Abstract
Introduction
Despite evidence from developing world trials that intravenous (IV) artesunate (AS) is superior to IV quinine (Q) in severe falciparum malaria (FM), IV AS remains unlicensed in the UK with national guidelines listing it as an acceptable alternative to IV Q as the drug of choice. We retrospectively evaluate the safety and effectiveness of IV AS in returning travellers with severe FM.
Methods
We identified adults admitted to the Infectious Diseases unit with severe FM and treated with IV Q (1991–2009) or IV AS (2009–2011). Outcomes included adverse events, mortality, length of stay, admission to intensive care and, where data were available, parasite/fever clearance time and hypoglycaemic events.
Results
Of 167 patients, 24 received IV AS and 143 IV Q. There was one potential AS-associated adverse event, a case of late onset haemolysis. Median length of stay (LOS) was significantly shorter for AS (3.5 versus 5 days, P = 0.017), even after adjusting for African ethnicity (for LOS ⩾3 days, mhor = 0.33, P = 0.027; crude OR = 0.29, P = 0.013). In the AS group, there were no fatalities (versus five in Q group, NS) and fewer intensive care unit (ICU) admissions (NS). Median parasite clearance was significantly faster in AS (65 versus 85 hours in Q, P = 0.0045) with no hypoglycaemic episodes (versus five in Q).
Discussion
We found IV AS to be safe and effective, with shorter LOS, faster parasite and fever clearance, no fatalities or hypoglycaemic events, and fewer ICU admissions versus IV Q. This corroborates both developing world trials and smaller European case series (although these lacked comparison groups). As well as obvious benefits for patients, there are potential resource savings. A case of late-onset haemolysis may represent an adverse event, particularly as it has been documented elsewhere, warranting further investigation. Nonetheless, our experience suggests IV AS should be first-line for treating severe FM in the UK.
Keywords: Falciparum malaria, Severe malaria, Artesunate, Quinine, Safety, Side effects, Adverse event, Hameolytic anaemia, Effectiveness, Clinical outcome, Length of stay, Parasite clearance, Returning traveler, UK, Non-endemic countries
Background
Currently, the UK reports >1500 cases of imported malaria annually, of which 70–80% are due to Plasmodium falciparum.1 Severe falciparum malaria (FM) is a medical emergency and often life-threatening, requiring urgent treatment. Non-immune returning travellers are at particular risk of developing severe FM and white ethnicity was shown to have increased risk of poor outcomes.2,3
British Infection Society guidelines advise parenteral treatment for patients who meet at least one of the criteria for severe FM or if they are unable to take oral medications.2,4,5 For many years intravenous (IV) quinine (Q) has been the standard treatment in this situation, despite its narrow therapeutic index and risk of inducing cardiac arrhythmias, hypoglycaemia, and cinchonism.5–10 The artemisinin derivative artesunate (AS) is an alternative IV antimalarial. Large randomized controlled trials on adults in Asia and Africa, and in African children, demonstrated that severe FM patients treated with parenteral AS had shorter parasite-clearance time, fewer side effects, and lower mortality compared to those treated with IV quinine, and an economic evaluation indicated AS use in South-East Asia was cost-effective.11–23
In light of this evidence, current WHO guidelines recommend IV AS as first-line treatment in severe FM.24,25 In UK guidelines, IV AS is currently listed as an alternative to IV Q as the drug of choice for treating severe FM in adults.4,5 It remains an unlicensed drug, with its formulation not having met good manufacturing practice (GMP).4,26,27 Furthermore, there are few data from centres in developed countries regarding safety and efficacy of AS in severe imported malaria.27–29
At Northwick Park Hospital, we changed from Q to AS in the treatment of severe FM in June 2009; we undertook a retrospective evaluation of our experience with both drugs.
Methods
Patients
Data from all adults (⩾16 years) admitted to the Northwick Park Infectious Diseases Unit for the treatment of severe FM from June 2009 to September 2011 were identified. FM was diagnosed via thin and thick film and dipstick HRP-2 antigen capture test. IV antimalarial treatment was given for hyperparasitaemia (⩾2% of red blood cells infected), or if the patient was vomiting, or in the presence of severity criteria: impaired consciousness, seizures, circulatory failure or prostration, pulmonary oedema, jaundice with bilirubin greater than 50 μmol/l, renal impairment, metabolic acidosis, or spontaneous bleeding.5
We selected comparable cases from our departmental database from July 1991 to May 2009. From this group of patients, we selected all cases classified as severe or complicated malaria, by the same severity criteria. These patients were all treated with IV quinine. Cases were excluded where there was insufficient clinical information regarding disease severity or outcome.
