Abstract
Background. Cryptococcal meningitis (CM) is a leading cause of HIV-associated mortality globally. High fungal burden in cerebrospinal fluid (CSF) at diagnosis and poor fungal clearance during treatment are recognized adverse prognostic markers; however, the underlying pathogenic factors that drive these clinical manifestations are incompletely understood. We profiled a large set of clinical isolates for established cryptococcal virulence traits to evaluate the contribution of C. neoformans phenotypic diversity to clinical presentation and outcome in human cryptococcosis.
Methods. Sixty-five C. neoformans isolates from clinical trial patients with matched clinical data were assayed in vitro to determine murine macrophage uptake, intracellular proliferation rate (IPR), capsule induction, and laccase activity. Analysis of the correlation between prognostic clinical and host immune parameters and fungal phenotypes was performed using Spearman’s r, while the fungal-dependent impact on long-term survival was determined by Cox regression analysis.
Results. High levels of fungal uptake by macrophages in vitro, but not the IPR, were associated with CSF fungal burden (r = 0.38, P = 0.002) and long-term patient survival (hazard ratio [HR] 2.6, 95% CI 1.2–5.5, P = 0.012). High-uptake strains were hypocapsular (r = –0.28, P = 0.05) and exhibited enhanced laccase activity (r = 0.36, P = 0.003). Fungal isolates with greater laccase activity exhibited heightened survival ex vivo in purified CSF (r = 0.49, P < 0.0001) and resistance to clearance following patient antifungal treatment (r = 0.39, P = 0.003).
Conclusion. These findings underscore the contribution of cryptococcal-phagocyte interactions and laccase-dependent melanin pathways to human clinical presentation and outcome. Furthermore, characterization of fungal-specific pathways that drive clinical manifestation provide potential targets for the development of therapeutics and the management of CM.
Funding. This work was made possible by funding from the Wellcome Trust (WT088148MF), the Medical Research Council (MR/J008176/1), the NIHR Surgical Reconstruction and Microbiology Research Centre and the Lister Institute for Preventive Medicine (to R.C. May), and a Wellcome Trust Intermediate fellowship (089966, to T. Bicanic). The C. neoformans isolates were collected within clinical trials funded by the British Infection Society (fellowship to T. Bicanic), the Wellcome Trust (research training fellowships WT069991, to A.E. Brouwer and WT081794, to J.N. Jarvis), and the Medical Research Council, United Kingdom (76201). The funding sources had no role in the design or conduct of this study, nor in preparation of the manuscript.
Introduction
Cryptococcal meningoencephalitis (CM), caused by the fungus C. neoformans, is a leading cause of mortality in HIV-infected individuals globally (1). Sub-Saharan Africa, with the highest HIV burden, is the most affected region, accounting for 80% of all CM-associated mortality, despite access to antiretroviral therapy (ART) (2, 3).
HIV-associated CM is characterized by a paucity of inflammation and a large fungal burden in the cerebrospinal fluid (CSF) at diagnosis (4, 5). In addition to high baseline fungal burden and a poor proinflammatory immune response, altered mental status and a slow rate of fungal clearance upon treatment are associated with acute mortality (6–8). Understanding the factors that link these prognostic markers with cryptococcal virulence is crucial for the development of effective anticryptococcal therapy.
Cryptococcal infection is acquired through inhalation of cryptococcal spores or desiccated yeast cells into the lung, where the fungus is either cleared or maintained in an asymptomatic latent state by effective host immunity (9). In immunocompromised hosts, however, cryptococci can disseminate from the lungs to other organs, in particular the central nervous system (CNS), where the fungus causes a usually fatal meningoencephalitis (10). The variation in CM severity and outcome between individual patients may be explained by underlying deficiencies in the host immune response (11, 12) and/or the virulence capacity of the infecting strain (13, 14). In addition to the ability to grow at physiological temperature (37°C), capsule expression, melanin production by laccase, and macrophage parasitism are all major virulence attributes that allow the pathogen to evade and overwhelm the immune system (15).
To date, most studies providing mechanistic links between cryptococcal virulence and outcome have been conducted in rodent models using laboratory reference strains. It remains unclear how these data relate to human infections. We report here for the first time, to our knowledge, a large-scale analysis of the relationship between the cryptococcal virulence factors (growth rate at 37°C, capsule expression, laccase activity, phagocytic uptake and intracellular proliferation in macrophages) and patient clinical parameters. We show that CNS fungal burden and consequent patient death are associated with high cryptococcal uptake by macrophages. Thus, effective phagocytosis counterintuitively predisposes to poor outcome upon cryptococcal infection. Finally, we show that laccase activity is associated with cryptococcal survival in human CSF ex vivo and poor in vivo CSF fungal clearance over a 2-week period of antifungal treatment.
Results
High cryptococcal uptake by macrophages is associated with high patient CSF fungal burden.
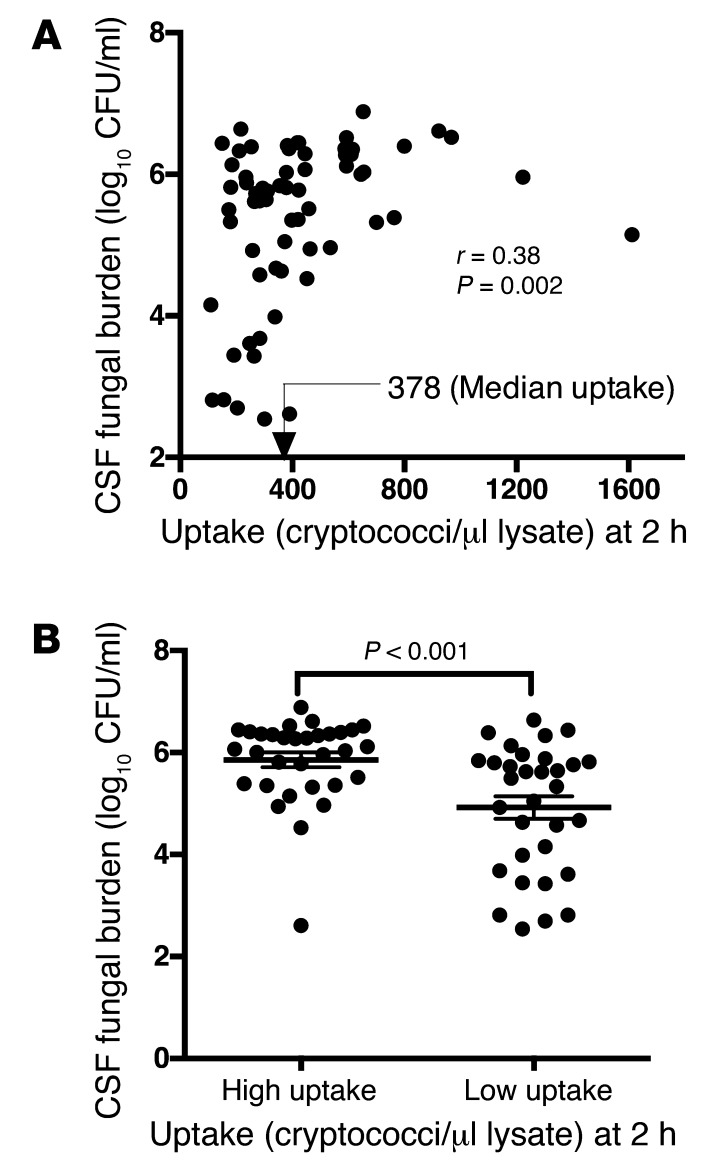
In Cryptococcus gattii infections of immunocompetent hosts, intracellular proliferation within phagocytes correlates with virulence in a murine model of cryptococcosis (16). Using a large patient-matched set of clinical isolates and the established murine macrophage–like cell line J774 (see Table 1 for a summary of patient baseline characteristics, isolate genotype, and in vitro phenotype), we tested whether a similar relationship exists for C. neoformans, but found no significant correlation between the intracellular proliferation rate (IPR) and patient fungal burden upon presentation (Spearman’s rank correlation r = –0.2, P = 0.22). However, we found a positive correlation between fungal burden in the CNS and phagocytic uptake of opsonized cryptococci (r = 0.38, P = 0.002; Figure 1A). We divided isolates into high- and low-uptake groups, based on median uptake (378 cryptococci/μl lysate), which confirmed that fungal burden in the high-uptake group of isolates was significantly higher than in the low-uptake isolates (P < 0.001, unpaired t test; Figure 1B).
