Abstract
The transcription factor steroidogenic factor 1 (SF-1; also known as NR5A1) is a crucial mediator of both steroidogenic and nonsteroidogenic tissue differentiation. Mutations within SF1 underlie different disorders of sexual development (DSD), including sex reversal, spermatogenic failure, ovarian insufficiency, and adrenocortical deficiency. Here, we identified a recessive mutation within SF1 that resulted in a substitution of arginine to glutamine at codon 103 (R103Q) in a child with both severe 46,XY-DSD and asplenia. The R103Q mutation decreased SF-1 transactivation of TLX1, a transcription factor that has been shown to be essential for murine spleen development. Additionally, the SF1 R103Q mutation impaired activation of steroidogenic genes, without affecting synergistic SF-1 and sex-determining region Y (SRY) coactivation of the testis development gene SOX9. Together, our data provide evidence that SF-1 is required for spleen development in humans via transactivation of TLX1 and that mutations that only impair steroidogenesis, without altering the SF1/SRY transactivation of SOX9, can lead to 46,XY-DSD.
Introduction
Steroidogenic factor 1 (SF-1; also known as NR5A1) is a transcription factor involved in steroidogenesis, reproduction, and sexual differentiation (1). In mice, it is expressed in all primary steroidogenic tissues and in the embryonic urogenital ridge. SF-1 participates with sex-determining region Y (SRY) and SOX-9 in mammalian sex determination and regulates Müllerian-inhibiting substance (AMH) (2, 3).
SF1 mutations lead to several phenotypes, including disorders of sexual development (DSD) with sex reversal, spermatogenetic failure, premature ovarian failure, and adrenocortical insufficiency (AI) (OMIM 184757). The only 2 recessive SF1 mutations described to date, D293N and R92Q (4, 5), caused severe 46,XY-DSD as well as adrenal failure in the R92Q homozygote. Here, we report a novel homozygous SF1 mutation, R103Q, presenting with 46,XY complete sex reversal and asplenia, but without AI. This mutation impaired SF-1 activation of the spleen development gene TLX1. It also impaired activation of steroid synthesis, but did not affect synergistic SF-1 and SRY activation of SOX9, a gene considered to be crucial for testicular differentiation.
Results and Discussion
Clinical features and laboratory studies.
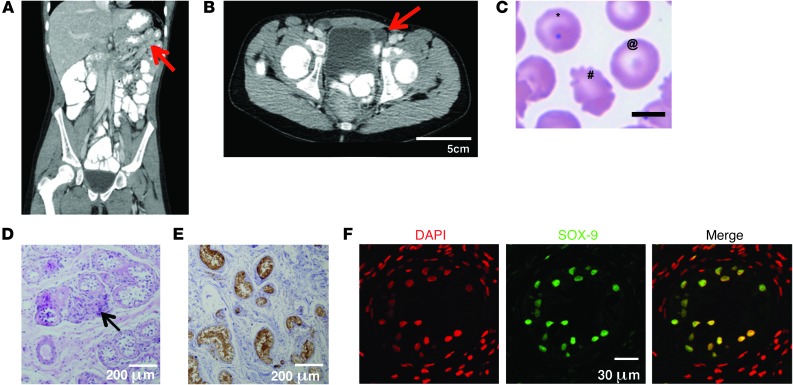
A 13.5-year-old girl of consanguineous Palestinian parents presented with abdominal pain ultimately ascribed to fecal impaction. History was notable only for delayed puberty and pneumococcal sepsis at 9 months of age. Ultrasound and CT imaging revealed asplenia, bilateral inguinal testes, and absence of the uterus, ovaries, and fallopian tubes (Figure 1, A and B). Peripheral blood smear revealed typical asplenia-associated Howell Jolly bodies and poikilocytosis (Figure 1C). Karyotype was 46,XY. Blood tests showed normal electrolytes, aldosterone, basal and adrenocorticotropic hormone–stimulated (ACTH-stimulated) cortisol, and 17-hydroxylase–progesterone. Dehydroepiandrosterone, androstenedione, and testosterone levels remained low to undetectable despite ACTH stimulation, while ACTH levels were slightly elevated (Supplemental Table 1; supplemental material available online with this article; doi: 10.1172/JCI73186DS1). Estrogen and progesterone levels were prepubertal. These results excluded a precursory major defect in steroid biosynthesis. 17-hydroxylase deficiency was thought to be an unlikely cause for the phenotype, given the patient’s normal serum electrolytes and normal blood pressure at 13.5 years of age. The severe sex reversal (46,XY-DSD) was therefore postulated to result from a genetic defect affecting testicular differentiation and gonadal function. The patient was reared as a female, given lack of male genitalia, and her gonads were surgically removed and studied for premalignant changes. Gonadal histopathology revealed normal and abundant Sertoli cells, few Leydig cells (consistent with low serum testosterone levels), absence of germinal or pregerminal cells, absence of staining for premalignant markers (e.g., placental-like alkaline phosphatase [PLAP]), and positive inhibin and SOX-9 staining in Sertoli cells (Figure 1, D–F).
