Abstract
The majority of mammalian cells have nonmotile primary cilia on their surface that act as antenna-like sensory organelles. Genetic defects that result in ciliary dysfunction are associated with obesity in humans and rodents, which suggests that functional cilia are important for controlling energy balance. Here we demonstrated that neuronal cilia lengths were selectively reduced in hypothalami of obese mice with leptin deficiency and leptin resistance. Treatment of N1 hypothalamic neuron cells with leptin stimulated cilia assembly via inhibition of the tumor suppressors PTEN and glycogen synthase kinase 3β (GSK3β). Induction of short cilia in the hypothalamus of adult mice increased food intake and decreased energy expenditure, leading to a positive energy balance. Moreover, mice with short hypothalamic cilia exhibited attenuated anorectic responses to leptin, insulin, and glucose, which indicates that leptin-induced cilia assembly is essential for sensing these satiety signals by hypothalamic neurons. These data suggest that leptin governs the sensitivity of hypothalamic neurons to metabolic signals by controlling the length of the cell’s antenna.
Introduction
Most mammalian cells have single nonmotile primary cilia, which were once thought to be vestigial but are now considered to be important signaling centers (1, 2). Accumulating evidence suggests a strong association between genetic ciliopathies and obesity in humans and animals (3). Obesity is a common manifestation observed in human genetic ciliopathies such as Bardet-Biedl syndrome (BBS) and Alström syndrome (4). Mice that lack BBS proteins and ciliary proteins such as the kinesin-2 subunit KIF3A and the intraflagellar transport protein 88 homolog (IFT88) are obese and hyperphagic (5–7). Furthermore, selective depletion of KIF3A in neurons, particularly in anorexigenic proopiomelanocortin-producing (POMC-producing) neurons, yields an obese phenotype (7), which suggests that neuronal cilia play a critical role in the maintenance of energy balance. Based on the association between genetic ciliopathies and obesity (3), we hypothesized that acquired or other types of genetic obesity may accompany ciliary defects in the hypothalamus, a key brain area involved in the sensation of peripheral metabolic signals and the orchestration of whole-body energy metabolism.
Results and Discussion
Reduced cilia length in the hypothalami of obese mice.
To investigate ciliary changes in the hypothalamus in mouse models of obesity, cilia were immunostained using an antibody against adenylate cyclase 3 (AC3) (8). Immunohistochemistry for somatostatin receptor 3 (SSTR3), another marker used to identify cilia in neurons (9), labeled only about 30%–50% of AC3-immunoreactive cilia in the hypothalamus (Supplemental Figure 1; supplemental material available online with this article; doi: 10.1172/JCI69395DS1). Nevertheless, the complete overlap of AC3 and SSTR3 staining along the entire length of the cilia confirmed that AC3 is a useful marker for analyzing cilia length. Dual staining of mouse brain sections with AC3 and either the neuron marker MAP2 or the glial marker GFAP revealed that most visible cilia belonged to neurons that had large nuclei (Supplemental Figure 2).
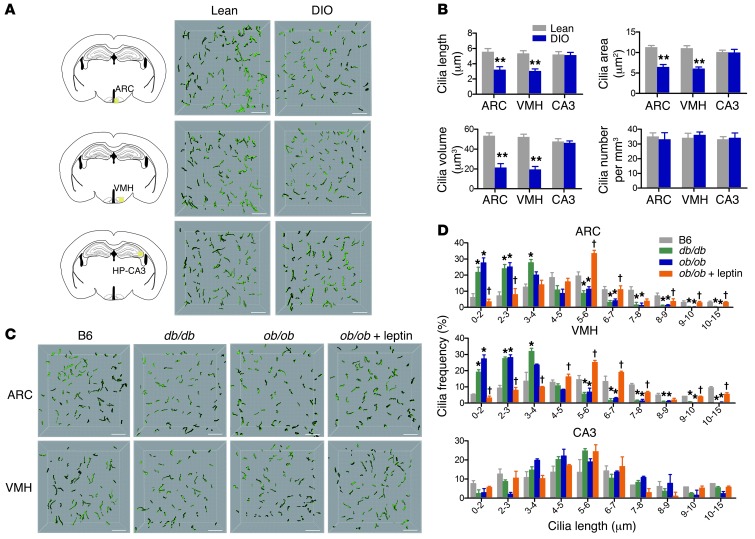