Table 1.
Patient baseline characteristics, isolate genotype, and phenotype
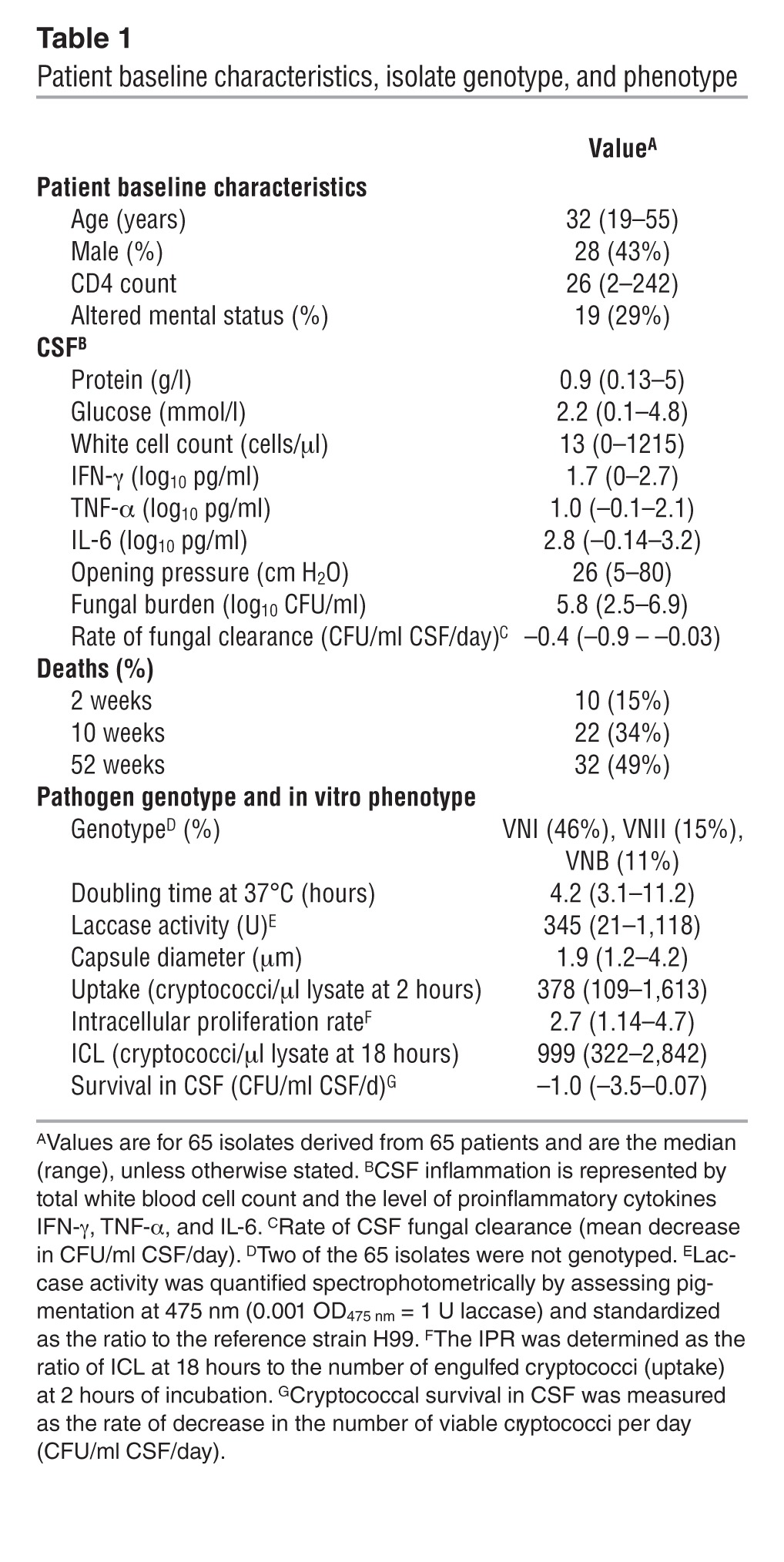
Figure 1. Association of cryptococcal uptake by macrophages with patient fungal burden.
(A) Positive correlation between cryptococcal uptake by J774 macrophages and patient CSF fungal burden. (B) The average log CSF fungal burden was significantly higher in the high-uptake than in the low-uptake group of isolates. Mean ± SEM, 5.856 ± 0.1457 and 4.922 ± 0.2189, respectively, P < 0.001. n = 32 in each group of isolates.
Macrophage uptake, not intracellular proliferation, drives intracellular fungal load in C. neoformans.
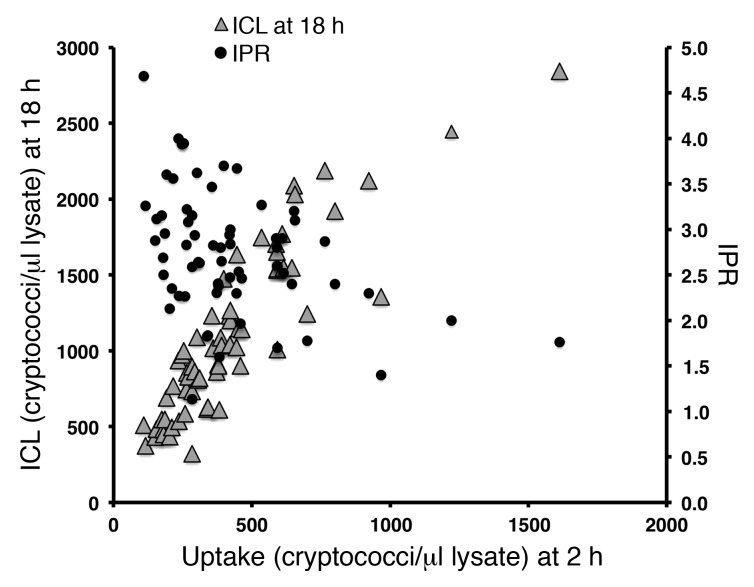
To dissect this relationship, we analyzed the relationship between uptake and intracellular proliferation. This analysis showed that uptake and IPR are inversely correlated in this set of isolates (r = –0.37, P = 0.003), implying that highly phagocytosed isolates have lower intracellular proliferation rates. However, high-uptake isolates had a larger quantity of intracellular cryptococci at 18 hours than the low-uptake counterparts, exhibiting a strong positive correlation between uptake at 2 hours and intracellular cryptococcal load (ICL) at 18 hours of incubation (r = 0.90, P < 0.0001). High phagocytosis coupled with minimal proliferation was enough to drive intracellular fungal burden in the high-uptake isolates, suggesting a high retention and survival of the initially phagocytosed cryptococci inside the macrophage. Thus, for C. neoformans, the intracellular burden of cryptococci within macrophages appeared to be driven primarily by the rate at which the pathogen was engulfed and not by subsequent proliferation within macrophages (Figure 2).
Figure 2. Relationship between uptake, IPR (r = –0.37, P = 0.003), and ICL.
Uptake was inversely correlated with IPR and positively correlated with ICL (r = 0.90, P < 0.001).