Figure 1. Phenotypic features of the patient.
(A) CT scan of the patient’s abdomen, revealing asplenia (arrow) and lack of uterus. (B) CT scan of the inguinal region, revealing inguinal testes (arrow). (C) Patient’s peripheral blood smear, with Howell-Jolly bodies (*), target cells (@), and poikilocytosis (#) indicative of asplenia. (D) H&E staining showed paucity of Leydig cells (arrow), lack of germinal cells, and abundance of Sertoli cells. (E) Paraffin-embedded sections of the patient’s testes immunostained with anti-inhibin antibody (brown). (F) Immunofluorescent staining of the proband’s testis with anti–SOX-9 antibody (green) and general nuclear staining with DAPI (red). Positive SOX-9 staining in Sertoli cells was similar in strength in the patient’s testes and in the control testes, which had a higher total number of cells, consisting mostly of germinal and pregerminal cells (not shown). Scale bars: 5 μm (C); 200 μm (D and E); 30 μm (F).
Genetic analysis.
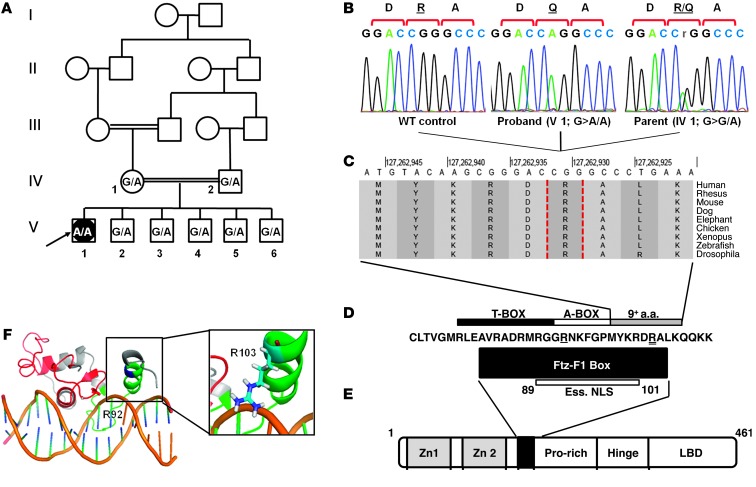
Analysis for XY-DSD included testing for SRY (by PCR) and sequencing of STAR and SF1 as candidates for nonclassic steroidogenic failure or gonadal dysgenesis. SRY was present, and no mutations were identified in STAR, consistent with the patient’s normal mineralocorticoid and glucocorticoid levels (Supplemental Table 1). However, in SF1, we identified a homozygous G-to-A transition (c.308G>A) resulting in a substitution of arginine to glutamine at codon 103 (p.R103Q). Both parents and all 5 unaffected brothers of the patient were heterozygous for this mutation (Figure 2, A–C), which was not detected in 190 ethnically matched controls (Supplemental Figure 1). This novel amino acid substitution, the third recessive SF1 mutation reported to date, results in loss of positive charge in the otherwise basic sub-domain of the cross-species conserved Fushi-tarazu (Ftz-F1) box of SF1 (Figure 2, D and E, and ref. 6). In a 3D model derived from SF-1 solution structure, the positively charged R103 appears to be in close proximity to the negatively charged sugar-phosphate backbone of the DNA site bound by SF-1 (Figure 2F and ref. 7). Replacement with a noncharged residue is likely to affect DNA binding, as previously shown for similar substitutions in the Ftz-F1 box (8).
