Abstract
T cell activation is regulated by the interactions of surface receptors with stimulatory and inhibitory ligands. Programmed death-1 homolog (PD-1H, also called VISTA) is a member of the CD28 family of proteins and has been shown to act as a coinhibitory ligand on APCs that suppress T cell responses. Here, we determined that PD-1H functions as a coinhibitory receptor for CD4+ T cells. CD4+ T cells in mice lacking PD-1H exhibited a dramatically increased response to antigen stimulation. Furthermore, delivery of a PD-1H–specific agonist mAb directly inhibited CD4+ T cell activation both in vitro and in vivo, validating a coinhibitory function of PD-1H. In a murine model of acute hepatitis, administration of a PD-1H agonist mAb suppressed CD4+ T cell–mediated acute inflammation. PD-1H–deficient animals were highly resistant to tumor induction in a murine brain glioma model, and depletion of CD4+ T cells, but not CD8+ T cells, promoted tumor formation. Together, our findings suggest that PD-1H has potential as a target of immune modulation in the treatment of human inflammation and malignancies.
Introduction
Activation of naive T cells is initiated by TCR engagement of specific peptides that are presented by MHC molecules. The outcome of this antigen recognition is determined by an array of cell-surface coreceptors that are either costimulatory or coinhibitory. Costimulatory receptors on T cell surfaces can induce positive intracellular signaling pathways, while coinhibitory signals can either induce negative signaling pathways or disrupt signaling mechanisms after binding a ligand or a counterreceptor on APCs or other cell types (1). Coinhibitory molecules, including PD-1, Tim-3, BTLA, CTLA-4, Lag-3, and CD160, play critical roles in the negative regulation of T cell responses in lymphoid organs and peripheral nonlymphoid tissues to control immune responses and inflammation (1–4). With few exceptions, coinhibitory receptors and/or ligands are induced after T cell activation and serve as a negative feedback mechanism that controls T cell responses. Using antibodies and soluble receptors/ligands to manipulate coinhibitory molecules has shown promise in the treatment of cancer and autoimmune diseases (5). In addition, blocking the interaction of CD28/B7-1/B7-2 with soluble CTLA-4 Ig fusion protein (ORENCIA; Abatacept) is an effective treatment for rheumatoid arthritis, psoriasis and other autoimmune diseases (6). Anti–CTLA-4 mAb enhances systemic immunity with survival benefits in 10%–15% of advanced melanoma patients (7). More recently, mAbs have been used to block the PD-1/B7-H1 pathway, causing a more dramatic therapeutic efficacy, which affects a broader range of advanced human cancers, including melanoma, non–small cell lung carcinoma, and renal cell carcinoma. These antibodies act with minimal toxicity by specifically blocking interactions in the tumor microenvironment (8, 9).
Programmed death-1 homolog (PD-1H, also called VISTA) is an IgV domain–containing cell-surface molecule that is constitutively expressed on several hematopoietic cell subsets, including the majority of naive T cells, NK cells, macrophages, and dendritic cells, but not on B cells (10, 11). Based on its primary amino acid sequence, our studies suggest that PD-1H is a member of the CD28 receptor family and is most closely related to PD-1 (10). When expressed on APCs, PD-1H negatively regulates T cell responses by acting as a ligand that interacts with an unknown T cell receptor (11). This notion is supported by the in vitro inhibition of T cell responses that is caused by recombinant PD-1H Ig fusion protein (11). In addition, administration of a neutralizing mAb to PD-1H exacerbates experimental autoimmune encephalomyelitis in mice (11), while an anti–PD-1H agonist mAb has a potent inhibitory effect in graft-versus-host diseases (10). In this study, we utilize a newly generated PD-1H–deficient mouse and a mouse anti-mouse PD-1H agonist mAb to explore the functions of PD-1H expressed on CD4+ T cells and their potential therapeutic applications.
Results
Characterization of PD-1H–deficient mice.
PD-1H–deficient mice (Pd1h–/–, herein referred to as PD-1H–KO) were generated using standard gene targeting/homologous recombination methods and were confirmed to be deficient in PD-1H expression (Lexicon Pharmaceuticals; see Methods). PD-1H–KO mice were initially characterized by Lexicon and Genentech Inc. (12) and had an increased percentage of CD4+ T cells in the blood compared with WT controls, but otherwise had no major phenotypic alterations ( http://mmrrc.mousebiology.org/phenotype/index.php).
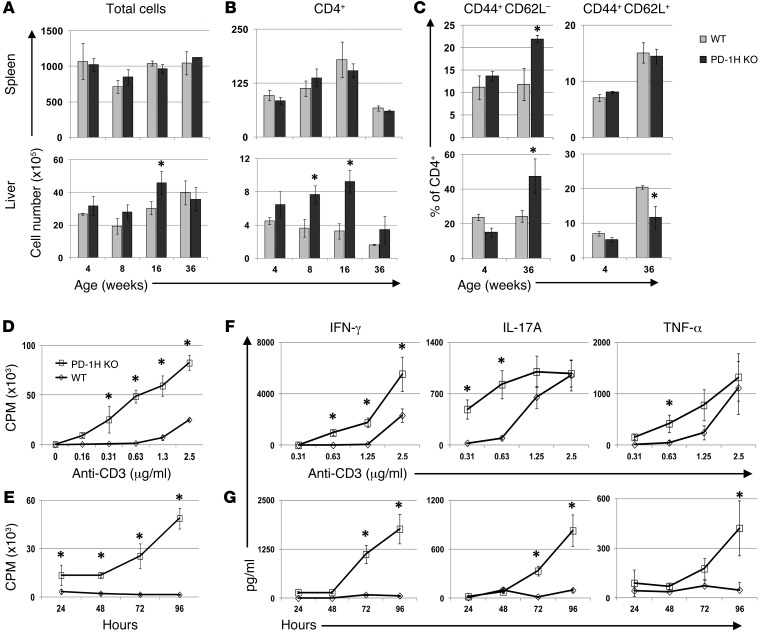
Hematopoietic cell subsets from the spleen, peripheral lymph nodes, mesenteric lymph nodes, and liver were analyzed by flow cytometry at 4, 8, 16, and 36 weeks in PD-1H–KO and WT littermates on a C57BL/6 background. PD-1H–KO mice at 4 weeks displayed normal total cell numbers, and the ratios of cells in all tissues examined were compared with those of their WT littermates (Figure 1A). Also, at 4 weeks of age, there were no differences in CD4+ and CD8+ T cell subsets, NKT cells, γδ T cells, NK cells, B cells, CD11b+ macrophages or Gr-1+ neutrophils observed in PD-1H–KOs compared with WT mice. These results indicate that PD-1H may not affect T cell development. In addition, CD62L expression on T cells at 4 weeks of age suggested no evidence of autonomous T cell activation. However, in comparison with WT mice, PD-1H–KO mice displayed elevated CD4+ T cells in the liver starting at 8 weeks, while CD4+ T cells in the spleen were similar to those in WT mice (Figure 1B). Interestingly, CD4+ T cells in both the spleen and liver also had increased levels of CD44+CD62L– effector memory phenotype T cells in aged mice, while liver CD4+ T cells in PD-1H–KO mice also had a decreased number of CD44+CD62L+ central memory cells (Figure 1C). The number of CD8+ T cells was also elevated in the liver of PD-1H–KO mice, but there was no change in the population of memory cells. PD-1H–KO mice had normal litters and no obvious pathologic abnormalities; their life spans were similar to those of WT mice. Based on these initial findings, we chose to specifically investigate the role of PD-1H expressed on CD4+ T cells.