In chow diet–fed lean mice, the average cilia length was 5.5 ± 0.44 μm in the hypothalamic arcuate nucleus (ARC) and 5.3 ± 0.39 μm in the ventromedial hypothalamus (VMH). Notably, average cilia length, surface area, and volume were reduced ∼42%, ∼44%, and ∼62%, respectively, in the hypothalami of diet-induced obese (DIO) mice, which had been fed with a high-fat, high-sucrose diet for 14 weeks and had developed leptin resistance (Figure 1, A and B, and Supplemental Figure 3). When these data were further analyzed by plotting the frequencies of different cilia lengths, we found that the frequency of short cilia (< 3 μm) was dramatically increased in DIO compared with lean hypothalami (ARC, 50.6% ± 5.1% vs. 13.5% ± 2.8%; VMH, 63.1% ± 5.1% vs. 9.5% ± 1.0%; Supplemental Figure 4). In addition, the frequency of long cilia (≥ 5 μm) was reduced in DIO versus lean hypothalami (ARC, 13.6% ± 1.7% vs. 55.6% ± 3.3%; VMH, 9.3% ± 1.1% vs. 52.5% ± 4.5%). Conversely, the number of cilia per cubic millimeter did not differ between lean and DIO mice (Figure 1B). Furthermore, DIO mice displayed normal patterns of cilia distribution in hippocampal region CA3 (Figure 1, A and B, and Supplemental Figure 4), which suggests that obesity-associated changes in neuronal cilia occur selectively in the hypothalamus.
Figure 1. Reduced cilia length in the hypothalamus of adult obese mice.
(A and B) 3D-reconstructed cilia images (A) and analysis of cilia parameters (B) in ARC, VMH, and hippocampal region CA3 of 20-week-old lean or DIO mice (n = 5 per group). (C and D) AC3-immunoreactive cilia images (C) and cilia distribution (D) in ARC, VMH, and hippocampal region CA3 in 12-week-old lean C57BL/6 (B6) or obese (ob/ob and db/db) mice and in ob/ob mice treated with leptin (10 μg/d) for 7 days (n = 3–4 per group). Data represent mean ± SEM. *P < 0.05, **P < 0.005 vs. lean control; †P < 0.05 vs. untreated ob/ob. Scale bars: 15 μm.
We next examined hypothalamic ciliary changes in leptin-deficient ob/ob mice and leptin receptor–deficient (LEPR-deficient) db/db mice. Similar to DIO mice, the number, surface area, and volume of hypothalamic neuron cilia were reduced in both ob/ob and db/db mice compared with those in age-matched lean C57BL/6 mice (Figure 1C and Supplemental Figure 5). Moreover, the number of short cilia was significantly increased in the hypothalami of both ob/ob and db/db mice (Figure 1D). When leptin (10 μg/d) was infused into the third cerebroventricle of ob/ob mice for 7 days prior to sacrifice, hypothalamic cilia lengths reverted to near normal (Figure 1, C and D, and Supplemental Figure 5). Further analysis of POMC neuron cilia showed similar ciliary changes in ob/ob mice, with and without leptin treatment, and in db/db mice (Supplemental Figure 6). These data led us to hypothesize that leptin may actively regulate hypothalamic neuron cilia length in adulthood, and that decreased leptin signaling in these neurons may lead to the short cilia phenotype in obese mice. To further study the effect of nutrient availability on hypothalamic neuron cilia lengths, brains were collected from C57BL/6 mice subjected to a 36-hour fast, or a 36-hour fast followed by 6-hour refeeding, prior to sacrifice. Compared to the 6-hour refed mice, 36-hour starved mice showed an increased frequency of shorter cilia and a decreased frequency of long cilia in their hypothalami (Supplemental Figure 7). These differences resembled the ciliary changes observed in obese mice. These data indicated that hypothalamic neuron cilia lengths are affected by feeding state.
Leptin controls cilia length in hypothalamic neuron cells.