Figure 2. Characterization of the SF1 mutation in the patient’s family.
(A) Pedigree of the patient’s consanguineous family. The proband (V1, arrow) a 46,XY female, has healthy first-cousin parents (IV1 and IV2) and 5 siblings (V2–V6). SF1 c.308 genotype (G, WT; A, mutant) is shown for each. (B) DNA sequencing chromatograms of the c.308G>A mutation (p.R103Q): homozygous in the proband, heterozygous in the mother, absent in a control (subject numbering as in A). (C) Cross-species conservation of the residues adjacent to R103 (dashed vertical outlines). Data were obtained from the UCSC human genome browser (24). (D) Conserved sequence of the Ftz-F1 box, the T-box and A-box subdomains, and the essential nuclear localization signal (Ess. NLS) of SF1 (6, 25). Residues mutated in the R92Q and R103Q mutations are denoted by single and double underline, respectively. (E) SF1 protein domains, including 2 zinc finger motifs, Ftz-F1 box (black box), proline-rich domain, hinge domain, and ligand-binding domain (LBD). (F) 3D model (generated using PyMol software; ref. 26) based on the solution structure of SF1 (7), showing the DNA-binding domain bound to its target sequence in the inhibin α subunit promoter. The 2 zinc finger motifs are shown in red, Ftz-F1 box is green, and R103 is blue. Inset shows the orientation of the R103 side chain, with the positive charge adjacent to the negatively charged DNA backbone.
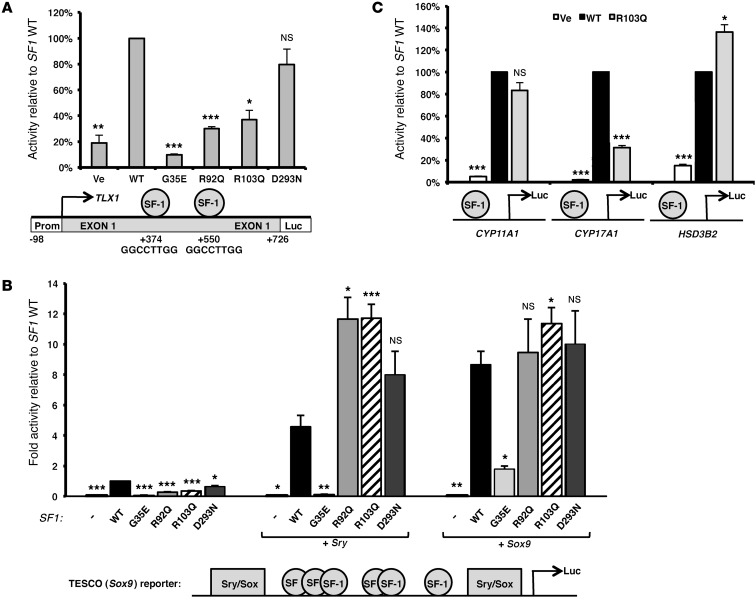
The unique finding of asplenia in this patient (Figure 1A), together with impaired spleen development reported in Sf1 knockout mice (9), raised the hypothesis that the R103Q mutation may alter expression of SF-1–regulated genes important for spleen development. We therefore searched for SF-1 recognition elements in genes previously implicated in spleen development, and identified 2 such bona fide elements in the first exon of TLX1 (OMIM 186770). A similar cluster of SF-1 binding sites was not found in the promoters of other spleen-development genes (PBX-1, NKX2-5, NKX3-2, WT-1, and POD-1). TLX-1 is a homeodomain-containing transcription factor critical for embryonic spleen development, as Tlx1 knockout mice have isolated asplenia (10, 11); in contrast, the other above-described spleen development genes also affect the development of other organs. To determine whether SF-1 regulates TLX1, and whether this effect is altered by the SF1 R103Q mutation, we engineered and tested the activity of a luciferase reporter construct controlled by the minimal promoter and first exon of TLX1 (Figure 3A). Whereas WT SF1 promoted TLX1 transcription, the SF1 R103Q mutation dramatically decreased this transcriptional activity by 2.7-fold in COS-7 cells and similarly in CHO cells (Figure 3A and Supplemental Figure 2). Interestingly, previously reported SF1 mutations, G35E and R92Q, also decreased SF-1 activation of the TLX1 promoter, whereas the D293N mutation, associated with a milder phenotype, had no significant effect (Figure 3A and refs. 5, 12, 13). This may explain the early demise of a homozygous R92Q patient, who died of sepsis, a well-known complication of hyposplenism, at 4–5 months of age (14). While SF-1 is characterized as a transcription factor involved in human gonadal and adrenal development, it was not previously known to be important for human spleen development. Such a role for SF-1 was surmised from tissue expression studies (15) and from the splenic phenotype of Sf1 knockout mice, which have small and maldeveloped spleens, but not complete asplenia (9). Interestingly, another case of asplenia in a patient with a SF1 mutation was presented after our study was completed (16). Our finding that the SF1 R103Q mutation impaired SF-1 transactivation of the TLX1 promoter provides a mechanism for the observed asplenia and suggests a role for SF-1 as a facilitator of normal spleen development in humans.