Figure 1. Analysis of CD4+ T cells in PD-1H–deficient mice.
(A) Total cell numbers calculated in the spleen and liver of 4-, 8-, 16-, and 36-week-old PD-1H–KO mice and WT littermates. (B) Absolute CD4+ cell numbers were determined by total cell count multiplied by percentage of total cells/100 as determined by flow cytometry. (C) CD44+CD62L– and CD44+CD62L+ populations were determined as a percentage of CD4+ T cells at 4 and 36 weeks in both the spleen and liver. 5 to 6 mice were analyzed per group at each time point. PD-1H–deficient CD4+ T cell proliferation and cytokine production are enhanced in vitro. 96-well plates were coated overnight with anti-CD3 mAb, and purified CD4+ T cells were added to the wells. Plates were pulsed overnight with 3-HTdR thymidine. (D) Proliferation of PD-1H–KO or WT CD4+ T cells at serial dilutions of anti-CD3 was determined at 96 hours. Other time points were similar. (E) Proliferation at an anti-CD3 mAb of 0.63 μg/ml is shown at 24, 48, 72, and 96 hours. (F and G) Supernatants were harvested from 96-well proliferation plates at 24, 48, 72, and 96 hours and analyzed for IFN-γ, IL-17A, and TNF-α. In F, cytokine concentrations at 96 hours are shown at serial dilutions of anti-CD3 mAb. In E, cytokine concentrations are shown at 24, 48, 72 and 96 hours at a single anti-CD3 concentration. All experiments were repeated at least 3 times in triplicate. *P < 0.05.
PD-1H–deficient CD4+ T cells have an increased response to TCR-mediated stimulation in vitro.
Our previous study using a PD-1H–specific mAb demonstrated that PD-1H is constitutively expressed on naive CD4+ T cells (10). We used PD-1H–KO mice to specifically investigate the function of PD-1H when expressed on CD4+ T cells. CD4+ T cells (>98%) from PD-1H–KO mice and WT littermates were purified to analyze their responses to polyclonal TCR stimulation using a plate-bound anti-CD3 mAb. Purified WT and PD-1H–KO CD4+ T cells were primarily naive T cells (>98%) based on CD44 expression. The proliferation of PD-1H–KO CD4+ T cells in response to anti-CD3 was substantially higher than that of the control WT T cells in the presence of anti-CD3, and the extent of PD-1H–KO CD4+ T cell proliferation was dependent on anti-CD3 dose (Figure 1D). This enhanced response was also sustained compared with that of WT CD4+ T cells, as indicated by time course studies (Figure 1E). Cultured supernatants from anti-CD3–stimulated PD-1H–KO CD4+ T cells contained increased levels of IFN-γ and IL-17 at from 24 to 96 hours, while increases in TNF-α were less significant (Figure 1, F and G). Therefore, PD-1H expressed on CD4+ T cells functioned as an inhibitor of APC-independent polyclonal TCR stimulation. Next, we sought to determine, more directly, whether PD-1H functions as a receptor that directly inhibits CD4+ T cells.
PD-1H is a coinhibitory receptor on CD4+ T cells.
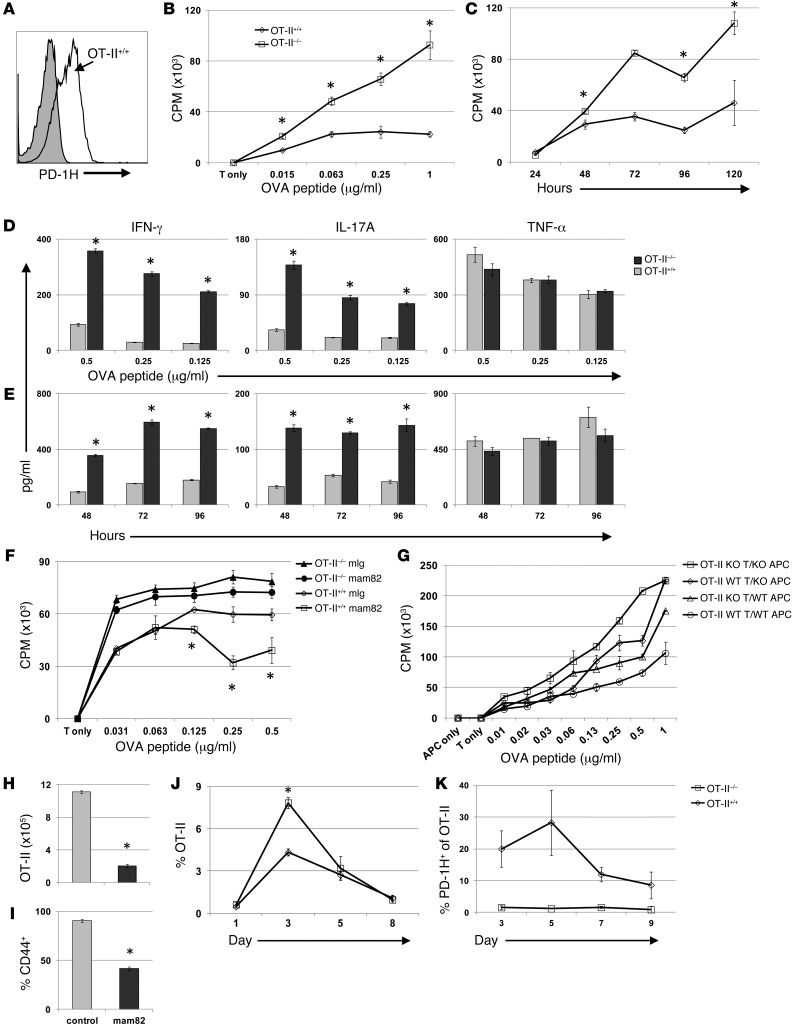
To facilitate further analysis, we backcrossed PD-1H–KO with OT-II TCR transgenic mice in which monoclonal CD4+ T cells recognize a chicken OVA peptide (OVA 323–339) in the context of the MHC class II molecule I-Ab. We confirmed the constitutive expression of PD-1H on the majority of naive (CD44–) WT OT-II CD4+ T cells (Figure 2A). When purified PD-1H–KO OT-II T cells (OT-II Pd1h–/–, herein referred to as OT-II–/–) were stimulated with OVA peptide–pulsed PD-1H–KO APCs, proliferation was greatly enhanced in a dose-dependent fashion (Figure 2B) and the response was sustained compared with that in WT OT-II T cells (Pd1h+/+, herein referred to as OT-II+/+) (Figure 2C). APCs were deficient in PD-1H, which indicates that the PD-1H expressed on OT-II T cells was responsible for the limited proliferation of OT-II+/+ T cells. IFN-γ and IL-17 production were also significantly enhanced (Figure 2D) and sustained (Figure 2E) in OT-II–/– culture compared with OT-II+/+, while no difference was observed in TNF-α production.