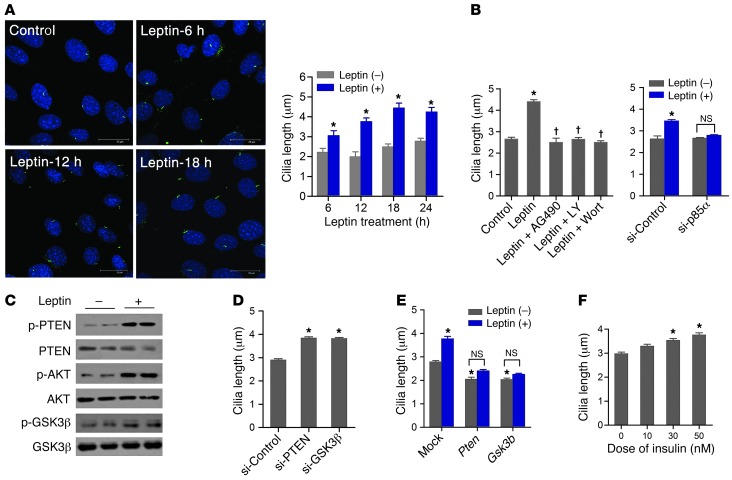
We next investigated the regulation of cilia length by leptin in N1 hypothalamic neuron cells. Cilia were analyzed using both AC3 and acetylated α-tubulin, a component of the ciliary axoneme. When grown in serum-free medium for 48 hours, about 50%–70% of N1 cells were ciliated, and the average cilia length was 2–2.5 μm. Immunocytochemistry of acetylated α-tubulin revealed that treatment of N1 cells with leptin (10–100 nM) for 6–18 hours increased cilia length by 36%–55% (Figure 2A). AC3 staining also demonstrated that leptin treatment increased the length of N1 cell cilia in a dose-dependent manner (Supplemental Figure 8). There was also a trend for leptin treatment to increase the percentage of ciliated cells, but this effect was not statistically significant (Supplemental Figure 9). Similarly, treatment of primary cultured hypothalamic neuron cells with leptin (100 nM for 12 hours) led to increased cilia length (Supplemental Figure 10), although the percentage of ciliated cells was lower (20%–30%) in these cells.
Figure 2. Leptin stimulates cilia assembly in hypothalamic neuron cells.
(A) Acetylated α-tubulin immunocytochemistry and cilia length measurement in N1 hypothalamic neuron cells treated or not with 100 nM leptin for the indicated times. Scale bars: 25 μm. (B) Changes in cilia length in N1 cells treated with leptin (100 nM for 18 hours) and/or AG490 (1 μM), LY294002 (1 nM), or wortmannin (10 nM), or in cells transfected with control siRNA (si-Control) or Pik3r1 siRNA (si-p85α). (C) Leptin treatment (100 nM for 30 minutes) of N1 cells caused serial phosphorylation of PTEN, AKT, and GSK3β in N1 cells. (D) siRNA-mediated knockdown of PTEN and GSK3β increased cilia length in N1 cells. (E) Effect of leptin treatment (100 nM for 18 hours) on cilia length in N1 cells overexpressing Pten and Gsk3b. (F) Effect of insulin treatment (18 hours) on cilia length in N1 cells. Data are mean ± SEM. *P < 0.05 vs. control; †P < 0.01 vs. leptin alone.
Leptin-induced elongation of cilia was completely blocked by cotreatment with the JAK2 inhibitor AG490 and the PI3K inhibitors LY294002 and wortmannin, as well as by depletion of the PI3K p85α subunit using siRNA specific for murine Pik3r1 (Figure 2B). These data suggest that JAK2-mediated PI3K activation is essential for leptin-stimulated cilia elongation. Leptin treatment induced serial phosphorylation of the PI3K downstream signaling molecules phosphatase and tensin homolog (PTEN), AKT, and glycogen synthase kinase 3β (GSK3β) in N1 cells (Figure 2C), resulting in activation of AKT and inactivation of both PTEN and GSK3β. To examine the roles of PTEN and GSK3β in the leptin-mediated regulation of cilia length, PTEN and GSK3β were depleted by transfection with siRNAs specific for murine Pten and Gsk3b. siRNA-mediated PTEN and GSK3β knockdown significantly increased cilia length (Figure 2D). Conversely, Pten and Gsk3b overexpression decreased cilia length and blocked the effects of leptin treatment on hypothalamic neuron cilia (Figure 2E). These data suggest that PTEN and GSK3β are downstream mediators of the regulation of cilia length by leptin. As insulin activates the PI3K signaling pathway in hypothalamic neurons (10), the effect of insulin treatment on the cilia of N1 cells was investigated. Average cilia length was increased up to 25% by insulin treatment (50 nM for 12 hours; Figure 2F), which suggests that insulin may also act on hypothalamic neurons to promote cilia growth.
Relationship between cilia length and central leptin signaling.