Figure 3. Functional studies of the SF1 R103Q mutant.
Transcriptional activation of spleen-specific (A), testes-specific (B), and steroidogenic (C) promoters by WT or mutant SF1 expression vectors were assayed by transient cotransfection of the expression vectors using the Promega Dual Luciferase assay system. For SF-1 binding elements in the reporters, see Supplemental Table 3. (A) Transcriptional activation of the spleen development–specific TLX1 promoter by WT and mutant SF1 constructs was studied in COS-7 cells. Ve, empty vector control. The TLX1 promoter–luciferase reporter construct is shown below, with the TLX1 promoter, transcription start site (arrow), exon 1 harboring 2 SF-1 binding sequences (spheres), and the luciferase reporter gene (Luc). Numbering is relative to the TLX1 transcription start site, at position +1. (B) Activity of the SOX9 testis-specific TESCO-luciferase enhancer, harboring both SF-1 and SRY/SOX binding sites, was measured in COS-7 cells. Transfections were performed using empty vector control (–) or WT or mutant SF1 expression vectors, either alone or together with Sry-myc or Sox9 expression vectors (3). A schematic illustration of the reporter construct is shown below. (C) Activity of the steroidogenic CYP11A1 (left), CYP17A1 (middle), and HSD3B2 (right) promoter reporter constructs (27) transfected with the SF1 constructs into nonsteroidogenic HEK293 cells. Results represent mean ± SEM relative luciferase activity of 4–5 independent experiments performed in duplicate. *P < 0.05, **P < 0.01, ***P < 0.001 vs. WT.
SF-1 plays a critical role in many aspects of gonadal development and testicular differentiation, including steroidogenesis (17). SF-1 is thought to induce SRY expression in the early gonad, leading to synergistic activation (by SRY and SF-1) of SOX9 transcription through its testis-specific enhancer, TES, and when sufficient levels of SOX9 are achieved, SOX9 replaces SRY and binds its own enhancer together with SF-1 to help maintain its own expression. Failure of this SF-1–induced SOX9 activation may lead to sex reversal (3, 18). In the next stage of testicular differentiation, SF-1 in Leydig cell progenitors also regulates expression of key testosterone synthesis genes, including STAR and CYP17A1. SF1 mutations can lead to XY sex reversal by impairing any of these functions, but most reported mutations have not been evaluated for their effects on SOX9 expression.
Based on the developmental roles of SF-1, we examined the effects of SF1 mutations R103Q, G35E, R92Q, and D293N on SOX9 gene expression using the luciferase reporter system designed by Sekido et al. (3), which assays transactivation of the core mouse SOX9 enhancer, TESCO. In the absence of SF-1, neither SRY nor SOX-9 overexpression stimulated TESCO-dependent reporter activity (Figure 3B). Recapitulating the known sequence of testicular development requiring a synergistic effect of SF-1 and SRY on the SOX9 promoter, expression of SRY or SOX-9 together with WT SF1 stimulated TESCO reporter activity ∼4.5- and ∼8.5-fold, respectively (Figure 3B).
