathology core facility of the Duke School of Medicine and the Duke Cancer Institute.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Note regarding evaluation of this manuscript: Manuscripts authored by scientists associated with Duke University, The University of North Carolina at Chapel Hill, Duke-NUS, and the Sanford-Burnham Medical Research Institute are handled not by members of the editorial board but rather by the science editors, who consult with selected external editors and reviewers.
Citation for this article:J Clin Invest. 2014;124(5):2198–2203. doi:10.1172/JCI61368.
References
- 1.Muller DN, et al. Immunosuppressive treatment protects against angiotensin II-induced renal damage. Am J Pathol. 2002;161(5):1679–1693. doi: 10.1016/S0002-9440(10)64445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burne MJ, et al. Identification of the CD4(+) T cell as a major pathogenic factor in ischemic acute renal failure. J Clin Invest. 2001;108(9):1283–1290. doi: 10.1172/JCI200112080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee S, et al. Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol. 2011;22(2):317–326. doi: 10.1681/ASN.2009060615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin SL, Castano AP, Nowlin BT, Lupher ML, Jr, Duffield JS. Bone marrow Ly6Chigh monocytes are selectively recruited to injured kidney and differentiate into functionally distinct populations. J Immunol. 2009;183(10):6733–6743. doi: 10.4049/jimmunol.0901473. [DOI] [PubMed] [Google Scholar]
- 5.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18(7):1028–1040. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ricardo SD, van Goor H, Eddy AA. Macrophage diversity in renal injury and repair. J Clin Invest. 2008;118(11):3522–3530. doi: 10.1172/JCI36150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Border WA, Noble NA. Interactions of transforming growth factor-beta and angiotensin II in renal fibrosis. Hypertension. 1998;31(1 pt 2):181–188. doi: 10.1161/01.HYP.31.1.181. [DOI] [PubMed] [Google Scholar]
- 8.Brenner BM, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345(12):861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 9.Ozawa Y, Kobori H, Suzaki Y, Navar LG. Sustained renal interstitial macrophage infiltration following chronic angiotensin II infusions. Am J Physiol Renal Physiol. 2007;292(1):F330–F339. doi: 10.1152/ajprenal.00059.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nataraj C, et al. Angiotensin II regulates cellular immune responses through a calcineurin-dependent pathway. J Clin Invest. 1999;104(12):1693–1701. doi: 10.1172/JCI7451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jurewicz M, et al. Human T and Natural Killer cells possess a functional renin-angiotensin system: further mechanisms of angiotensin II-induced inflammation. J Am Soc Nephrol. 2007;18(4):1093–1102. doi: 10.1681/ASN.2006070707. [DOI] [PubMed] [Google Scholar]
- 12.Pimentel JL, Jr, Montero A, Wang S, Yosipiv I, el-Dahr S, Martinez-Maldonado M. Sequential changes in renal expression of renin-angiotensin system genes in acute unilateral ureteral obstruction. Kidney Int. 1995;48(4):1247–1253. doi: 10.1038/ki.1995.408. [DOI] [PubMed] [Google Scholar]
- 13.Kitagawa K, et al. Blockade of CCR2 ameliorates progressive fibrosis in kidney. Am J Pathol. 2004;165(1):237–246. doi: 10.1016/S0002-9440(10)63292-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Satoh M, et al. Renal interstitial fibrosis is reduced in angiotensin II type 1a receptor-deficient mice. J Am Soc Nephrol. 2001;12(2):317–325. doi: 10.1681/ASN.V122317. [DOI] [PubMed] [Google Scholar]
- 15.Nishida M, et al. Absence of angiotensin II type 1 receptor in bone marrow-derived cells is detrimental in the evolution of renal fibrosis. J Clin Invest. 2002;110(12):1859–1868. doi: 10.1172/JCI200215045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crowley SD, et al. A role for angiotensin II type 1 receptors on bone marrow-derived cells in the pathogenesis of angiotensin II-dependent hypertension. Hypertension. 2010;55(1):99–108. doi: 10.1161/HYPERTENSIONAHA.109.144964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramachandran P, et al. Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc Natl Acad Sci U S A. 2012;109(46):E3186–E3195. doi: 10.1073/pnas.1119964109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haq M, Norman J, Saba SR, Ramirez G, Rabb H. Role of IL-1 in renal ischemic reperfusion injury. J Am Soc Nephrol. 1998;9(4):614–619. doi: 10.1681/ASN.V94614. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.