Figure 2. PD-1H inhibits antigen-specific CD4+ T cell responses in vitro.
OT-II+/+ and OT-II–/– T cells were evaluated for response to antigen. (A) Expression of PD-1H on naive OT-II+/+ T cells (white) and OT-II–/– T cells (gray). (B–F) Purified PD-1H–/– or PD-1H+/+ OT-II T cells were added to 96-well plates with irradiated PD-1H–KO splenocytes. Proliferation was assessed at specific peptide concentration (B) and at specific time points (C). Supernatants from proliferation assays were assessed for cytokines at specific peptide concentrations (D) and at time points using 0.5 μg/ml OVA peptide (E). (F) Proliferation was assessed in the presence of 2 μg/ml of PD-1H mAb or mouse Ig. (G) Irradiated WT APCs or PD1H KO APCs were cultured with either OT-II+/+ or OT-II–/– T cells and analyzed for proliferation. (H and I) Naive purified OT-II+/+ T cells were adoptively transferred (i.v.) on day –1 to PD-1H–KO recipient mice. OVA and polyI:C were injected on day 0 with either mouse Ig or anti–PD-1H. OT-II T cell numbers (H) and activation (I) as determined by CD44 expression were assessed on day 3. (J and K) Naive OT-II+/+ or OT-II–/– T cells were purified and identical numbers were transferred (i.v.) on day –1 to PD-1H–KO mice. OVA and polyI:C were injected on day 0. Mice bled at indicated time points were analyzed for percentage of OT-II T cells of total CD4+ T cells (J) and PD-1H expression (K). All experiments were repeated a minimum of 3 times. *P < 0.05.
Our results indicate that PD-1H on CD4+ T cells could function independently of APCs to suppress T cell responses to antigen, suggesting that PD-1H acts as a suppressive receptor on CD4+ T cells. However, it is still possible that PD-1H on T cells could function as a ligand to suppress neighboring T cells via an unknown receptor (T-T interaction). Because the counterreceptor of PD-1H is unidentified, a direct test of this interaction is not possible. To address this issue, we generated an agonist anti–PD-1H mAb that could mimic the ligand and directly suppress CD4+ T cell function. To do so, we immunized PD-1H–KO mice with purified murine PD-1H Ig recombinant fusion protein. Upon extensive screening of over 100 hybridomas, we obtained a new mouse anti-mouse PD-1H mAb, designated as clone mam82. This IgG1 isotype mAb specifically binds to PD-1H, as determined by ELISA, by staining PD-1H–transfected P815 mastocytoma and by staining of WT and PD-1H–KO T cells; no background mam82 staining was observed in any of the cell populations.
Using the OT-II T cell proliferation assay, as described above, with irradiated PD-1H–KO APCs, and either including a control mouse IgG or a mam82 mAb, we found that mam82 significantly inhibits WT OT-II T cell proliferation at high OVA peptide concentrations (Figure 2F). Because APCs in this assay are PD-1H–deficient and only the OT-II+/+ T cells express PD-1H, the effect of mam82 is dependent on binding OT-II+/+ T cells. Consistent with this finding, PD-1H–KO OT-II (OT-II–/–) T cells were also examined in the presence of mam82 or control mouse IgG. Our results show that mam82 has no effect in the absence of PD-1H in the culture. The effect of mam82 could not be interpreted as blocking the interaction of PD-1H and its counterreceptor because this would lead to an enhanced OT-II response, not a suppressed OT-II response. Together, our results demonstrate that PD-1H is a coinhibitory receptor that could directly inhibit CD4+ T cell proliferation.
PD-1H (VISTA) expressed on APCs inhibits CD4+ T cell functions via an unidentified receptor (11). To determine the role of PD-1H on APC versus T cells, we performed a “crisscross” experiment to examine the potential dual inhibitory function of PD-1H on both CD4+ T cells and APCs. This experiment was performed as above, but this time using 4 combinations of OT-II T cells and irradiated APCs: OT-II–/– T cells and KO APCs, OT-II–/– T cells and WT APCs, OT-II+/+ T cells and KO APCs, and OT-II+/+ T cells and WT APCs. The combination of OT-II–/– T cells with KO APCs resulted in the strongest T cell proliferation, and the OT-II+/+ T cells with WT APCs had the lowest proliferation, while combinations in which PD-1H was deficient on either OT-II T cells or APCs had an intermediate effect (Figure 2G). These results suggest that PD-1H on both CD4+ T cells and APCs contributes to the inhibition of T cell proliferation via specific pathways.
PD-1H suppresses early phase CD4+ T cell expansion in vivo.
In contrast with the inducible nature of many coinhibitory molecules, PD-1H is constitutively expressed on naive CD4+ T cells. Our in vitro results suggested that a possible role for PD-1H is to limit T cell activation. To test this in vivo, we transferred OT-II+/+ T cells into PD-1H–KO mice so that only OT-II T cells expressed PD-1H. Mice were subsequently immunized with OVA protein plus polyI:C as an adjuvant to activate OT-II T cells. Administration of mam82 at the time of OVA protein immunization inhibited the expansion (Figure 2H) and activation (Figure 2I) of OT-II T cells at 72 hours compared with control IgG-treated mice.
We then examined PD-1H–deficient OT-II T cell response to antigen in vivo. We transferred identical numbers of either OT-II–/– or OT-II+/+ T cells into PD-1H–KO mice, followed by i.p. immunization with OVA protein and polyI:C 24 hours later. OT-II T cells in the blood on days 1, 3, 5, and 8 indicated that OT-II–/– T cell expansion was significantly greater by day 3 compared with OT-II+/+ T cell expansion (Figure 2J). However, there was no difference in OT-II–/– and OT-II+/+ percentages by day 5, suggesting that PD-1H on T cells primarily limits early activation and expansion. Indeed, PD-1H expression on OT-II+/+ T cells appears highest on naive and early activated T cells but quickly declines following activation (Figure 2K). These findings were consistent with our in vitro results and further suggested that PD-1H functions as an inhibitory receptor that suppresses CD4+ T cell activation.
PD-1H suppresses acute inflammation in a model of experimental hepatitis.