SF1 mutations, in the absence of SRY or SOX-9, reduced TESCO activation: R103Q reduced activation compared with WT SF1 (by 65%), as did the R92Q and D293N mutations (by 72% and 39%, respectively), and G35E drastically decreased TESCO activity by 93% (Figure 3B). However, the synergistic effect of SF-1 with either SRY or SOX9 was not impaired by the novel R103Q mutation or by the 2 previously clinically reported recessive mutations, R92Q and D293N (Figure 3B).
These findings were consistent with the normal histological appearance and inhibin staining of Sertoli cells in the gonads removed from the patient. Furthermore, staining of these gonadal sections with antibodies against SOX-9, the critical protein for male gonadal sexual development (19), demonstrated, for the first time, SOX-9 protein expression in gonadal Sertoli cells of a 46,XY DSD patient (Figure 1F). In contrast to the recessive SF1 mutations, impairment of TESCO activation by G35E (which is known to cause autosomal-dominant DSD; ref. 20) remained even with coexpression of SRY or SOX-9, and similar results were obtained in CHO cells (Figure 3B and Supplemental Figure 3). This qualitative difference between recessive mutations and a severe dominant SF1 mutation was not distinguishable in a previous study conducted with the human TES (hTES) element, most probably because SRY alone is sufficient for hTES activation (21).
Finally, to understand the XY sex reversal phenotype and the patient’s lack of blood testosterone, we studied the effect of the SF1 R103Q mutation on transcriptional activation of 3 steroidogenic gene promoters: CYP11A1, CYP17A1, and HSD3B2. Compared with WT SF1, R103Q reduced by 3-fold activation of the CYP17A1 promoter, which lacks SRY/SOX-9 binding sites. Activation of CYP11A1 was not significantly affected by the mutation, and HSD3B2 promoter activity was slightly elevated (1.3-fold; Figure 3C). It therefore appeared that XY sex reversal in this patient was due to a defect in steroidogenesis, rather than impaired SOX9 activation. This is consistent with the general observation that the degree of hypoandrogenism in SF1 mutant patients is greater than the degree of testicular dysgenesis, as well as with examples of sex-limited dominant inheritance of SF1 mutations from unaffected mothers to affected sons (22).
We hypothesize that the different phenotypes observed with SF1 mutations may be explained by their different effects on SRY and SOX9 activation and testicular steroidogenesis. The recessive SF1 mutations R92Q and D293N may be similar to R103Q in enabling sufficient activation of SRY to thus activate SOX9 (Figure 1E and Figure 3B), leading to early gonadal and testicular differentiation and inhibin expression (Figure 1, E and F). However, the defect in testosterone synthesis caused by the R103Q mutation was apparently sufficiently severe to lead to undescended testes and phenotypic sex reversal. Severe mutations such as G35E may act earlier in development to curtail SOX9 activation altogether.
In conclusion, our present study describes a unique clinical phenotype of severe 46,XY-DSD with asplenia, caused by a novel homozygous SF1 mutation, R103Q. Transactivation studies of TLX1 demonstrated for the first time that SF-1 is required for spleen development in humans, which shows that SF1-DSD and asplenia are not simply coincidental. We also found that SF1 mutations can lead to sex reversal due to a defect in testosterone synthesis without disrupting the SF1-SRY-SOX9 activation cascade. Because asplenia has therapeutic consequences, the splenic status of individuals with DSD harboring SF1 mutations should be actively examined.
Methods
Further information can be found in Supplemental Methods.
Genetic studies.
Standard Sanger sequencing was performed on genomic DNA extracted from the proband and her nuclear family. We sequenced the coding regions of both SF1 and STAR (Supplemental Table 2). The SF1 mutation identified was tested in ethnically matched controls by PCR and ApaI digestion (Supplemental Figure 1).
Antibody staining: SOX9 and inhibin.
Paraffin-embedded sections of the patient’s gonads were stained with mouse anti–SOX-9 and monoclonal mouse anti-inhibin, as previously described (23), and visualized by fluorescence and DAB staining, respectively.
Functional analysis of the SF1 R103Q mutation.
Promoter activity assays were performed by transfecting luciferase reporter vectors controlled by various gene promoters into cells, and used to assay for SF1 activity as a transcription factor.
Statistics.
For transfection experiments, 2-tailed paired t test was used to compare WT and mutant SF1 vectors. A P value less than 0.05 was considered significant.