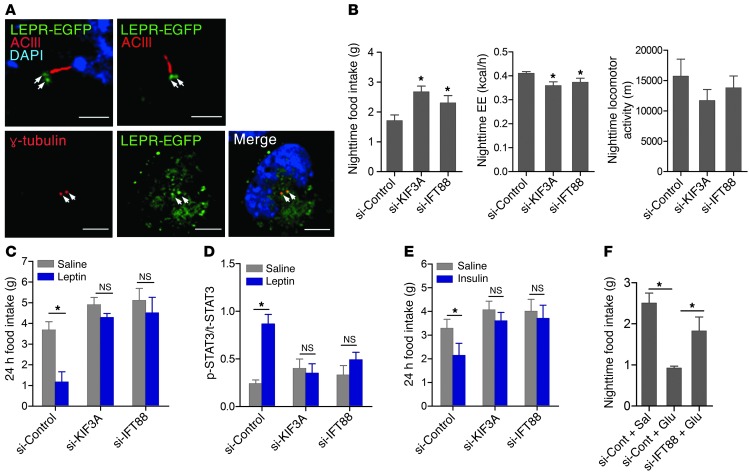
Finally, we sought to understand the physiological relevance of leptin-mediated control of cilia length in the hypothalamus. Mice with ciliary dysfunctions develop leptin resistance prior to becoming obese (6), which suggests the involvement of cilia in leptin’s signal transduction. To demonstrate the anatomical relationship between cilia and leptin signaling, a functional LEPR fluorescent fusion protein, LEPR-EGFP, was expressed in N1 cells. Immunocytochemistry for AC3 or the basal body marker γ-tubulin in LEPR-EGFP–expressing cells demonstrated that LEPR-EGFP localized to the γ-tubulin–stained basal body (Figure 3A), which indicates that the leptin signaling machinery is anatomically linked to cilia–basal body complexes.
Figure 3. Induction of short hypothalamic cilia in adult mice disrupts energy balance.
(A) Immunocytochemistry for AC3 and γ-tubulin, a basal body marker, in N1 cells expressing a LEPR-EGFP fusion protein. Arrows indicate colocalization of LEPR and γ-tubulin. Scale bars: 6.25 μm. (B) Nocturnal food intake, energy expenditure (EE), and locomotor activity in mice with siRNA-mediated knockdown of KIF3A or IFT88 (n = 4–5 per group). (C and D) Effect of i.c.v. leptin (3 μg) on food intake (C) and hypothalamic STAT3 phosphorylation (D; shown relative to total STAT3) in mice with siRNA-mediated knockdown of KIF3A or IFT88 (n = 4–5 per group). (E and F) Effects of i.c.v. insulin and glucose on 24-hour (E) and nocturnal (F) food intake in mice with short hypothalamic cilia (n = 4 per group). Data are mean ± SEM. *P < 0.05 vs. respective control or as indicated.
To further understand the relationship between cilia length and leptin signaling, short cilia were artificially induced in the hypothalamus using stereotaxic microinjection technology. Bilateral injection of siRNA specific to Kif3a or Ift88 into the mediobasal hypothalamus successfully increased the frequency of short cilia (<3 μm) in the injected area (control siRNA, 10% ± 0.3%; Kif3a siRNA, 58% ± 1.2%; Ift88 siRNA, 55% ± 2.0%; Supplemental Figures 11 and 12). Notably, mice with siRNA-mediated KIF3A or IFT88 knockdown displayed increased food intake, decreased energy expenditure, and a tendency of reduced locomotor activity during the dark period (Figure 3B), which promoted weight gain (control siRNA, 0.08 ± 1.16 g change during 0–48 h; Kif3a siRNA, 1.01 ± 0.25 g; Ift88 siRNA, 0.81 ± 0.23 g). Moreover, they were less responsive to exogenous leptin than mice that received control siRNA, as measured by feeding behavior and hypothalamic STAT3 phosphorylation (Figure 3, C and D, and Supplemental Figure 13). Consistently, POMC neuronal activity, as assessed by c-fos staining, increased after leptin administration in control siRNA–injected mice, but this effect was blunted in mice injected with Kif3a siRNA (Supplemental Figure 14). Notably, the anorexic responses to insulin and glucose were also reduced in mice with siRNA-mediated knockdown of KIF3A and IFT88 (Figure 3, E and F). These findings support the theory that leptin reinforces the responsiveness of hypothalamic neurons to anorexigenic hormones and nutrients by positively regulating cilia length. In this model, short neuronal cilia in the hypothalamus of obese mice would further compromise the ability of the animals to sense satiety signals, resulting in overeating.