Drugs
IV AS (Guilin Pharmaceuticals, Shanghai, China) was administered at a dose of 2.4 mg/kg body weight per dose 12 hourly ×2 doses, then 24 hourly until scanty or no trophozoites were detected on thin films, followed by oral Malarone® (atovaquone/proguanil), four tablets daily for three days, or oral doxycycline 100 mg 12 hourly for 1 day, then 100 mg daily for 6 days.
As our departmental policy, IV Q was given at a dose of 10 mg/kg 8 hourly until sufficiently recovered to tolerate an oral regimen, usually doxycycline, as above. To avoid any excessive risk of toxicity, one Q dose of 20 mg/kg (loading dose), followed by 10 mg/kg 8 hourly was only considered for cases of life threatening hyperparasitaemia of >10%,9 not responding to treatment with exchange transfusion.
Departmental and hospital electronic databases and case records were reviewed for details of AS administration and for evidence of biochemical toxicity. If IV Q had been administered at a referring hospital before we could start AS treatment, the number of Q doses was recorded.
Baseline results and outcome measures
Inpatient clinical and laboratory parameters were recorded for both groups. Outcome measures were: length of hospital stay (days); death in hospital and admission to/length of stay in intensive care unit (ICU). For those patients treated from October 2002 onwards, electronically-recorded data enabled us to examine additional outcomes: parasite clearance time (no trophozoites detected; hours from admission; parasite clearance at 48 hours); fever clearance time (hours from admission to the start of the first 24-hour period where patient’s body temperature was <38°C); and occurrence of hypoglycaemic episodes.
Statistical methods
Stata IC10.1 (StataCorp LP, USA) statistics software was used for data analysis, comparing baseline parameter means (two-sample t-test, adjusted for unequal variances where sample sizes differed; Welch test), differences between medians of two sample distributions (non-parametric rank-sum test for independent samples) and categorical variables (crude odds ratio, OR; stratified analysis by ethnicity, via Mantel–Haenszel OR). Association between year of admission and outcome measures was excluded by linear regression analysis.
Results
We identified 185 severe malaria cases treated July 1991–September 2011. Eighteen were excluded due to lack of clinical information; of the remaining 167 patients comprising our case series, 24 received IV AS and 143 received IV Q. Demographic, and baseline clinical and laboratory data were similar in both groups, except that the Q group was younger, more in the AS group were Africans and had travelled to West Africa, whereas admission plasma glucose was lower in the AS group (Table 1). All 24 patients in the AS group, and 140 in the Q group, acquired their malaria in Africa. Overall, West Africa was the region most visited and the majority of patients were of African ethnicity. Visiting friends and relatives and work-related travel were common reasons for travel. Two-thirds of patients were recorded as having taken malaria prophylaxis. Eight of 24 patients received a dose of IV Q in their referring centre before we provided treatment with IV AS.
Table 1. Means of demographic baseline clinical and laboratory parameters (admission).
| All (n = 167) | AS (n = 24) | Q (n = 143) | P value (95% confidence interval) | |
| Demographic features | ||||
| Male (%) | 107 (64.1%) | 17 (70.8%) | 90 (62.9%) | 0.5a |
| Age (years) (SD; range) | 39.3 (±14.81; 16–81) | 46.5 (±15.03; 24–81) | 38.1 (±14.48; 16–73) | 0.01b (2.03, 14.71) |