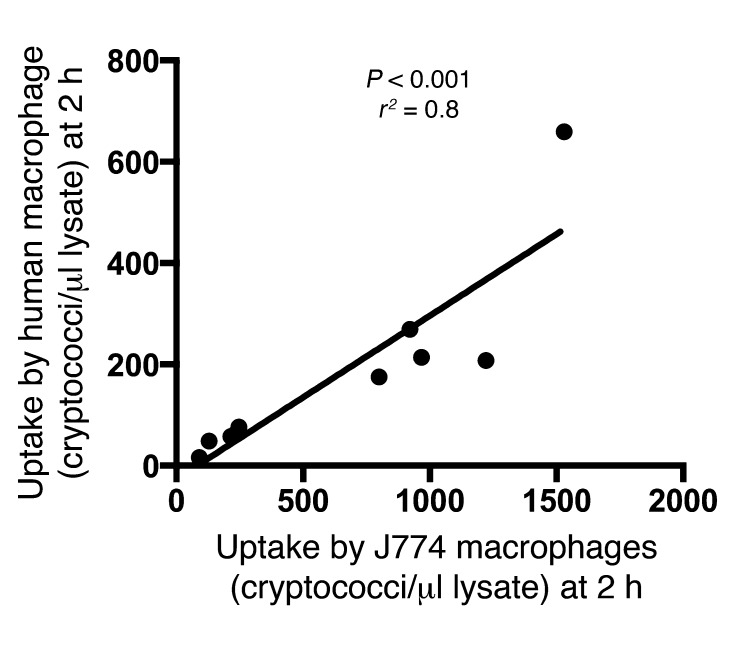
To test whether the uptake phenotype is host specific, we repeated the phagocytosis assay using a selection of high-uptake and low-uptake isolates (5 strains from each group) and human primary monocyte–derived macrophages. This analysis showed a significant (r2 = 0.8, P < 0.001, linear regression) correlation with uptake by J774 macrophages, suggesting that the traits mediating cryptococcal uptake by macrophages were conserved in these isolates and could be recognized by both murine and human macrophages (Figure 3).
Figure 3. Cryptococcal uptake by J774 macrophages correlates with uptake by human macrophages.
High-uptake (n = 5) and low-uptake (n = 5) isolates were independently exposed to murine macrophage–like cell line J774 and primary human macrophages for 2 hours at 37°C. The rate of uptake in J774 macrophages was consistent with that in human macrophages (P < 0.001, linear regression).
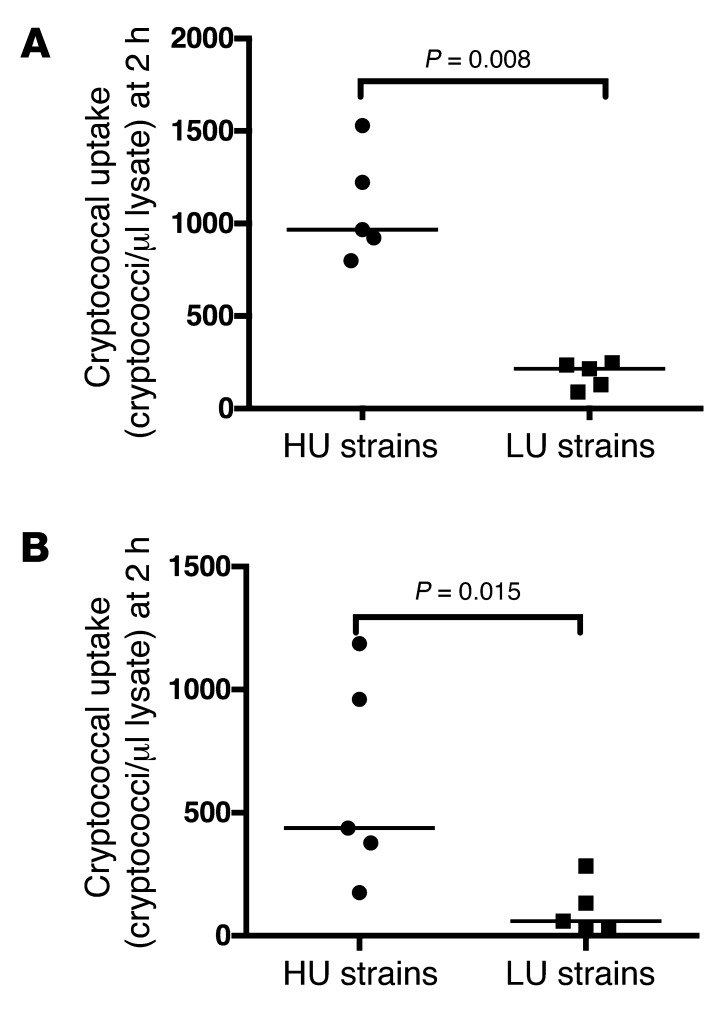
We next tested whether the high-/low-uptake effect was opsonin dependent. Using the same set of 10 strains (tested in both J774 and human primary macrophages), we examined uptake following 2 hours of macrophage infection in the absence of any opsonin (i.e., in serum-free media without the addition of anticapsule mAb 18B7). High-uptake strains again showed better engulfment than low-uptake strains (median 438 vs. 60 cryptococci/μl lysate after a 2-hour exposure to macrophages, P = 0.02, Mann-Whitney U test), suggesting that this phenotype is an intrinsic feature of the pathogen and not a reflection of variable opsonin deposition (Figure 4).
Figure 4. Cryptococcal uptake by macrophages in the absence of either antibody or complement opsonization.
(A) High-uptake (HU) strains were significantly more engulfed by macrophages in the presence of opsonin (anticapsule antibody 18B7) and (B) in the absence of opsonin compared with low-uptake (LU) strains. Solid black lines represent the median uptake within a group of strains for four experimental repeats.
Cryptococcal uptake by macrophages and CSF IFN-γ levels are independently associated with fungal burden.
To gain insight into other host factors that could influence fungal burden, we performed univariable analysis of patient CSF immune parameters, including total CSF white blood cell count and CSF cytokines and chemokines IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, IL-17, GM-CSF, MCP1, MIP1α, RANTES, VEGF, IFN-γ, and TNF-α, at the time of CM diagnosis. CSF IFN-γ levels were significantly associated with fungal burden (P = 0.001, linear regression). We then used a multivariable regression model to ask whether in vivo IFN-γ and in vitro cryptococcal uptake were interdependent in influencing fungal burden. Both uptake and IFN-γ remained independently associated with CSF fungal burden (P = 0.003 and 0.004, respectively).
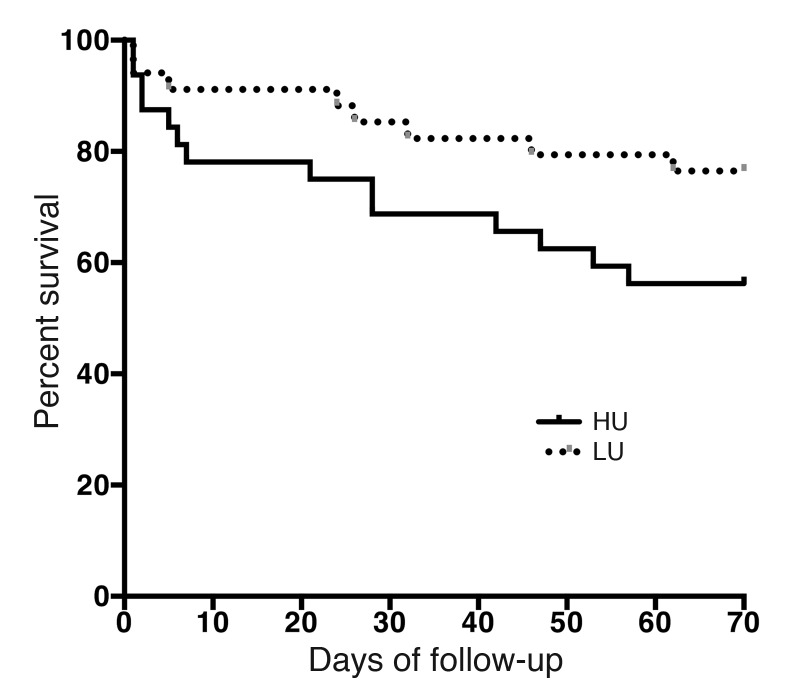
Infection with high-uptake strains increases the risk of death.
Using Cox regression analysis, we explored the impact of the macrophage-cryptococcal uptake relationship on patient survival, including an adjustment for fungal burden. Ten weeks after diagnosis, there was a trend toward worse survival in the high-uptake group (hazard ratio [HR] 2.2, 95% CI 0.9–5.6, P = 0.095, Figure 5), which became significant on long-term follow-up (HR 2.6, 95% CI 1.2–5.5, P = 0.012). The link between macrophage cryptococcal uptake and patient survival is likely partly mediated through fungal burden. Adjusting for CSF fungal burden rendered the 10-week uptake-survival association nonsignificant (HR 2.0, 95% CI 0.8–5.3, P = 0.14).