Because PD-1H is expressed on naive and early activated CD4+ T cells, we proposed that in addition to limiting TCR-dependent CD4+ T cell stimulation, PD-1H may also regulate T cell responses during acute inflammation. To test this possibility, we used a model of Concanavalin A–induced (Con A–induced) hepatitis in which infusion of Con A induces acute liver inflammation that is largely dependent on polyclonal activation of CD4+ T cells and NKT cells (13–15).
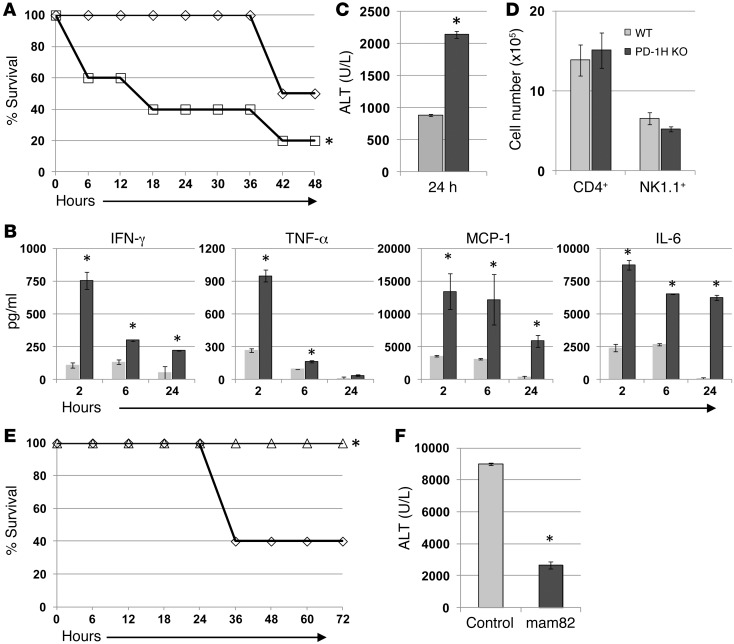
Four- to six-week-old mice were used for all hepatitis studies because cell subsets in this age group are indistinguishable in WT and PD-1H–KO mice (Figure 1). PD-1H–KO or WT C57BL/6 littermates (WT) were injected with a high dose (30 mg/kg) of Con A, and mice were monitored for survival. PD-1H–KO mice were more susceptible to Con A–induced lethality than WT mice, and the majority of mice (80%) died of hepatitis (Figure 3A). Treatment with sublethal Con A (15 mg/kg), which induces a similar pathology in the liver without lethality, allowed for the examination of several parameters associated with disease pathology. Analysis of serum cytokine levels from 2 to 24 hours after Con A treatment showed that PD-1H–KO mice had significantly elevated levels of IFN-γ, TNF-α, MCP-1, and IL-6 compared with WT mice (Figure 3B). Serum alanine aminotransferase (ALT) levels, a proxy for hepatocyte death and liver damage, more than doubled in PD-1H–KO mice compared with WT controls (Figure 3C). Interestingly, no difference was observed in the absolute number of CD4+ T cells or NK1.1+ cells in the livers of PD-1H–KO and WT mice 24 hours after Con A infusion (Figure 3D). These results support the supposition that enhanced pathology and mortality observed in PD-1H–KO mice are due to excessive activation of resident liver cells rather than changes in cellular infiltration in the liver.
Figure 3. PD-1H–deficient mice are more susceptible to acute Con A–induced hepatitis.
(A) PD-1H–KO or WT C57BL/6 littermates received i.v. injections of Con A at 30 mg/kg and were monitored for survival. (B) PD-1H–KO or WT C57BL/6 littermates received sublethal i.v. injections of Con A at 15 mg/kg,and serum was isolated at the indicated times to analyze cytokine levels. (C) As in B, serum at 24 hours was analyzed for ALT concentration. (D) The livers of PD-1H–KO or littermates receiving 15 mg/kg Con A were isolated at 24 hours, and the cells were stained with anti-CD4 and anti-NK1.1 mAbs for calculation of absolute cell numbers. (E) C57BL/6 mice were pretreated with i.p. injections of control mouse Ig or anti–PD-1H (mam82) 3 hours before i.v. injections of Con A at 30 mg/kg and monitored for survival. (F) As in E, mice were bled at 20 hours, and the serum was analyzed for ALT concentration. The experiments were repeated 3 to 5 times with 5 mice/group. *P < 0.05.
The increased susceptibility of PD-1H–KO mice to Con A–induced acute inflammation suggested that PD-1H limits acute inflammation. Therefore, we predicted the agonist mam82 mAb would suppress Con A pathology and lethality. To test this, we treated WT C57BL/6 mice with either mam82 mAb or control IgG 3 hours before a lethal injection (30 mg/kg) of Con A and monitored their survival. As shown in Figure 3E, mam82 mAb treatment protected mice, while 60% of control mice died within 36 hours of treatment. Concordant with the agonist function of mam82, serum ALT levels were significantly decreased in mam82-treated mice (Figure 3F). Our results thus indicate a crucial role of PD-1H in the control of acute experimental hepatitis.
PD-1H deficiency on hematopoietic cells is sufficient to exacerbate acute inflammation in vivo.
PD-1H is expressed and may function on several hematopoietic cell subsets, while the expression and function of PD-1H on nonhematopoietic cells is unknown. To evaluate the contribution of PD-1H on hematopoietic and nonhematopoietic cells in Con A–mediated acute inflammation, we used BM chimeric models in which lethally irradiated mice (recipients) were reconstituted with T cell–depleted BM (TCD-BM) from either PD-1H–KO mice (BM–/–) or WT littermates (BM+/+) donors (Figure 4A).
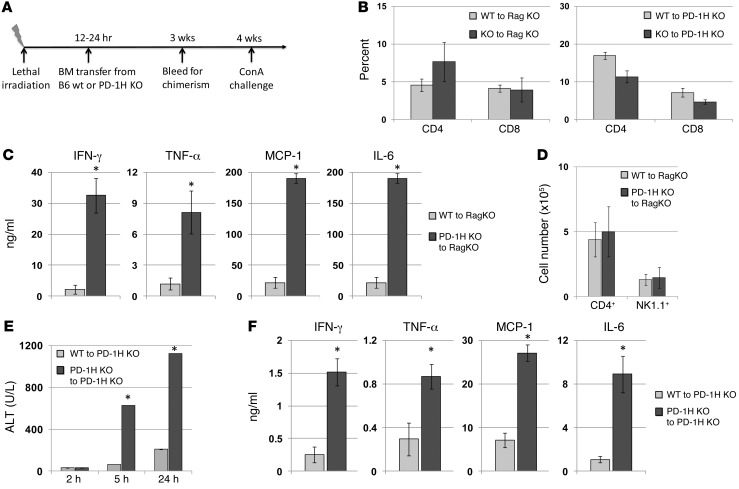
Figure 4. PD-1H expression on hematopoietic cells regulates the severity of acute Con A–induced hepatitis.