| African ethnic background | 91 (54.5%) | 18 (75%) | 73 (51%) | 0.05a |
| West Africa as travel destination | 92 (55.1%) | 20 (83.3%) | 72 (50.4%) | 0.003a |
| East/North East Africa as travel destination | 45 (27.0%) | 2 (8.3%) | 43 (30.1%) | 0.124a |
| Visiting friends and relatives | 58 (34.8%) | 15 (62.5%) | 43 (30.1%) | 0.007c |
| Work related visit of endemic region | 39 (23.4%) | 4 (16.7%) | 35 (24.5%) | 0.6a |
| Malaria prophylaxis taken | 105 (62.9%) | 16 (66.7%) | 89 (62.2%) | 0.789a |
| Admission clinical features | ||||
| Temperature, °C (SD; range) | 38.1 (±1.21; 35.6–42) | 37.9* (±1.10; 36.2–39.8) | 38.1* (±1.22; 35.6–42) | 0.54b (−0.88, 0.47) |
| Heart rate, min−1 (SD; range) | 102 (±18.28; 62–160) | 110* (±19.53; 71–140) | 102* (18.02; 62–160) | 0.09b (−1.43, 18.76) |
| Systolic blood pressure, mmHg (SD; range) | 114 (±18.99; 72–190) | 121* (±24.92; 72–156) | 114* (±18.23; 80–190) | 0.18b (−3.48, 17.58) |
| Diastolic bld. pressure, mmHg (SD; range) | 69 (±12.12; 40–104) | 71* (±15.232; 45–104) | 68* (±11.784; 40–100) | 0.44b (−4.12, 9.38) |
| Respiratory rate, min−1 (SD; range) | 21.3 (±5.947; 10–60) | 20* (±2.58; 16–24) | 22* (±6.18; 10– 60) | 0.053d (−3.54, 0.03) |
| Blood glucose, mmol/l (SD; range) | 6.867 (±3.07; 2.8–23.2) | 5.8 (±1.55; 2.8–11) | 7.1** (±3.26; 3.5–23.2) | 0.004d (−2.19, −0.44) |
| Admission laboratory features | ||||
| Parasitaemia, % (SD; range) | 5.9 (±6.577; 0–42) | 7.7 (±7.42; 1–32) | 5.6 (±6.41; 0–42) | 0.21b (−1.21, 5.36) |
| Haemoglobin, g/dl (SD; range) | 12.6 (±2.428; 5.6–17.1) | 12.2 (±1.91; 6.9–16) | 12.6 (±2.51; 5.6–17.1) | 0.5b (−1.42, 0.70) |
| Platelets, μl−1 (SD; range) | 72 (±54.001; 3–340) | 61 (±37.57; 9–143) | 74 (±56.20; 3–340) | 0.17d (−30.72, 5.57) |
| Serum urea, mmol/l (SD; range) | 9.0 (±10.368; 1.3–94) | 11.1 (±11.68; 3.6–41.1) | 8.6 (±10.13; 1.3–94) | 0.29b (−2.08, 6.95) |
| Creatinine, μmol/l (SD; range) | 125 (±116.42; 56–1189) | 139 (±125.02; 56– 607) | 123 (±115.21; 59–1189) | 0.51b (−33.89, 67.70) |
| Serum bilirubin, mol/l (SD; range) | 44.8 (±37.224; 5–282) | 42.7 (±36.78; 10–157) | 45.2 (±37.42; 5–282) | 0.77b (−18.70, 13.81) |
| Alanine aminotransferase, IU/l (SD; range) | 53.92 (±41.265; 11–296) | 44.7 (±29.79; 13–151) | 55.47 (±42.78; 11–296) | 0.13d (−25.05, 3.50) |
| Prothrombin time, second (SD; range) | 15.61 (±2.078; 11.2–25) | 15.5 (±2.26; 13–21) | 15.6† (±2.05; 11.2–25) | 0.79b (−0.06, 0.80) |
| Serum lactate, mol/l (SD; range) | 2.78 (±2.645; 0.7–21.5) | 2.27†† (±1.12; 0.8– 4.9) | 2.98†† (±3.03; 0.7–21.5) | 0.17d (−1.71, 0.30) |
Note: aExact test (Fisher).
bt-test for equal variances (t-test).
cChi-squared test.
dt-test for unequal variances (Welch test).
*Vital parameter results available from 14 AS and 130 Q patients.
**Blood glucose results available from 115 Q patients.
†Prothrombin time results available from 110 Q patients.
††Serum lactate results available from 19 AS and 49 Q patients.
Adverse events among AS recipients
We identified only one potential AS-associated adverse event, of late-onset haemolytic anaemia. A 78-year-old white man had P. falciparum parasitaemia of 27%, complicated by renal failure (creatinine: 359 μmol/l). His admission haemoglobin was 13.2 g/dl. During his 3-day admission to ICU, where he did not require organ support, he received IV AS and his parasites cleared by 35 hours. He was discharged on day 12, clinically well. On day 15, he was re-admitted with breathlessness and a haemoglobin 6.4 g/dl. We diagnosed Coombs test positive immune-mediated haemolysis, requiring 9 units of red cell concentrate. Haemolysis ceased after starting prednisolone 40 mg daily.