Figure 5. Kaplan-Meier survival curve estimates of patients infected with high-uptake (solid line) versus low-uptake (dotted line) isolates out to 10-week follow-up (HR 2.2, 95% CI 0.9–5.6, P = 0.095).
High CSF fungal burden is most likely a reflection of meningeal or brain parenchymal infection, not replication in situ.
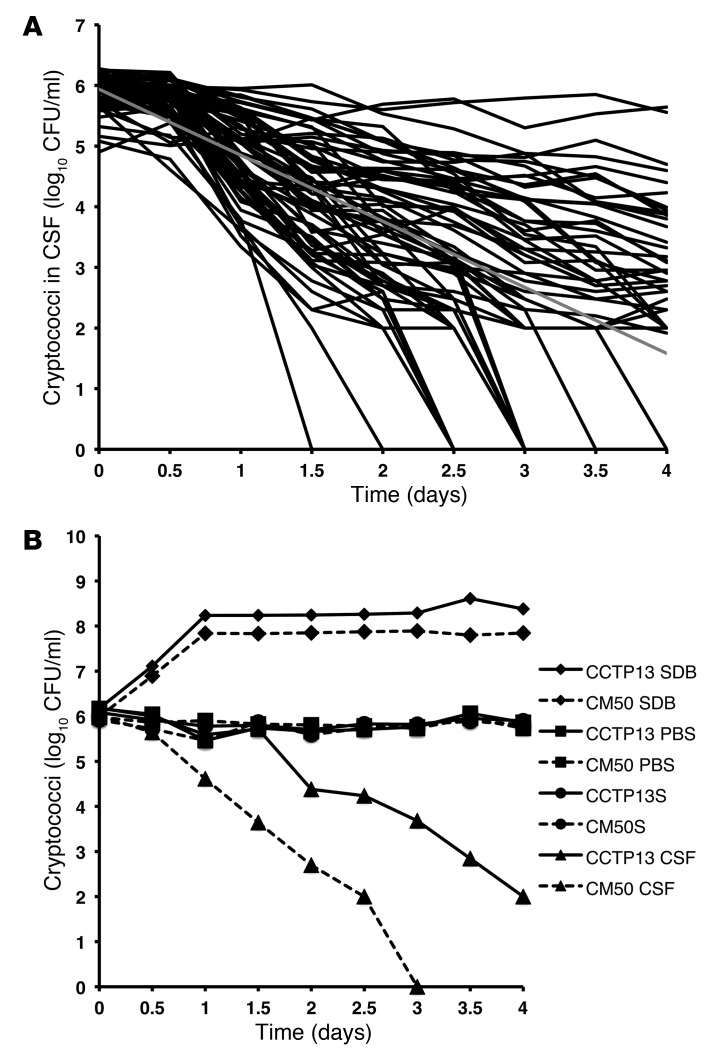
At the time of CM diagnosis, there is often a high organism load in the CSF, an indication either of cryptococcal “immigration” from other body sites, or an ability to proliferate within the CNS. We tested the latter possibility through ex vivo exposure of the strains to normal human CSF. None of the isolates grew in CSF, and in most cases, fungal burden dropped significantly during incubation over a 96-hour period (mean rate of decrease of –1.1 log10 CFU/ml per day of incubation), suggesting that high fungal burden in the CNS may be due to replication in the brain parenchyma rather than in the CSF, which may just reflect “spillover” from the brain and meninges (Figure 6A). As controls, isolates were grown in nutrient-rich Saboraud dextrose broth (SDB) nutrient–deficient PBS and in 1% saline at pH 5.6, 7.4, 8.5, and 10. Isolates grew efficiently in SDB (mean ± SD rate of increase 0.47 ± 0.11 CFU/ml SBD/day) and had comparatively better survival in PBS (0.012 ± 0.067) and saline (–0.001 ± 0.033) than in CSF, indicating that cryptococcal failure to grow in CSF was neither nutrient nor pH dependent (Figure 6B).
Figure 6. Cryptococcal survival in ex vivo human CSF.
(A) Survival of 65 clinical C. neoformans strains exposed to human CSF over 4 days. None of the strains proliferated in CSF; each black line represents the survival of one isolate over 4 days, and the gray line represents the mean survival for all strains. Survival slope was calculated as the mean rate of increase or decrease of CSF cryptococcal counts per day of incubation, derived by averaging the slope of the linear regression of log10 CFU/ml over time for each isolate. (B) Testing for whether the growth inhibition effect of CSF was nutrient or pH dependent. Two clinical strains (CCTP13 and CM50) were grown in SDB (positive control), nutrient-deficient PBS, and 1% saline (average per strain of survival at pH 5.6, 7.4, 8.5, and 10). Strains grew exponentially in SDB and maintained a stable population in PBS and saline but were gradually killed in CSF.
High-uptake strains are hypocapsular.
High-uptake and low-uptake strains showed similar in vitro growth rates in SDB at 37°C (mean ± SD doubling time of 4.1 ± 0.16 hours and 4.9 ± 0.36 hours, respectively, P = 0.1, unpaired t test), which correlated neither with uptake nor with patient CSF fungal burden. Following capsule induction for all strains, the average capsule diameter was inversely correlated with macrophage uptake (r = –0.28, P = 0.05; mean ± SD diameter 1.9 ± 0.7 μm for high-uptake and 2.2 ± 0.5 μm for low-uptake strains). Thus, as previously reported, the capsule represents a major inhibitor of phagocytic uptake in both opsonic and nonopsonic conditions (17). We did not find any correlation between induced capsular diameter and ex vivo quantification of shed capsular material (cryptococcal antigen titer; n = 47, Spearman’s r –0.07, P = 0.59), nor did the cryptococcal antigen titer in CSF correlate with phagocytic uptake in vitro (n = 47, Spearman’s r = 0.14, P = 0.27) or patients’ CSF opening pressure (n = 56, Spearman’s r –0.08. P = 0.56), in line with our previous findings in a larger cohort (18). Thus, a reduced capsular diameter is likely to drive more effective uptake by host phagocytes without necessarily impacting the systemic immunomodulatory effects that result from the shedding of capsular material.
High-uptake strains showed high laccase activity, separate from an effect of melanin production.
The cryptococcal enzyme laccase is a well-characterized virulence factor, which oxidizes both iron and polyphenols, producing a protective melanin cell wall coat (19), and modifies prostaglandin synthesis (20). We tested laccase activity in vitro by quantifying the production of black melanin-like pigment upon exposure to L-3,4-dihydroxphenylalanine (L-DOPA) medium. Laccase activity was positively correlated with uptake (r = 0.36, P = 0.003), indicating that the more effectively phagocytosed isolates had higher laccase activity. To demonstrate whether this was mediated through differences in melanin production, we quantified cell wall melanin by generating melanin ghosts using acid digestion (21) for ten clinical strains with the highest (n = 5) and the lowest (n = 5) laccase activity. We found no significant correlation between cell wall melanin levels and uptake (P = 0.1). Given the small sample size, this does not entirely rule out the association of melanization with phagocytosis. Alternatively, the association of laccase activity with efficient phagocytosis may be mediated by an additional role of the laccase enzyme (e.g., in iron scavenging or inflammatory regulation), separate from its effect on melanin.