(A) Depiction of BM chimeric models for the Con A hepatitis experiments and analysis of chimerism in blood for CD4+ and CD8+ T cell reconstitution at 3 weeks. (B) Chimeric mice were bled 3 weeks after reconstitution, and CD4+ and CD8+ percentages were determined. (C) Rag-1–KO mice adoptively transferred with either PD-1H–KO or WT TCD-BM and injected with 15 mg/kg Con A were bled at 3 hours for analysis of serum cytokine levels as indicated. (D) As in C, liver lymphocytes were stained with anti-CD4 and anti-NK1.1 to determine absolute cell numbers in liver. (E) PD-1H–KO mice reconstituted with PD-1H–KO or WT TCD-BM were injected (i.v.) with 15 mg/kg of Con A and bled at 2, 5, and 24 hours for analysis of serum ALT concentrations. (F) As in C, serum at the 2- hour time point was analyzed for cytokines. *P < 0.05.
First, TCD-BM–/– or TCD-BM+/+ was transferred to lethally irradiated Rag-1–deficient recipients (Rag-KO) that lack T and B cells. In this model, PD-1H was deficient on hematopoietic cells from mice that were reconstituted with BM–/–, while somatic cells and radiation-resistant cells remained PD-1H sufficient. After BM transfer, mice were allowed to reconstitute for 4 weeks. Mice were bled at 3 weeks and BM–/– and BM+/+ mice were found to have similar and reasonable levels of CD4+ and CD8+ T cells (Figure 4B). At 4 weeks after reconstitution, mice were injected with 15 mg/kg of Con A. Compared with BM+/+ mice, the mice reconstituted with BM–/– had drastically elevated levels of serum cytokines, including IFN-γ, TNF-α, MCP-1, and IL-6 (Figure 4C). Flow cytometry analysis of liver lymphocytes 24 hours after Con A treatment revealed that chimeric mice reconstituted with BM–/– or BM+/+ had similar levels of CD4+ and NK1.1+ cells (Figure 4D).
In a second chimeric model, lethally irradiated PD-1H–KO mice were used as recipients and were reconstituted with TCD-BM–/– or TCD-BM+/+ as above. In this model, PD-1H would only be expressed on hematopoietic cells in mice that were reconstituted with BM+/+, while BM–/– reconstituted mice served as PD-1H–KO controls. Reconstitution of PD-1H–KO recipient mice with either BM+/+ or BM–/– resulted in similar levels of CD4+ and CD8+ T cells when analyzed 4 weeks after reconstitution (Figure 4B). As expected, mice reconstituted with PD-1H–deficient BM had significantly elevated ALT (Figure 4E) and proinflammatory cytokine levels (Figure 4F) compared with mice reconstituted with WT BM when injected with 15 mg/kg of Con A. Together, these results confirmed that PD-1H expression on hematopoietic cells alone is sufficient to modulate the severity of Con A–induced acute inflammation. Because CD4+ T cells and NKT cells are primarily responsible for Con A–induced pathology (13–15), these results suggested that PD-1H on CD4+ T cells, and potentially NKT cells, played a central role in limiting the extent of Con A–induced inflammation.
PD-1H selectively suppressed CD4+ T cell–mediated tumor immunity in a murine glioma model.
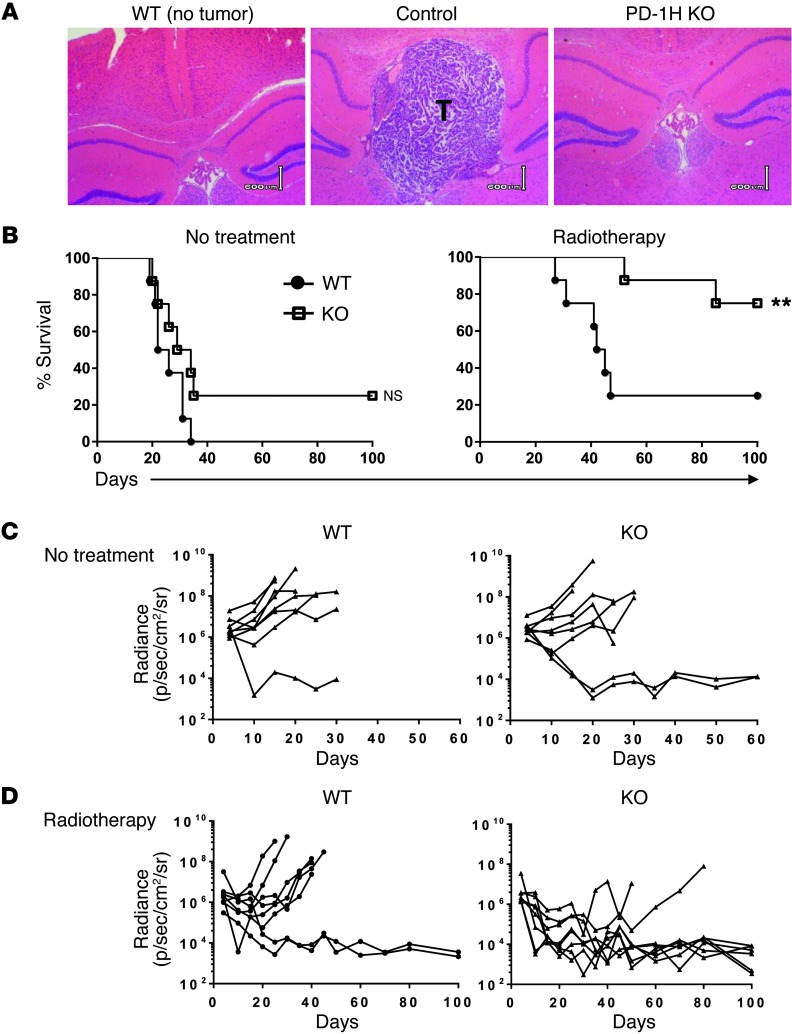
With PD-1H’s profound effect on CD4+ T cell suppression, we tested PD-1H’s role in regulating antitumor immunity. In a murine glioma model, GL261-luc cells were directly inoculated into the left hemisphere of the brains of control littermates (WT) or PD-1H–KO mice and the real time growth of their brain tumors was monitored and analyzed using an in vivo imaging system (Supplemental Figures 1–3; supplemental material available online with this article; doi: 10.1172/JCI74589DS1); the data were validated by histopathology (Figure 5A). WT mice inoculated with GL261-luc tumors died within 35 days. Ionizing irradiation of the whole brain with a total dose of 4 Gy could extend the survival in approximately 20% of mice while the majority of them died within 50 days (Figure 5B). Interestingly, PD-1H–KO mice treated with ionizing radiation had significantly extended survival compared with WT mice (Figure 5B), which correlated with decreased intracranial radiance levels, or a reduction in tumor volume (Figure 5C and Supplemental Figure 2). Our results indicate that elimination of PD-1H confers resistance to GL261 brain tumor growth in a low-dose radiotherapy setting.