Comparison of outcome measures in AS and Q groups
Median length of stay (LOS) was 3.5 days in the AS group (n = 24) versus 5 days in the Q group (n = 143; P = 0.017). We found no association between year of admission and LOS, length of stay in ICU or parasitaemia clearance time (linear regression analysis, P = 0.098, P = 0.192 and P = 0.067; Tables 2 and 3). No deaths occurred in the AS group, versus five deaths in the Q group (NS). Fewer admissions to ICU and shorter length of stay in ICU were seen in the AS group (Table 2; NS). No exchange transfusion was required in any of the 24 AS patients.
Table 2. Primary outcome measures for all patients (n = 167) .
| All | AS | Q | P | P | |
| n = 167 | n = 24 | n = 143 | OR (CI) | Associationc adm.year&OC | |
| Median length of stay, day (mean±SD) | 5 (6.1±8.6) | 3.5 (4.4±3.0) | 5 (6.4±9.2) | P = 0.017a | 0.098c |
| Length of stay ⩾3 days (%) | 145 (87%) | 17 (71%) | 128 (90%) | P = 0.013b OR = 0.29 (0.10, 0.82) | |
| Length of stay ⩾5 days (%) | 84 (50%) | 7 (29%) | 77 (54%) | P = 0.026b OR = 0.35 (0.14, 0.92) | |
| Median length of stay (ICU), day (mean±SD; range) | 0 (1.5±6.3; 0–78) | 0 (0.6±1.6; 0–6) | 0 (1.6±6.8; 0–78) | P = 0.187a | 0.192 c |
| ICU admission (%) | 45 (27%) | 3 (13%) | 42 (29%) | P = 0.086b OR = 0.34 (0.10, 1.23) | |
| Death during stay (%) | 5(3%) | 0 (0%) | 5 (3.5%) | P = 0.354b |
Note: aWilcoxon rank-sum test.
bOdds ratio.
cLinear regression analysis, Prob>F (showing no association between year of admission and numerical outcome measures).
Table 3. Primary and further outcome measures for sub-group of patients (n = 70).
| All | AS | Q | P | P | |
| n = 70 | n = 22 | n = 48 | OR (CI) | Associationc adm.year&OC | |
| Median length of stay, day (mean±SD; range) | 4 (4.7±2.5; 2–12) | 3.5 (4.5±3.1; 2–12) | 5 (4.9±2.1; 2–12) | P = 0.103a | 0.588c |
| Length of stay ⩾3 days (%) | 59 (84%) | 16 (73%) | 43 (90%) | P = 0.074b OR = 0.31 (0.08, 1.21) | |
| Length of stay ⩾5 days (%) | 32 (46%) | 7 (32%) | 25 (52%) | P = 0.117b OR = 0.43 (0.15, 1.28) | |
| Med. parasite clearance time, hours (mean±SD; range) | 69 (79±32.3; 27–200) | 62 (65±36.0; 27–200) | 80 (85±28.7; 41–145) | P = 0.005a | 0.067 c |
| Parasite clearance time ⩾48 hours (%) | 57 (81%) | 12 (55%) | 45 (94%) | P<0.001b OR = 0.08 (0.016, 0.41) | |
| Hypoglycaemia episodeblood gluc⩽3.6 mmol/l (%) | 5 (16%)* | 0 (0%)* | 5 (45%)* | P<0.001b | |
| Med.fever clearance<38°C time, hour (mean±SD; range) | 25† (31±36; 0–130) | 0† (7.3±13.3; 0–41) | 41† (56.2±36; 8–130) | P<0.001a | 0.002c |
Note: aWilcoxon rank sum test.
bOdds ratio.
cLinear regression analysis, Prob>F (showing no association between year of admission and numerical outcome measures).
*episode of blood glucose ⩽3.6 mmol/l after admission: data available in 22 AS and 11 Q patients.
†Start of full 24-hour episode where body temperature <38°C (noted in hours after admission): data available from 12 AS and 11 Q patients.
We had sufficient data to determine parasite clearance in 48 cases treated with IV Q from October 2002 onwards, and in 22 of the AS group. We found significantly reduced median parasite clearance time in the AS cases (65 hours) compared to the Q group (85 hours; P = 0.0045, Wilcoxon rank-sum test; Table 3 and Fig. 1). In 33 patients (22 AS group and 11 Q group), data on regular blood glucose checks were available; we found no hypoglycaemic episodes (blood glucose of ⩽3.6 mmol/l) after admission in the AS group, compared to hypoglycaemic episodes in six patients of the Q group. In 23 patients, data on regular body temperature measurements were available, and a significantly shorter fever clearance time (18 hours versus 54 hours) was seen in AS compared to Q recipients (Table 3).