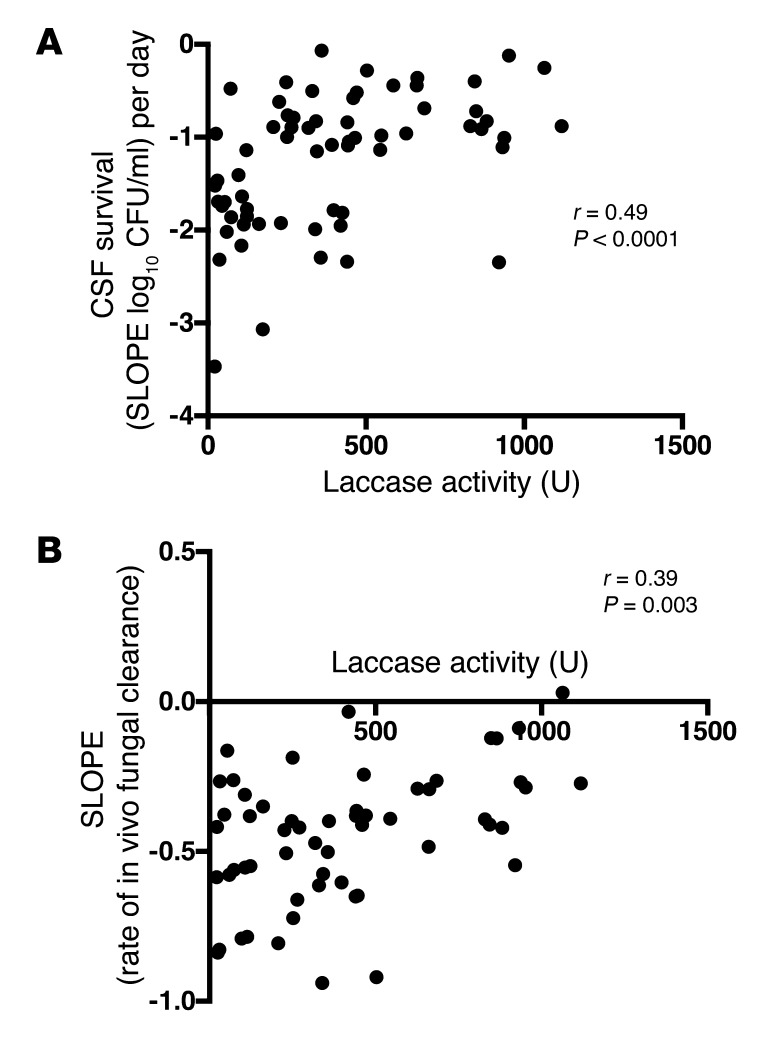
Cryptococcal laccase activity is associated with ex vivo CSF survival and the in vivo rate of fungal clearance.
Laccase activity and survival in human CSF showed a significant positive correlation (r = 0.49 and P < 0.0001) (Figure 7A). Interestingly, we also observed a significant correlation between laccase activity and the in vivo rate of CSF fungal clearance on amphotericin-based antifungal therapy over a 2-week treatment period (r = 0.39, P = 0.003) (Figure 7B). Thus, high laccase activity appears to enhance survival within human CSF, both in the presence and absence of antifungals.
Figure 7. Association of laccase activity with ex vivo cryptococcal survival in CSF and the in vivo rate of fungal clearance.
(A) Higher survival in CSF by high laccase activity isolates. (B) High laccase activity isolates were cleared less effectively from patient CSF during antifungal treatment.
Discussion
In this study of the virulence phenotype of clinical C. neoformans isolates, we have shown an association of two cryptococcal virulence traits, macrophage uptake and laccase activity, with fungal burden and rate of clearance of infection, two in vivo adverse prognostic markers in CM, and mortality. Infection with cryptococcal strains exhibiting high-uptake and high laccase activity was correlated with higher pretreatment CSF fungal burden and poor cryptococcal clearance from CSF on antifungal therapy, significantly increasing the likelihood of death from CM. We have demonstrated, to our knowledge, the first direct link between these pathogen virulence factors and poor clinical outcome in HIV-associated CM. Importantly, by drawing on isolates from five clinical trials, four of which were conducted at different times and in different regions of South Africa and the fourth conducted in Thailand (4, 22–25), our analysis includes a diverse set of human hosts, pathogen genotypes, and commonly used antifungal treatment regimens. Our findings are thus likely to reflect common virulence traits associated with clinical phenotypes in patients across a wide spectrum of cryptococcal disease.
The key, and perhaps surprising, observation arising from this study is that strains showing high engulfment by phagocytes in vitro were associated with higher fungal burden in patients. Interestingly, this correlation does not reflect higher replication rates either extracellularly in nutrient-rich medium or intracellularly, as measured by doubling time at 37°C, or higher IPRs; nor did we see evidence of growth using an ex vivo CSF model. Instead, we hypothesize that strains that are more easily phagocytosed have a higher chance of intracellular survival within phagocytes and thus of more efficient dissemination to the brain, resulting ultimately in a higher CNS fungal burden and poorer patient outcome. In this context, it is interesting to note that Rohatgi et al. very recently reported that a higher-affinity variant of the phagocytic receptor FcγRIIIA is a risk factor for HIV-associated cryptococcal disease (26). Thus, both host factors (26) and pathogen factors (this study) that enhance phagocytic uptake appear to increase the risk of (severe) cryptococcal disease.
Cryptococcal nonlytic escape and spread from cell to cell has been described in in vitro cultures of both murine and human macrophages (27–29) and has been recently confirmed to occur in vivo in a murine model of cryptococcosis (30). Moreover, cryptococci-laden macrophages have been observed in the brain capillaries and leptomeninges of a mouse with severe cryptococcal meningitis (31). The ability of monocytes to systemically disseminate the fungus to the brain has further been demonstrated in a mouse model of cryptococcosis (32). These observations point to the potential of phagocytes to drive host fungal burden through the uptake of fungal cells, providing a niche for cryptococcal survival in the host and spreading it from the site of infection to the brain in a “Trojan horse” model.
The cryptococcal capsule is well described for its role in resisting phagocytosis (17), and thus possession of a smaller capsule may partly explain why high-uptake isolates were easily engulfed by macrophages. In the environment, such a phenotype is likely to be deleterious, since fungi will be effectively engulfed and killed by soil amoebae and other predators. Thus, cryptococcal strains causing human CM are most likely a selected subset of a more diverse environmental population and happen to exhibit a combination of rapid engulfment but good intracellular survival.
We also observed that strains exhibiting high phagocytic uptake also exhibited higher laccase activity. Laccase is a critical enzyme for the synthesis of melanin, a pigment that is protective against both macrophage antimicrobial activities (such as the reactive oxygen burst) and amphotericin B, a drug that is the basis of the induction of CM therapy, including in our patients (33–35). Acid extraction of a small subset of these strains showed no significant correlation between phagocytic uptake and cell wall melanin. This may have been due to the small sample size (n = 10); alternatively, the link between laccase activity and phagocytic uptake in this group of isolates may reflect one of the other functions of this enzyme. In particular, it is tempting to speculate that the production of prostaglandins by cryptococci, which is a laccase-dependent phenotype (20), may be driving differences in phagocytic efficacy. Of note, our laccase activity assay used L-DOPA as a medium, and in vitro melanization may be different from in vitro polymerization of L-DOPA. Several possible substrates exist for melanization by cryptococcal laccase, including the indole compounds described by Kwon-Chung in 1983 (36).
Our aim was to look for pathogen virulence factors that are linked to patient clinical phenotype and to interrogate relationships with host factors that are important for the control of infection. Patients’ CSF fungal burden and rate of clearance have been demonstrated to be modulated by the nature of the host phagocyte response and proinflammatory cytokines, in particular IFN-γ, both within and outside the CNS (11, 12). We observed that CSF fungal burden was negatively associated with CSF IFN-γ levels. Taken together, these associations, in the HIV-infected host, are indicative of a host environment with inactivated phagocytes (37) due to a paucity of IFN-γ, in which the easily phagocytosed cryptococcal strains thrive and proliferate, resulting in a greater fungal burden. This is in contrast to C. gattii infection in the immunocompetent host, in which a strong adaptive immune response results in appropriate macrophage activation and in which the ability to rapidly replicate in macrophages is likely to be more important for virulence (16). In HIV-infected patients with CM, we propose that the ease of uptake of cryptococci by macrophages, coupled with the inability to orchestrate an effective IFN-γ–activated fungicidal macrophage response, results in unchecked proliferation and survival of the fungus, with dissemination to the CNS yielding high fungal burden. We also suggest that high laccase activity in C. neoformans increases cryptococcal intracellular (macrophage) and extracellular (CSF) survival and confers resistance to antifungal killing. Although we did not demonstrate a clear relationship between laccase activity and cell wall melanin production, this may have been due to the limitations of the in vitro assay and the small sample size. Melanization has been demonstrated in cryptococci in human brain tissue (38). The catecholamine substrates for melanization in the human brain include more than just L-DOPA, so it remains possible that the association between laccase activity and clearance of infection during amphotericin treatment is still mediated through an effect on in vivo melanin production. Alternatively, laccase, through alternative pathways, may protect Cryptococcus from the toxic free radicals and proinflammatory cytokines generated by amphotericin B (39).