Figure 5. PD-1H–KO mice are resistant to the growth of GL261 glioma.
3 × 105 GL261-luc cells were injected into the left hemisphere of the brains of PD-1H–KO or WT mice. Mice were subsequently treated with or without 4 Gy radiotherapy of the whole brain on day 5 after tumor inoculation. (A) Whole-brain tissues were isolated on day 15 for H&E staining. The brain from a WT mouse without tumor inoculation was used as a control. T, tumor area. Scale bars: 600 μm. (B) Survival of WT or PD-1H–KO mice with or without radiotherapy was monitored daily (n = 8). (C) Growth of GL261 tumors in individual mice was measured using a Lumina XR imaging system every 5 days and presented as bioluminescence of tumor in radiance (n = 8). **P < 0.01.
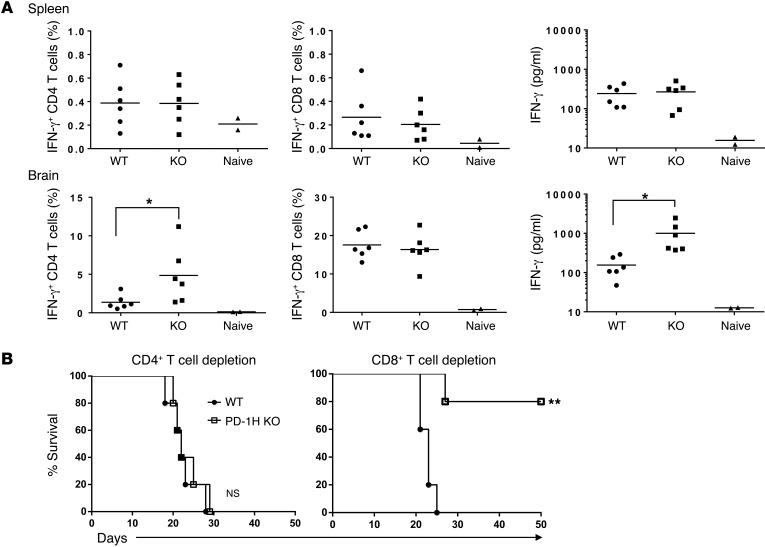
To evaluate the role of CD4+ T cells in this model, we first enumerated antigen-experienced T cells in freshly isolated brain and spleen specimens by staining for IFN-γ+CD4+ and IFN-γ+CD8+ T cells after a brief restimulation with irradiated WT GL261. As shown in Figure 6A, a significantly increased percentage of IFN-γ+CD4+ T cells was found in the brains, but not in the spleens of tumor-bearing PD-1H–KO mice; meanwhile, there were no significant differences in IFN-γ+CD8+ T cells in either brains or spleens. This finding is consistent with increased IFN-γ levels in culture supernatant after restimulation with irradiated GL261 cells in vitro (Figure 6A). Importantly, depletion of CD4+ T cells in vivo using anti-CD4 mAbs resulted in the elimination of tumor resistance in PD-1H–KO mice treated with radiotherapy, whereas depletion of CD8+ T cells by anti-CD8a mAb had no impact on tumor growth (Supplemental Figure 3) or on overall survival (Figure 6B). Therefore, we conclude from these studies that PD-1H selectively suppresses CD4+ T cell–mediated tumor immunity in this mouse glioma model.
Figure 6. Resistance to GL261 tumor growth in PD-1H–KO mice is mediated by CD4+ T cell immunity.
3 × 105 GL261-luc tumor cells were injected into the left hemisphere of the brains of PD-1H–KO or WT mice. Mice were treated with a total dose of 4 Gy radiotherapy of the whole brain on day 5 after tumor inoculation. (A) Ten days after radiotherapy, splenocytes and brain lymphocytes were isolated and restimulated with irradiated GL261 cells for 5 days. Percentages of IFN-γ–producing CD4+ T cells of total CD4+ T cells (left panels) or IFN-γ–producing CD8+ T cells of total CD8+ T cells (middle panels) were detected by intracellular staining. IFN-γ levels in culture supernatant were detected by CBA (right panels). One point indicates the result from 1 mouse (n = 6). Naive mice (n = 2) without tumor inoculation were used as negative control. (B) CD4+ T cells or CD8+ T cells were depleted by i.p. injection of anti-CD4 (GK1.5) or anti-CD8a (53-6.72) antibody at 500 μg every 5 to 7 days from 5 days before tumor inoculation. Survival of mice was monitored daily up to 50 days (n = 5). *P < 0.05; **P < 0.01.
Discussion
Experiments using PD-1H–KO mice indicate that PD-1H expressed on CD4+ T cells has an inhibitory function. In addition, studies utilizing an agonist mAb targeting PD-1H point toward the ability of PD-1H to function as a receptor on CD4+ T cells. Together, our experiments support the hypothesis that PD-1H is an inhibitory receptor on CD4+ T cells that limits activation of CD4+ T cell responses to antigen-specific TCR-dependent T cell responses. By manipulating PD-1H in mouse models, either by elimination with gene targeting or by enforcement with agonistic mAb, we provide evidence that targeting this molecular pathway suppresses acute inflammation and enhances antitumor immunity.
A previous study by Wang et al. showed that PD-1H (VISTA) expressed on dendritic cells or tumor cells inhibited T cell responses and the authors postulated that this effect was mediated through a yet-to-be-identified receptor that is expressed on CD4+ T cells (11). Previously, we showed that PD-1H is constitutively expressed and can be further upregulated on CD4+ T cells (10). Furthermore, a mAb targeting PD-1H broadly inhibited mouse graft-versus-host disease through the inhibition of allogeneic T cell responses (10). While this finding suggests a role for PD-1H on T cells, it remains unclear whether PD-1H works as a receptor that suppresses CD4+ T cell function. Meanwhile, our molecular and genetic analyses indicate that PD-1H is most closely related to the coinhibitory receptor PD-1, rather than the PD-1 ligands B7-H1 (PD-L1) and B7-DC (PD-L2) or other B7 family ligands (10). Here, we have utilized PD-1H–KO mice and a new agonist mAb that targets PD-1H to show that PD-1H expressed on CD4+ T cells functions as a coinhibitory receptor that limits CD4+ T cell activation and function.
Using anti-CD3 to stimulate CD4+ T cells, in an APC-independent process, we found that the absence of PD-1H on CD4+ T cells caused enhanced proliferation and cytokine production. In addition, we used PD-1H–deficient APCs to stimulate antigen-specific CD4+ OT-II T cells, again showing that PD-1H deficiency on OT-II T cells causes enhanced OT-II proliferation and cytokine production compared with WT OT-II T cells. Both of these results support the idea that PD-1H acts as a receptor on CD4+ T cells to transmit a negative signal. In addition, we developed an agonist mAb against PD-1H to mimic the function of the PD-1H ligand. This agonist mAb inhibited CD4+ T cell function both in vitro and in vivo. These findings indicate that PD-1H functions as a coinhibitory receptor on CD4+ T cells because if mam82 mAb was blocking (antagonistic), enhanced expansion of CD4+ T cell should have resulted, which is similar to the results we obtained using PD-1H–deficient CD4+ T cells. Our findings provide for the possibility of elucidating the mechanisms of this new receptor in suppressing T cell immune responses.