Study approval.
Histological and molecular analysis of the patient’s tissues and blood, as well as genetic analysis of the patient’s family, were under informed written consent of the subjects themselves and/or the legal guardians thereof. The study was approved by the institutional Helsinki board of Shaare Zedek Medical Center (approval no. 20/10).
Supplementary Material
Acknowledgments
This study was supported by the Legacy Heritage Biomedical Program of the Israel Science Foundation (grant 1531/2009; to D. Zangen) and by the US Agency for International Development (USAID) program for Middle East Regional Cooperation (grant TA-MOU-10-M30-021; to E. Levy-Lahad and M. Kanaan). We thank Ryohei Sekido for providing the SRY-MYC, SOX9, and SF1 expression vectors as well as the TESCO-luc reporter vector.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article:J Clin Invest. 2014;124(5):2071–2075. doi:10.1172/JCI73186.
References
- 1.Luo X, Ikeda Y, Parker KL. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell. 1994;77(4):481–490. doi: 10.1016/0092-8674(94)90211-9. [DOI] [PubMed] [Google Scholar]
- 2.Shen WH, Moore CC, Ikeda Y, Parker KL, Ingraham HA. Nuclear receptor steroidogenic factor 1 regulates the mullerian inhibiting substance gene: a link to the sex determination cascade. Cell. 1994;77(5):651–661. doi: 10.1016/0092-8674(94)90050-7. [DOI] [PubMed] [Google Scholar]
- 3.Sekido R, Lovell-Badge R. Sex determination involves synergistic action of SRY and SF1 on a specific Sox9 enhancer. Nature. 2008;453(7197):930–934. doi: 10.1038/nature06944. [DOI] [PubMed] [Google Scholar]
- 4.Soardi FC, Coeli FB, Maciel-Guerra AT, Guerra-Junior G, Mello MP. Complete XY gonadal dysgenesis due to p.D293N homozygous mutation in the NR5A1 gene: a case study. J Appl Genet. 2010;51(2):223–224. doi: 10.1007/BF03195733. [DOI] [PubMed] [Google Scholar]
- 5.Achermann JC, et al. Gonadal determination and adrenal development are regulated by the orphan nuclear receptor steroidogenic factor-1, in a dose-dependent manner. J Clin Endocrinol Metab. 2002;87(4):1829–1833. doi: 10.1210/jcem.87.4.8376. [DOI] [PubMed] [Google Scholar]
- 6.Wilson TE, Fahrner TJ, Milbrandt J. The orphan receptors NGFI-B and steroidogenic factor 1 establish monomer binding as a third paradigm of nuclear receptor-DNA interaction. Mol Cell Biol. 1993;13(9):5794–5804. doi: 10.1128/mcb.13.9.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Little TH, et al. Sequence-specific deoxyribonucleic acid (DNA) recognition by steroidogenic factor 1: a helix at the carboxy terminus of the DNA binding domain is necessary for complex stability. Mol Endocrinol. 2006;20(4):831–843. doi: 10.1210/me.2005-0384. [DOI] [PubMed] [Google Scholar]
- 8.Ueda H, Sun GC, Murata T, Hirose S. A novel DNA-binding motif abuts the zinc finger domain of insect nuclear hormone receptor FTZ-F1 and mouse embryonal long terminal repeat-binding protein. Mol Cell Biol. 1992;12(12):5667–5672. doi: 10.1128/mcb.12.12.5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morohashi K, et al. Structural and functional abnormalities in the spleen of an mFtz-F1 gene-disrupted mouse. Blood. 1999;93(5):1586–1594. [PubMed] [Google Scholar]
- 10.Lu M, Gong ZY, Shen WF, Ho AD. The tcl-3 proto-oncogene altered by chromosomal translocation in T-cell leukemia codes for a homeobox protein. EMBO J. 1991;10(10):2905–2910. doi: 10.1002/j.1460-2075.1991.tb07840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roberts CW, Shutter JR, Korsmeyer SJ. Hox11 controls the genesis of the spleen. Nature. 1994;368(6473):747–749. doi: 10.1038/368747a0. [DOI] [PubMed] [Google Scholar]