Notably, cilia length in terminally differentiated neurons is actively regulated by metabolic alterations such as leptin treatment, feeding-fasting, and diet-induced obesity, and this appears to selectively occur in hypothalamic neurons. Given the effects of leptin on hypothalamic neuron cilia, cilia length may reflect the intensity of leptin signaling to target neurons.
A remaining question is why long cilia are essential for sensing metabolic signals such as leptin. Neuronal cilia have been suggested to be an extrasynaptic signaling center because they contain several receptors, signaling enzymes, and ion channels (11). The surfaces of neurons are covered with dense glycocalyx (12), which might interfere with access of hormones and nutrients to surface receptors. Furthermore, extracellular fluids (ECFs) adjacent to the cell surface may be poorly mixed due to the electrostatic effects of charged lipids that comprise plasma membranes (13). Therefore, neurons may require receptors that are distant from the cell surface, such as those on cilia, to accurately sense hormonal and nutritional changes in ECFs.
How do cilia modulate leptin signaling? BBS proteins, which are implicated in protein transport to the cilia–basal body complex, are involved in LEPR trafficking (6). Furthermore, we demonstrated that LEPR long isoform (LEPRb) proteins localize to basal bodies. Thus, an as-yet unidentified form of cilia-mediated signaling might function to amplify leptin signaling in basal bodies. Indeed, mice that lack AC3, a ciliary enzyme that catalyzes cAMP production, are obese and insensitive to leptin (14), demonstrating a connection between cilia-mediated cAMP signaling and leptin signaling. Alternatively, a small subpool of LEPRb proteins might travel along the cilia and efficiently generate leptin signaling in the limited space of the cilia matrix. Although future studies are necessary to test these possibilities, fine-tuning of cilia lengths represents a novel strategy by which leptin can regulate the sensitivities of hypothalamic neurons to metabolic signals.
Methods
Further information can be found in Supplemental Methods.
Animals.
12-week-old male C57BL/6, ob/ob, and db/db mice (Japan SLC) were used for cilia analysis. Either saline or leptin (R&D Systems) was infused i.c.v. into ob/ob mice for 7 days using an osmotic minipump (Alzet). DIO mice were obtained by feeding mice a high-fat, high-sucrose diet (Research Diet Co.).
Cell culture.
N1 hypothalamic neuron cells were maintained in DMEM supplemented with 10% FBS. To induce ciliogenesis, cells were grown to approximately 90% confluence, then cultured in serum-free medium for 48 hours before cilia analysis.
Cilia analysis.
For staining of neuron cilia in mice, whole brains were collected after 15-minute cardiac perfusion with 4% PFA. 3 coronal brain slices per animal (150 μm thick), which included the hypothalamus and the hippocampus, were obtained using a cryostat and incubated with primary antibody against AC3 (1:500 dilution; Santa Cruz). Cilia images were taken by z stacking using a confocal microscope (Carl Zeiss), reconstructed to 3D projections, and analyzed with IMARIS software (Bitplane AG). For cilia analysis of N1 cells, cells were fixed with 4% PFA for 15 minutes at room temperature and stained with primary antibodies against AC3 or acetylated α-tubulin at 4°C for 48 hours. For the staining of basal body, cells were fixed with 100% methanol for 4 minutes at –20°C and incubated with anti–γ-tubulin antibody at room temperature for 1 hour.
Immunoblotting.
Cell and tissue lysates (50 μg total proteins) were subjected to immunoblotting using antibodies directed against total and phosphorylated PTEN, AKT, GSK3β, and STAT3 (all 1:1,000 dilution; Cell Signaling).
Transfection.
N1 cells were cultured in 12-well plates and transfected with plasmids expressing LEPR-EGFP, FLAG-Pten, or Gsk3b (300 ng) using Lipofectamine (Invitrogen). Transfections were performed in quadruplicate, and each experiment was repeated at least twice.
siRNA treatment.
N1 cells were transfected with nontargeting scrambled control siRNA or siRNAs specific for murine Ift88, Kif3a (Dharmacon), or Pik3r1 (Santa Cruz) (all 50 nM). For in vivo administration, siRNA was resuspended in RNase-free water and injected bilaterally into the mediobasal hypothalamus as previously described (15).
Statistics.