Our data and the data from Wang et al. (10, 11) now indicate that PD-1H functions on both APCs and CD4+ T cells. To address the relative contribution of PD-1H on APCs and CD4+ T cells, we performed an experiment in which PD-1H was expressed on both OT-II T cells and APCs, on only OT-II T cells or APCs, or on neither (completely PD-1H deficient). Here, we found that PD-1H deficiency on both OT-II T cells and APCs yielded the strongest proliferation, while PD-1H–sufficient APCs and OT-II T cells resulted in the poorest proliferation. These results showed that PD-1H on both T cells and APCs contributes to suppression and may have a synergistic effect. It is likely that PD-1H possesses dual functionality as both a receptor and a ligand, reminiscent of other coinhibitory molecular interactions, such as B7-H1/PD-1 and B7-1/CTLA-4 (1). Indeed, the yet-to-be-identified PD-1H counterreceptor also functions as an inhibitory receptor on T cells, suggesting that this is a potent bidirectional, and synergistic, inhibitory axis of molecular interaction.
It is particularly interesting that PD-1H–KO mice are highly resistant to the growth of transplanted mouse GL261 glioma. GL261, upon injection into the brain, grows rapidly and kills the majority of mice within 6 weeks. Importantly, we observed a brain-specific increase in IFN-γ+CD4+ T cells, indicating a selective activation of CD4+ T cells in the brain of PD-1H–KO mice. Elimination of PD-1H expression in KO mice is less effective in the absence of radiation treatment, likely because low-dose ionizing radiation of the brain induces localized inflammation and increased infiltration of lymphocytes (16–19); these effects may be modulated by PD-1H inhibitory function. GL261 induces CD8+ T cell responses upon cancer vaccine treatment (20). However, we did not see any sign of selective CD8+ T cell activation in PD-1H–KO mice during the growth of GL261 tumors. In sharp contrast to the dominant role of CD8+ T cells in the majority of tumor models, depletion of CD8+ T cells in our GL261 tumor model did not eliminate immune resistance in PD-1H–KO mice, whereas elimination of CD4+ T cells completely abrogated the effect. This finding further supports the possibility that PD-1H suppresses the functions of CD4+ T cells.
While most coinhibitory receptors are expressed at suboptimal levels and are upregulated after T cell activation, PD-1H is constitutively expressed at significant levels on naive T cells. PD-1H may limit early T cell activation without completely abrogating T cell responses. As such, it is interesting to speculate on the effects of targeting PD-1H in combination with PD-1, CTLA-4, or other coinhibitory molecules in therapeutic disease models. The evidence presented here indicates PD-1H functions as an inhibitory receptor on CD4+ T cells. In support of these observations, the PD-1H cytoplasmic tail contains tyrosine residues in potential protein kinase C–binding sites (10). Finally, because PD-1H is expressed on several myeloid and lymphoid cell subsets, it is likely that PD-1H has broad immunological functions that have only begun to be elucidated. Therefore, PD-1H may be a useful therapeutic target for human diseases.
Methods
Mice.
PD-1H-KO (Genbank gene NM_028732; GenBank protein JN602184) mice were purchased from the mutant mouse regional resource center (MMRRC, University of California–Davis, Davis, California, USA). Mice were generated in 129/ScEvBrd-derived embryonic stem cells by targeting coding exon 1. Mice were subsequently backcrossed with C57BL/6N mice for more than 12 generations in our laboratory. Additional information on PD-1H–KO mice and primers used for screening can be found at the MMRRC site ( www.mmrrc.org/catalog/sds.php?mmrrc_id=31656). PD-1H homozygote (+/+) C57BL/6N mice generated from PD-1H heterozygotes were bred and maintained in conditions identical to those of PD-1H–KO mice and were used as controls (WT) for PD-1H–KO studies. OT-II mice were purchased from Jackson Laboratory and backcrossed to PD-1H–KO mice to generate PD-1H–deficient OT-II mice.
Generation of murine PD-1H mAb.
A PD-1H–KO mouse was immunized with mouse PD-1H Ig fusion protein and adjuvant, as previously described (10). Hybridomas were screened by both PD-1H Ig–specific ELISA and by flow cytometry using PD-1H–transfected P815 cells (10). Isotyping was performed by cytometric bead array (CBA) (BD Biosciences). PD-1H mAb was produced and purified as previously described (10).
T cell proliferation assay in vitro.
For anti-CD3–induced T cell proliferation assays, anti-CD3 (clone 145-2C11, BD Biosciences) was coated onto 96-well plates overnight at 4°C. CD4+ T cells were enriched to more than 95% purity by negative selection using the MACS CD4+ T cell Isolation Kit II and LD columns (Miltenyi Biotec). Cells were added at 3 × 105 cells/well, and the supernatant was harvested prior to the addition of 1 μCi 3H-TdR for 8 hours. Cells were harvested from 96-well plates using a Filtermate Cell Harvester (PerkinElmer), and 3H-TdR incorporation was analyzed using a MicroBeta2 Plate Counter (PerkinElmer).
Flow cytometry, cytokine, and ALT analysis.
PD-1H flow cytometry was performed in 3 stages using a previously developed hamster anti-mouse PD-1H mAb (10) followed by anti-hamster Ig–biotin and streptavidin-APC (eBioscience). All other fluorescently labeled antibodies were from e-Bioscience. Cytokine analysis was performed using mouse inflammation or Th1/2/17 CBA kits. ALT serum analysis was performed using a COBAS Mira Plus (Roche).
OT-II T cell experiments.
For in vitro OT-II T cell proliferation assays, chicken egg OVA peptide 323–339 (Peptides International) was added to 96-well plates followed by the addition of 3 × 105 PD-1H–KO splenocytes irradiated with a total dose of 40 Gy. OT-II T cells were isolated by CD4+ negative selection and added to the wells at 1 × 105 or 5 × 104 cells/well. OT-II T cells were determined by flow cytometry analysis with CD4+ staining and mAbs specific for TCR chains Vα2 and Vβ5. For the in vivo OT-II T cell expansion study, OT-II T cells were transferred to PD-1H–KO mice by i.v. tail vein injection at 5 × 105 cells/mouse. Mice were immunized using i.p. injections of 300 μg OVA (Sigma-Aldrich) and 150 μg polyI:C.
Mouse model of acute hepatitis.