- 12.Lourenco D, et al. Mutations in NR5A1 associated with ovarian insufficiency. N Engl J Med. 2009;360(12):1200–1210. doi: 10.1056/NEJMoa0806228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Achermann JC, Ito M, Hindmarsh PC, Jameson JL. A mutation in the gene encoding steroidogenic factor-1 causes XY sex reversal and adrenal failure in humans. Nat Genet. 1999;22(2):125–126. doi: 10.1038/9629. [DOI] [PubMed] [Google Scholar]
- 14.Sills RH. Splenic function: physiology and splenic hypofunction. Crit Rev Oncol Hematol. 1987;7(1):1–36. doi: 10.1016/S1040-8428(87)80012-4. [DOI] [PubMed] [Google Scholar]
- 15.Ninomiya Y, Okada M, Kotomura N, Suzuki K, Tsukiyama T, Niwa O. Genomic organization and isoforms of the mouse ELP gene. J Biochem. 1995;118(2):380–389. doi: 10.1093/oxfordjournals.jbchem.a124918. [DOI] [PubMed] [Google Scholar]
- 16. Hua-Mei M, Zhe S, Yan-Hong L. P2-d2-1111: A de novo novel heterozygous deletion mutation in steroidogenic factor 1 (SF1, NR5A1) in a 46,XY patient with primary adrenal failure and splenic hypoplasia/aplasia. Presented at: 9th Joint Meeting of Paediatric Endocrinology; September 19–22, 2013; Milan, Italy. [Google Scholar]
- 17.Sekido R, Lovell-Badge R. Genetic control of testis development. Sex Dev. 2013;7(1–3):21–32. doi: 10.1159/000342221. [DOI] [PubMed] [Google Scholar]
- 18.Pilon N, et al. Porcine SRY promoter is a target for steroidogenic factor 1. Biol Reprod. 2003;68(4):1098–1106. doi: 10.1095/biolreprod.102.010884. [DOI] [PubMed] [Google Scholar]
- 19.Kanai Y, Hiramatsu R, Matoba S, Kidokoro T. From SRY to SOX9: mammalian testis differentiation. J Biochem. 2005;138(1):13–19. doi: 10.1093/jb/mvi098. [DOI] [PubMed] [Google Scholar]
- 20.Tremblay JJ, Viger RS. A mutated form of steroidogenic factor 1 (SF-1 G35E) that causes sex reversal in humans fails to synergize with transcription factor GATA-4. J Biol Chem. 2003;278(43):42637–42642. doi: 10.1074/jbc.M305485200. [DOI] [PubMed] [Google Scholar]
- 21.Knower KC, et al. Failure of SOX9 regulation in 46XY disorders of sex development with SRY, SOX9 and SF1 mutations. PLoS One. 2011;6(3):e17751. doi: 10.1371/journal.pone.0017751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin L, Achermann JC. Steroidogenic factor-1 (SF-1, Ad4BP, NR5A1) and disorders of testis development. Sex Dev. 2008;2(4–5):200–209. doi: 10.1159/000152036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anuka E, et al. Infarct-induced steroidogenic acute regulatory protein: a survival role in cardiac fibroblasts. Mol Endocrinol. 2013;27(9):1502–1517. doi: 10.1210/me.2013-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kent WJ, et al. The human genome browser at UCSC. Genome Res. 2002;12(6):996–1006. doi: 10.1101/gr.229102.ArticlepublishedonlinebeforeprintinMay2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li LA, Chiang EF, Chen JC, Hsu NC, Chen YJ, Chung BC. Function of steroidogenic factor 1 domains in nuclear localization, transactivation, and interaction with transcription factor TFIIB and c-Jun. Mol Endocrinol. 1999;13(9):1588–1598. doi: 10.1210/mend.13.9.0349. [DOI] [PubMed] [Google Scholar]
- 26. Schrodinger LLC. 2010. The PyMOL Molecular Graphics System, Version 1.3r1. PyMOL Web site. http://www.pymol.org/ . Accessed March 7, 2014.
- 27.Camats N, et al. Ten novel mutations in the NR5A1 gene cause disordered sex development in 46,XY and ovarian insufficiency in 46,XX individuals. J Clin Endocrinol Metab. 2012;97(7):E1294–E1306. doi: 10.1210/jc.2011-3169. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.