In summary, our study has shown that the relationship between the macrophage-cryptococcal interaction and poor clinical outcome is mediated through increased patient fungal burden. Building on a recent study linking macrophage-cryptococcal interaction to high mortality at 1 year of follow-up in HIV-associated CM (14), we have shown an association with mortality at 10 weeks in our cohort, a time when most mortality is attributed to CM as the major cause of death. Furthermore, we are the first to demonstrate an association of laccase activity and poor CSF fungal clearance during amphotericin-based treatment of human CM, which may either be mediated through an effect on melanization in vivo or through an alternative immunomodulatory effect of laccase. These findings underscore the importance of cryptococcal-phagocyte interactions and the laccase-dependent melanin pathway and their relevance to human clinical outcome and identify these as important targets for future therapeutics and management of CM.
Methods
Patients and isolates
A total of 65 cryptococcal isolates were assayed from a cohort of more than 300 clinically characterized HIV-associated CM patients from five completed clinical trials in Thailand and South Africa (4, 22–25). Participants provided written informed consent, and all trials were approved by the local research ethics committees as well as the ethics committee of Wandsworth (London, United Kingdom). Isolates for this study were selected to reflect the range of clinical trials, geographic locations, and isolate genotypes of serotype A C. neoformans var. grubii. Genotypes were determined using multilocus sequence typing (MLST) according to the ISHAM (International Society for Human and Animal Mycology) consensus typing scheme (40). All isolates included were from patients who were ART naive at enrollment and treated for a first episode of CM with amphotericin B–based therapies (sometimes in combination with fluconazole, flucytosine, or adjuvant IFN-γ). Patient baseline characteristics including mental status, CSF white cell count, protein levels, cytokine levels, opening pressure, and fungal burden were determined before therapeutic intervention.
Cryptococcal culture
The reference serotype A C. neoformans var. grubii strain H99 (obtained from R.C. May’s laboratory at the University of Birmingham) was used as the control strain in all assays. Prior to assay, clinical isolates and H99 were propagated either in yeast peptone dextrose (YPD) broth (1% yeast extract, 1% peptone, and 2% glucose; Sigma-Aldrich) or on SDA (Thermo Fisher Scientific) at 30°C. Infection inocula were made from 24-hour starter cultures in YPD broth or SDB (Thermo Fisher Scientific) at 37°C with rotation.
Macrophage culture
The murine-derived J774 macrophage cell line and human monocyte–derived macrophages were used for the study. Prior to use, 1.0 × 105 J774 macrophages were grown in 24-well tissue culture plates (Greiner) containing DMEM supplemented with 10% FBS, 1 mM L-glutamine, and 1% penicillin-streptomycin (Sigma-Aldrich) for 24 hours at 37°C with 5% CO2. The macrophage batches used were kept within the range of three passages to limit passage-to-passage variations.
Primary human macrophages were derived from peripheral blood monocytes from healthy donor buffy coats (National Blood Service, United Kingdom). PBMCs were isolated by Ficoll (Sigma-Aldrich) gradient centrifugation, and monocytes were purified from the PBMC population by adherence to plastic following overnight culture in tissue culture flasks (T75 Greiner) in RPMI 1640 medium with L-glutamine (Gibco), supplemented with either 10% AB male human serum (First Link) or 10% FBS (Sigma-Aldrich) plus 1% penicillin-streptomycin, in the presence of 1,000 U/ml GM-CSF. Monocytes were detached and counted by hemocytometer to achieve a final cell density of 5 × 105 cells per well in a 24-well tissue culture plate (Corning). Cells were maintained for 7 to 14 days in RPMI 1640 medium supplemented with 10% FBS and 1% penicillin-streptomycin for differentiation into macrophages. The medium was changed every 3 days.
Phagocytosis and intracellular proliferation assay
Isolates were tested for the rate at which they were phagocytosed by (uptake) — and proliferated inside (IPR) — macrophages. Prior to infection, J774 macrophages were activated with 15 μg/ml PMA for 30 to 40 minutes in serum-free medium (DMEM with L-glutamine and 1% penicillin-streptomycin). Cryptococci were harvested from the 24-hour starter culture by centrifugation, washed twice in PBS, and opsonized with 1 μg/ml of the anticapsule mAb 18B7 (provided by Arturo Casadevall, Albert Einstein College of Medicine, New York, New York, USA) for 30 to 60 minutes at room temperature. Macrophages were then exposed to the opsonized cryptococci at a 1:10 ratio and incubated for 2 hours at 37°C with 5% CO2 to promote phagocytosis. After 2 hours, the medium was removed, and macrophages were extensively washed (4–5 times) with sterile PBS to remove extracellular cryptococci. Part of the culture (time point 0) was lysed with 200 μl of sterile water for 20 minutes at 37°C to release intracellular cryptococci, which were counted using a hemocytometer. The remaining culture was maintained in serum-free medium at 37°C, and cells were lysed and intracellular cryptococci were counted at 18, 24, and 48 hours after infection. The rate of phagocytosis (uptake) was determined as the number of cryptococci engulfed by macrophages 2 hours after infection. The IPR was determined as the ratio of the number of intracellular cryptococci at 18 hours to the number of intracellular cryptococci at 2 hours. Results were expressed as the mean of 5 to 7 experimental repeats. Ten isolates were selected from the J774 macrophage uptake profile, representing the top 5 (high-uptake) and bottom 5 (low-uptake) strains and then tested in primary human macrophages. The human monocyte–derived macrophages were stimulated with 1,000 U/ml human recombinant IFN-γ (Immunotools) and 10 μg/ml LPS (Sigma-Aldrich) 24 hours prior to infection. The rate of cryptococcal uptake and the IPR were determined as described above with J774 macrophages. To evaluate opsonin influence on cryptococcal uptake, the experiment was repeated with nonopsonized cryptococci, and the rate of uptake was determined after 2 hours of incubation at 37°C with 5% CO2.
Laccase activity
Isolates were grown for 12 hours in SDB at 37°C with shaking at 200 rpm. The growth medium was removed, and the yeast cells were washed twice in PBS, counted by hemocytometry, and adjusted to achieve an inoculum of 4 × 106 cells/ml. Cryptococci were then incubated in L-DOPA medium (0.1% glucose anhydrous, 0.1% L-asparagine, 0.3% KH2PO4, 0.025% MgSO4•7H2O, and 0.01% L-DOPA, pH 5.56) for 16 hours at 37°C and then 24 hours at 25°C, with shaking at 250 rpm to induce melanin production. After incubation, the supernatants were harvested at 4,000 g for 5 minutes, and the amount of pigment produced was determined spectrophotometrically at a 475-nm wavelength and converted into laccase units (U) by a factor of 0.001 OD = 1 U (41). Assays were repeated three to four times. To adjust for interexperimental variation, laccase activity of the clinical isolates was expressed as a ratio to H99 (positive control), and results were reported as the median of the repeats.
Cell wall melanin quantification
Acid-insoluble melanin production was quantified following the protocol of Wang and colleagues (21). Ten strains (5 with low laccase activity: IFN16, RCT7, RCT33, CM50, and CM36, and 5 with high laccase activity: CCTP2, CCTP3, CCTP10, CCTP13, and CCTP30) were subcultured on YPD agar and then cultured in 3 ml of YPD broth with rotation (20 rpm) at 25°C for 24 hours. 1.0 × 108 yeast cells were then transferred into 100 ml of defined minimal media (41) and incubated in the dark on a shaking incubator at 200 rpm at 30°C, for 10 days.