Con A (Sigma-Aldrich) was dissolved in PBS and administered in a total volume of 300 μl by i.v. tail-vein injection. In the experiments in which anti–PD-1H mAb was used, mice received 200 μg mam82 mAb via i.p. injections 3 hours prior to Con A injection. Liver lymphocytes were isolated with a GentleMACS dissociator (Miltenyi Biotec). To generate chimerism, mice were lethally irradiated with a total dose of 10 Gy radiation followed by T cell–depleted BM reconstitution with 1 × 107 cells transferred via the tail vein approximately 12 hours later. BM was harvested and T cells were removed with MACS thy1.2 microbeads (Miltenyi Biotec).
GL261 brain tumor model in mice.
Mice were anesthetized, and 3 × 105 GL261 tumor cells in a 20 μl volume were injected into the left frontal lobe of brains of PD-1H–KO or WT littermates using a Hamilton PB-600-1 Repeatable Dispenser. Low-dose irradiation (4 Gy) was performed on day 5 after tumor inoculation using an XRAD 320 irradiator with an adjustable beam collimator to reduce exposure field to include only the head of the mouse (approximately 2 cm2) and dose rate of approximately 2.25 Gy/min. Whole-brain tissues were taken on day 15 for H&E staining. Survival of the mice was monitored daily. For immune response analysis, splenocytes and brain lymphocytes were isolated at day 10 after radiotherapy and restimulated by irradiating WT GL261 cells for 5 days. IFN-γ–producing CD4+ T cells or CD8+ T cells were detected by intracellular staining. IFN-γ levels in the culture supernatant were also detected using a mouse CBA inflammation kit. For depletion of T cells in vivo, mice were injected with anti-CD4 (GK1.5) or anti-CD8a (53-6.72) mAb at 500 μg i.p. every 5 to 7 days starting at day 5 before tumor inoculation.
In vivo imaging.
Prior to imaging, mice received i.p. injections of 3 mg luciferin substrate (PerkinElmer) approximately 5 minutes prior to being anesthetized using the XRT-8 gas (isofluorane) anesthesia system (PerkinElmer) according to the manufacturer’s protocol. Anesthesia was maintained while the mice were imaged for bioluminescence using a Lumina XR in vivo imaging system (PerkinElmer).
Statistics.
Data are presented as mean ± SD where error bars are shown. Statistical analyses were performed by an unpaired 2-tailed Student’s t test using Microsoft Excel or Graphpad Prism software. P values of less than 0.05 were considered significant.
Study approval.
All mouse procedures were performed in Yale University’s animal facility and all mouse studies were approved by Yale University’s IACUC.
Supplementary Material
Acknowledgments
We thank Beth Cadugan for editing the manuscript and K. Philbrick for technical assistance. This work was supported in part by NIH grant CA 121974 and postdoctoral fellowship T32 AI089704 to D.B. Flies, a dowry fund from Yale School of Medicine, and a United Technologies Corp. endowment to L. Chen.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article:J Clin Invest. 2014;124(5):1966–1975. doi:10.1172/JCI74589.
See the related Commentary beginning on page 1891.
References
- 1.Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13(4):227–242. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baitsch L, et al. Extended co-expression of inhibitory receptors by human CD8 T-cells depending on differentiation, antigen-specificity and anatomical localization. PLoS One. 2012;7(2):e30852. doi: 10.1371/journal.pone.0030852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riha P, Rudd CE. CD28 co-signaling in the adaptive immune response. Self Nonself. 2010;1(3):231–240. doi: 10.4161/self.1.3.12968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu Y, Yao S, Chen L. Cell surface signaling molecules in the control of immune responses: a tide model. Immunity. 2011;34(4):466–478. doi: 10.1016/j.immuni.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yao S, Zhu Y, Chen L. Advances in targeting cell surface signalling molecules for immune modulation. Nat Rev Drug Discov. 2013;12(2):130–146. doi: 10.1038/nrd3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Linsley PS, Nadler SG. The clinical utility of inhibiting CD28-mediated costimulation. Immunol Rev. 2009;229(1):307–321. doi: 10.1111/j.1600-065X.2009.00780.x. [DOI] [PubMed] [Google Scholar]
- 7.Ott PA, Hodi FS, Robert C. CTLA-4 and PD-1/PD-L1 blockade: new immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin Cancer Res. 2013;19(19):5300–5309. doi: 10.1158/1078-0432.CCR-13-0143. [DOI] [PubMed] [Google Scholar]
- 8.Brahmer JR, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Topalian SL, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flies DB, Wang S, Xu H, Chen L. Cutting edge: a monoclonal antibody specific for the programmed death-1 homolog prevents graft-versus-host disease in mouse models. J Immunol. 2011;187(4):1537–1541. doi: 10.4049/jimmunol.1100660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang L, et al. VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. J Exp Med. 2011;208(3):577–592. doi: 10.1084/jem.20100619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang T, et al. A mouse knockout library for secreted and transmembrane proteins. Nat Biotechnol. 2010;28(7):749–755. doi: 10.1038/nbt.1644. [DOI] [PubMed] [Google Scholar]
- 13.Cao Q, Batey R, Pang G, Russell A, Clancy R. IL-6, IFN-γ and TNF-α production by liver-associated T cells and acute liver injury in rats administered concanavalin A. Immunol Cell Biol. 1998;76(6):542–549. doi: 10.1046/j.1440-1711.1998.00779.x. [DOI] [PubMed] [Google Scholar]
- 14.Dennert G, Aswad F. The role of NKT cells in animal models of autoimmune hepatitis. Crit Rev Immunol. 2006;26(5):453–473. doi: 10.1615/CritRevImmunol.v26.i5.50. [DOI] [PubMed] [Google Scholar]
- 15.Tiegs G, Hentschel J, Wendel A. A T cell-dependent experimental liver injury in mice inducible by concanavalin A. J Clin Invest. 1992;90(1):196–203. doi: 10.1172/JCI115836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Durante M, Reppingen N, Held KD. Immunologically augmented cancer treatment using modern radiotherapy. Trends Mol Med. 2013;19(9):565–582. doi: 10.1016/j.molmed.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 17.Liu SZ. Cancer control related to stimulation of immunity by low-dose radiation. Dose Response. 2007;5(1):39–47. doi: 10.2203/dose-response.06-108.Liu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moravan MJ, Olschowka JA, Williams JP, O’Banion MK. Cranial irradiation leads to acute and persistent neuroinflammation with delayed increases in T-cell infiltration and CD11c expression in C57BL/6 mouse brain. Radiat Res. 2011;176(4):459–473. doi: 10.1667/RR2587.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Newcomb EW, et al. The combination of ionizing radiation and peripheral vaccination produces long-term survival of mice bearing established invasive GL261 gliomas. Clin Cancer Res. 2006;12(15):4730–4737. doi: 10.1158/1078-0432.CCR-06-0593. [DOI] [PubMed] [Google Scholar]
- 20.Maes W, Van Gool SW. Experimental immunotherapy for malignant glioma: lessons from two decades of research in the GL261 model. Cancer Immunol Immunother. 2011;60(2):153–160. doi: 10.1007/s00262-010-0946-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.