Data are presented as mean ± SEM. Statistical significance of differences among groups was tested using 1- or 2-way ANOVA followed by a post-hoc least-significant difference (LSD) test. Repeated ANOVA was used to compare cilia frequency between groups according to cilia length. A P value less than 0.05 was considered significant.
Study approval.
All animal procedures were approved by the IACUC of the Asan Institute for Life Sciences.
Supplementary Material
Acknowledgments
This study was supported by the National Research Foundation of Korea (grant nos. 2009-0079566, NRF-2013R1A1A3010137, and NRF-2013M3C7A1056024) and the Asan Institute for Life Sciences (grant no. 12-326).
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article:J Clin Invest. 2014;124(5):2193–2197. doi:10.1172/JCI69395.
References
- 1.Goetz SC, Anderson KV. The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet. 2010;11(5):331–344. doi: 10.1038/nrg2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singla V, Reiter JF. The primary cilium as the cell’s antenna: signaling at a sensory organelle. Science. 2006;313(5787):629–633. doi: 10.1126/science.1124534. [DOI] [PubMed] [Google Scholar]
- 3.Sen Gupta P, Prodromou NV, Chapple JP. Can faulty antennae increase adiposity? The link between cilia proteins and obesity. J Endocrinol. 2009;203(3):327–336. doi: 10.1677/JOE-09-0116. [DOI] [PubMed] [Google Scholar]
- 4.Badano JL, Mitsuma N, Beales PL, Katsanis N. The ciliopathies: an emerging class of human genetic disorders. Annu Rev Genomics Hum Genet. 2006;7:125–148. doi: 10.1146/annurev.genom.7.080505.115610. [DOI] [PubMed] [Google Scholar]
- 5.Rahmouni K, et al. Leptin resistance contributes to obesity and hypertension in mouse models of Bardet-Biedl syndrome. J Clin Invest. 2008;118(4):1458–1467. doi: 10.1172/JCI32357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seo S, Guo DF, Bugge K, Morgan DA, Rahmouni K, Sheffield VC. Requirement of Bardet-Biedl syndrome proteins for leptin receptor signaling. Hum Mol Genet. 2009;18(7):1323–1331. doi: 10.1093/hmg/ddp031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davenport JR, et al. Disruption of intraflagellar transport in adult mice leads to obesity and slow-onset cystic kidney disease. Curr Biol. 2007;17(18):1586–1594. doi: 10.1016/j.cub.2007.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bishop GA, Berbari NF, Lewis J, Mykytyn K. Type III adenylyl cyclase localizes to primary cilia throughout the adult mouse brain. J Comp Neurol. 2007;505(5):562–571. doi: 10.1002/cne.21510. [DOI] [PubMed] [Google Scholar]
- 9.Iwanaga T, Miki T, Takahashi-Iwanaga H. Restricted expression of somatostatin receptor 3 to primary cilia in the pancreatic islets and adenohypophysis of mice. Biomed Res. 2011;32(1):73–81. doi: 10.2220/biomedres.32.73. [DOI] [PubMed] [Google Scholar]
- 10.Niswender KD, et al. Insulin activation of phosphatidylinositol 3-kinase in the hypothalamic arcuate nucleus: a key mediator of insulin-induced anorexia. Diabetes. 2003;52(2):227–231. doi: 10.2337/diabetes.52.2.227. [DOI] [PubMed] [Google Scholar]
- 11.Whitfield JF. The neuronal primary cilium--an extrasynaptic signaling device. Cell Signal. 2004;16(7):763–767. doi: 10.1016/j.cellsig.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Debbage PL. A systematic histochemical investigation in mammals of the dense glycocalyx glycosylations common to all cells bordering the interstitial fluid compartment of the brain. Acta Histochem. 1996;98(1):9–28. doi: 10.1016/S0065-1281(96)80046-8. [DOI] [PubMed] [Google Scholar]
- 13.Marshall WF, Nonaka S. Cilia: tuning in to the cell’s antenna. Curr Biol. 2006;16(15):R604–R614. doi: 10.1016/j.cub.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 14.Wang Z, et al. Adult type 3 adenylyl cyclase-deficient mice are obese. PLoS One. 2009;4(9):e6979. doi: 10.1371/journal.pone.0006979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim MS, et al. Role of hypothalamic Foxo1 in the regulation of food intake and energy homeostasis. Nat Neurosci. 2006;9(7):901–906. doi: 10.1038/nn1731. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.