Yeast cells were then pelleted, washed once with 1.0 M sorbitol in 0.1 M sodium citrate (pH 5.0), and resuspended in 5 ml of this solution. The number of cells present in this resuspension was counted by hemocytometry before lysis in a Precellys cell homogenizer (two 45-second periods of lysis, separated by 1 minute on ice). The cell debris was collected by centrifugation and resuspended in 4.0 M guanidinium isothiocyanate, a protein denaturant, for 30 minutes at room temperature. Cell debris was pelleted by centrifugation and resuspended in 6.0 M HCl at 100°C for 30 minutes. This treatment dissolved cells completely, leaving a black/brown pellet of insoluble melanin. This pellet was washed in water and left to dry completely before being weighed and the mass per yeast cell calculated.
Capsule induction
To induce capsule production in vitro, C. neoformans isolates were inoculated into SDB and grown overnight at 37°C with shaking. The following day, 20 μl of culture was inoculated into 5-ml capsule-inducing medium (DMEM with 1% NCTC-109 medium and 10% heat-inactivated FBS; Sigma-Aldrich) and incubated for 48 hours at 37°C with 10% CO2. Cells were subsequently harvested by centrifugation at 941 g for 5 minutes and observed by counterstaining with India ink, using a ×40 bright-field objective. Cells were measured using ImageJ software, version 1.440 (NIH), and the capsule diameter was calculated as the average of: (total cell diameter – cell body diameter) / 2 for 50 to 100 cells.
CSF survival
Human CSF was obtained from patients undergoing therapeutic lumbar punctures for benign intracranial hypertension at the Neurosciences day unit of St. George’s Hospital NHS Trust in London. CSF parameters in these patients, including white cell count and protein and glucose levels, were within the normal range for human CSF and did not contain antifungal drugs.
CSF pH was determined before filter sterilization with a 0.2-μm filter and preserved at –80°C until use. Isolates were initially grown to the stationary phase in SDB at 37°C, with shaking at 150 rpm. Cultures were diluted in PBS and then inoculated into CSF at a concentration of 1 to 2 × 106 cells/ml. Cultures were then incubated at 37°C for 96 hours with shaking at 150 rpm. Aliquots were collected at time point 0 and subsequently at 12, 24, 36, 72, and 96 hours after inoculation and plated on SDA for CFU counts (42). In parallel, 1% saline at a different pH, PBS, and SDB were inoculated and monitored as pH, nutrient-deplete and nutrient-rich growth media controls, respectively. The survival slope was calculated as the mean rate of increase or decrease in CSF cryptococcal counts per day of incubation, derived by averaging the slope of the linear regression of log10 CFU/ml over time for each isolate.
Growth curves
Isolates were grown in SDB for 48 hours to reach the stationary phase. Cultures were then reinoculated into fresh SDB in 24-well flat-bottomed, transparent polystyrol plates (Corning) to achieve an initial OD of 0.2 at a 600-nm wavelength. The plate was then incubated at 37°C with a combination of orbital and linear shaking (3-mm amplitude) for 48 hours (Tecan i-control 1.7.1.12, Infinite 200 PRO Machine). OD was measured every 30 minutes, and the growth curves were plotted in Microsoft Excel, version 2010, after subtraction of the blank. Doubling time was then determined using the formula: doubling time = cell concentration at time 0*egrowthrate*time (Roth V. 2006, http://www.doubling-time.com/compute.php).
Statistics
Analyses were performed using Prism, version 6.0b (GraphPad Software) and Stata, version 11 (StataCorp). Associations were tested between the isolate virulence factors and patient clinical variables and outcome using linear regression for continuous variables and logistic regression for categorical variables. P values less than or equal to 0.05 were considered significant. Fungal burden and CSF cytokines were log10 transformed before analysis. Spearman’s rank correlation was used to determine the correlation between in vitro phenotypes and in vivo (patient) characteristics. Linear regression was used to determine the relationship between cryptococcal uptake by the murine macrophage-like cell line J774 and human primary monocyte–derived macrophages. Differences between groups were determined using the Mann-Whitney U test for non-normally distributed data and an unpaired t test (2-tailed) for normally distributed (log-transformed) data. To elucidate the relationship between different and potentially confounding variables, factors found to be significantly associated (P ≤ 0.05) with CSF fungal burden on univariable analysis were entered into a multivariable regression model, with fungal burden as the dependent variable. Cox regression was used to assess the risk of death for patients infected with high-uptake cryptococcal strains.
Study approval
Clinical cryptococcal isolates.
Isolates used in this study were obtained from patients enrolled in five published clinical trials in Thailand and South Africa (4, 22–25). All trials received approval from the ethics committee of Wandsworth, London, as well as from the local research ethics committees in the host countries. Patients gave written informed consent, including for the storage and use of their clinical isolates for future research.
Human CSF.
We consulted the UK National Research Ethics Service as well as the Research and Development Office at St. George’s University of London regarding the requirement for ethical review. Given that the lumbar punctures were being performed for a clinical indication, that the CSF used would otherwise have been discarded, that no clinical data were being collected, and that CSF samples were anonymized, rendered acellular by filtration, and pooled for experimental use, ethical approval was deemed to be unnecessary.
Supplementary Material
Acknowledgments
We gratefully acknowledge the support of the May and Bicanic groups in making this study possible.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article:J Clin Invest. 2014;124(5):2000–2008. doi:10.1172/JCI72950.
See the related Commentary beginning on page 1893.
References
- 1.Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23(4):525–530. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 2.Jarvis JN, et al. High ongoing burden of cryptococcal disease in Africa despite antiretroviral roll out. AIDS. 2009;23(9):1182–1183. doi: 10.1097/QAD.0b013e32832be0fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loyse A, et al. Cryptococcal meningitis: improving access to essential antifungal medicines in resource-poor countries. Lancet Infect Dis. 2013;13(7):629–637. doi: 10.1016/S1473-3099(13)70078-1. [DOI] [PubMed] [Google Scholar]
- 4.Brouwer AE, et al. Combination antifungal therapies for HIV-associated cryptococcal meningitis: a randomised trial. Lancet. 2004;363(9423):1764–1767. doi: 10.1016/S0140-6736(04)16301-0. [DOI] [PubMed] [Google Scholar]
- 5.Day JN, et al. Combination antifungal therapy for cryptococcal meningitis. N Engl J Med. 2013;368(14):1291–1302. doi: 10.1056/NEJMoa1110404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saag MS, et al. Comparison of amphotericin B with fluconazole in the treatment of acute AIDS-associated cryptococcal meningitis. The NIAID Mycoses Study Group and the AIDS Clinical Trials Group. N Engl J Med. 1992;326(2):83–89. doi: 10.1056/NEJM199201093260202. [DOI] [PubMed] [Google Scholar]
- 7.Jarvis JN, Harrison TS. HIV-associated cryptococcal meningitis. AIDS. 2007;21(16):2119–2129. doi: 10.1097/QAD.0b013e3282a4a64d. [DOI] [PubMed] [Google Scholar]
- 8.Bicanic T, et al. Independent association between rate of clearance of infection and clinical outcome of HIV-associated cryptococcal meningitis: analysis of a combined cohort of 262 patients. Clin Infect Dis. 2009;49(5):702–709. doi: 10.1086/604716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jarvis JN, Harrison TS. Pulmonary cryptococcosis. Semin Respir Crit Care Med. 2008;29(2):141–150. doi: 10.1055/s-2008-1063853. [DOI] [PubMed] [Google Scholar]
- 10.Sabiiti W, May RC. Mechanisms of infection by the human fungal pathogen Cryptococcus neoformans. Future Microbiol. 2012;7(11):1297–1313. doi: 10.2217/fmb.12.102. [DOI] [PubMed] [Google Scholar]
- 11.Jarvis JN, et al. The phenotype of the Cryptococcus-specific CD4+ memory T-cell response is associated with disease severity and outcome in HIV-associated cryptococcal meningitis. . J Infect Dis. 2013;207(12):1817–1828. doi: 10.1093/infdis/jit099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siddiqui AA, et al. IFN-γ at the site of infection determines rate of clearance of infection in cryptococcal meningitis. J Immunol. 2005;174(3):1746–1750. doi: 10.4049/jimmunol.174.3.1746. [DOI] [PubMed] [Google Scholar]
- 13.Wiesner DL, et al. Cryptococcal genotype influences immunologic response and human clinical outcome after meningitis. MBio. 2012;3(5):e00196-12. doi: 10.1128/mBio.00196-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alanio A, Desnos-Ollivier M, Dromer F. Dynamics of Cryptococcus neoformans-macrophage interactions reveal that fungal background influences outcome during cryptococcal meningoencephalitis in humans. MBio. 2011;2(4):e00158-11. doi: 10.1128/mBio.00158-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma H, May RC. Virulence in Cryptococcus species. Adv Appl Microbiol. 2009;67:131–190. doi: 10.1016/S0065-2164(08)01005-8. [DOI] [PubMed] [Google Scholar]
- 16.Ma H, et al. The fatal fungal outbreak on Vancouver Island is characterized by enhanced intracellular parasitism driven by mitochondrial regulation. Proc Natl Acad Sci U S A. 2009;106(31):12980–12985. doi: 10.1073/pnas.0902963106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.García-Rodas R, Zaragoza O. Catch me if you can: phagocytosis and killing avoidance by Cryptococcus neoformans. FEMS Immunol Med Microbiol. 2012;64(2):147–161. doi: 10.1111/j.1574-695X.2011.00871.x. [DOI] [PubMed] [Google Scholar]
- 18.Robertson EJ, et al. Cryptococcus neoformans ex vivo capsule size is associated with intracranial pressure and host immune response in HIV-associated cryptococcal meningitis. J Infect Dis. 2014;209(1):74–82. doi: 10.1093/infdis/jit435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu X, Williamson PR.Role of laccase in the biology and virulence of Cryptococcus neoformans. FEMS Yeast Res. 2004;5(1):1–10. doi: 10.1016/j.femsyr.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 20.Erb-Downward JR, Noggle RM, Williamson PR, Huffnagle GB. The role of laccase in prostaglandin production by Cryptococcus neoformans. Mol Microbiol. 2008;68(6):1428–1437. doi: 10.1111/j.1365-2958.2008.06245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Aisen P, Casadevall A. Melanin, melanin “ghosts,” and melanin composition in Cryptococcus neoformans. Infect Immun. 1996;64(7):2420–2424. doi: 10.1128/iai.64.7.2420-2424.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bicanic T, et al. Fungal burden, early fungicidal activity, and outcome in cryptococcal meningitis in antiretroviral-naive or antiretroviral-experienced patients treated with amphotericin B or fluconazole. Clin Infect Dis. 2007;45(1):76–80. doi: 10.1086/518607. [DOI] [PubMed] [Google Scholar]
- 23.Bicanic T, et al. High-dose amphotericin B with flucytosine for the treatment of cryptococcal meningitis in HIV-infected patients: a randomized trial. Clin Infect Dis. 2008;47(1):123–130. doi: 10.1086/588792. [DOI] [PubMed] [Google Scholar]
- 24.Loyse A, et al. Comparison of the early fungicidal activity of high-dose fluconazole, voriconazole, and flucytosine as second-line drugs given in combination with amphotericin B for the treatment of HIV-associated cryptococcal meningitis. Clin Infect Dis. 2012;54(1):121–128. doi: 10.1093/cid/cir745. [DOI] [PubMed] [Google Scholar]
- 25.Jarvis JN, et al. Adjunctive interferon-γ immunotherapy for the treatment of HIV-associated cryptococcal meningitis: a randomized controlled trial. AIDS. 2012;26(9):1105–1113. doi: 10.1097/QAD.0b013e3283536a93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rohatgi S, et al. Fcγ receptor 3A polymorphism and risk for HIV-associated cryptococcal disease. MBio. 2013;4(5):e00573-13. doi: 10.1128/mBio.00573-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma H, Croudace JE, Lammas DA, May RC. Expulsion of live pathogenic yeast by macrophages. Curr Biol. 2006;16(21):2156–2160. doi: 10.1016/j.cub.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 28.Alvarez M, Casadevall A. Phagosome extrusion and host-cell survival after Cryptococcus neoformans phagocytosis by macrophages. Curr Biol. 2006;16(21):2161–2165. doi: 10.1016/j.cub.2006.09.061. [DOI] [PubMed] [Google Scholar]
- 29.Ma H, Croudace JE, Lammas DA, May RC. Direct cell-to-cell spread of a pathogenic yeast. BMC Immunol. 2007;8:15. doi: 10.1186/1471-2172-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nicola AM, Robertson EJ, Albuquerque P, Derengowski Lda S, Casadevall A. Nonlytic exocytosis of Cryptococcus neoformans from macrophages occurs in vivo and is influenced by phagosomal pH. MBio. 2011;2(4):e00167-11. doi: 10.1128/mBio.00167-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chrétien F, Lortholary O, Kansau I, Neuville S, Gray F, Dromer F. Pathogenesis of cerebral Cryptococcus neoformans infection after fungemia. J Infect Dis. 2002;186(4):522–530. doi: 10.1086/341564. [DOI] [PubMed] [Google Scholar]
- 32.Charlier C, Nielsen K, Daou S, Brigitte M, Chretien F, Dromer F. Evidence of a role for monocytes in dissemination and brain invasion by Cryptococcus neoformans. Infect Immun. 2009;77(1):120–127. doi: 10.1128/IAI.01065-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ikeda R, Sugita T, Jacobson ES, Shinoda T. Effects of melanin upon susceptibility of Cryptococcus to antifungals. Microbiol Immunol. 2003;47(4):271–277. doi: 10.1111/j.1348-0421.2003.tb03395.x. [DOI] [PubMed] [Google Scholar]
- 34.van Duin D, Casadevall A, Nosanchuk JD. Melanization of Cryptococcus neoformans and Histoplasma capsulatum reduces their susceptibilities to amphotericin B and caspofungin. Antimicrob Agents Chemother. 2002;46(11):3394–3400. doi: 10.1128/AAC.46.11.3394-3400.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y, Casadevall A. Growth of Cryptococcus neoformans in presence of L-dopa decreases its susceptibility to amphotericin B. Antimicrob Agents Chemother. 1994;38(11):2648–2650. doi: 10.1128/AAC.38.11.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kwon-Chung KJ, Tom WK, Costa JL. Utilization of indole compounds by Cryptococcus neoformans to produce a melanin-like pigment. J Clin Microbiol. 1983;18(6):1419–1421. doi: 10.1128/jcm.18.6.1419-1421.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee SC, Dickson DW, Casadevall A. Pathology of cryptococcal meningoencephalitis: analysis of 27 patients with pathogenetic implications. Hum Pathol. 1996;27(8):839–847. doi: 10.1016/S0046-8177(96)90459-1. [DOI] [PubMed] [Google Scholar]
- 38.Nosanchuk JD, Rosas AL, Lee SC, Casadevall A. Melanisation of Cryptococcus neoformans in human brain tissue. Lancet. 2000;355(9220):2049–2050. doi: 10.1016/S0140-6736(00)02356-4. [DOI] [PubMed] [Google Scholar]
- 39.Mesa-Arango AC, Scorzoni L, Zaragoza O. It only takes one to do many jobs: Amphotericin B as antifungal and immunomodulatory drug. Front Microbiol. 2012;3:286. doi: 10.3389/fmicb.2012.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meyer W, et al. Consensus multi-locus sequence typing scheme for Cryptococcus neoformans and Cryptococcus gattii. Med Mycol. 2009;47(6):561–570. doi: 10.1080/13693780902953886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hicks JK, Bahn YS, Heitman J. Pde1 phosphodiesterase modulates cyclic AMP levels through a protein kinase A-mediated negative feedback loop in Cryptococcus neoformans. Eukaryot Cell. 2005;4(12):1971–1981. doi: 10.1128/EC.4.12.1971-1981.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee A, et al. Survival defects of Cryptococcus neoformans mutants exposed to human cerebrospinal fluid result in attenuated virulence in an experimental model of meningitis. Infect Immun. 2010;78(10):4213–4225. doi: 10.1128/IAI.00551-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.