Figure 1.
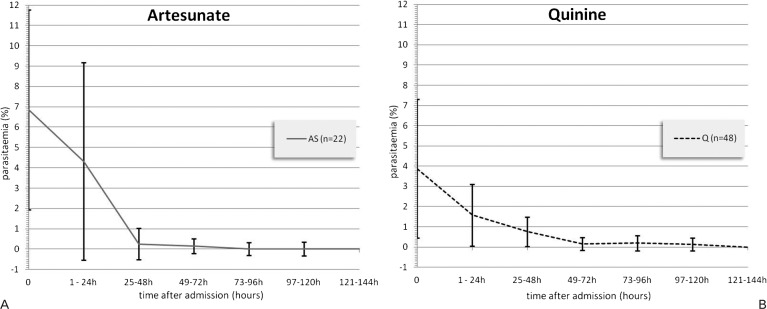
Mean parasitaemia rates (% of infected red blood cells): levels displayed by time intervals after admission, divided into (A) AS and (B) Q patients (n = 70).
Tables stratified by African versus non-African ethnicity (Mantel–Haenszel OR) show a confounding effect of African ethnicity though the differences in LOS between AS and Q remain significant (Table 4). A similar effect is seen regarding fewer ICU admissions in the AS group. Table 5 illustrates additional outcome measures stratified by ethnicity.
Table 4. Outcome parameters for patients (n = 167) treated with AS and Q, stratified by African versus non-African ethnicity.
| African ethnicity (n = 91) | Non-African ethnicity (n = 76) | |||||
| AS n = 18 | Q n = 73 | AS n = 6 | Q n = 70 | P mhor (95% CI) | P Crude OR (95% CI) | |
| Median length of stay, days (mean±SD) | 3 (3.8±2.1) | 4 (4.7±2.7) | 4.5 (6.2±4.7) | 6 (8.1±12.7) | ||
| Length of stay ⩾3 days (%) | 13 (72%) | 63 (86%) | 4 (67%) | 65 (93%) | P = 0.027a mhor = 0.33 (0.1, 0.9) | P = 0.013 OR = 0.29 (0.10, 0.82) |
| Length of stay ⩾5 days (%) | 4 (22%) | 30 (41%) | 3 (50%) | 47 (67%) | P = 0.090b mhor = 0.43 (0.2, 1.2) | P = 0.026 OR = 0.35 (0.14, 0.92) |
| Median length of stay (ICU), day (mean±SD) | 0 (0.4±1.4) | 0 (0.6±1.5) | 0 (1.3±2.2) | 0 (2.6±9.5) | ||
| ICU admission (%) | 1 (6%) | 13 (18%) | 2 (33%) | 29 (41%) | P = 0.219c mhor = 0.44 (0.1, 1.7) | P = 0.086 OR = 0.34 (0.10, 1.23) |
| Death during stay (%) | 0 (0%) | 0 (0%) | 0 (0%) | 5 (7%) | P>0.05d | |
Note: Numeric outcome measures; categorical measures examined for confounding effect by ethnicity via Mantel–Haenszel odds ratio (mhor), compared to crude (unstratified) odds ratio (OR).
aPhomogeneity of ORs = 0.401.
bPhomogeneity of ORs = 0.867.
cPhomogeneity of ORs = 0.489.
dPhomogeneity of ORs = not avalable for this calculation.
Table 5. Primary and further outcome measures for sub-group of patients (n = 70) treated with AS and Q, stratified by African versus non-African ethnicity.
| African ethnicity (n = 50) | Non-African ethnicity (n = 20) | |||||
| AS n = 16 | Q n = 34 | AS n = 6 | Q N = 14 | P mhor (95% CI) | P Crude OR (95% CI) | |
| Med. length of stay, day (mean±SD) | 3 (3.8±2.1) | 4 (4.6±2.1) | 4.5 (6.2±4.7) | 5 (5.6±2.1) | ||
| Length of stay ⩾3 days (%) | 12 (75%) | 30 (88%) | 4 (67%) | 13 (93%) | P = 0.077a mhor = 0.31 (0.1, 1.2) | P = 0.074 OR = 0.31 (0.08, 1.21) |
| Length of stay ⩾5 days (%) | 4 (25%) | 16 (47%) | 3 (50%) | 9 (64%) | P = 0.120b mhor = 0.42 (0.1, 1.3) | P = 0.117 OR = 0.43 (0.15, 1.28) |
| Median parasite clearance time, hour (mean±SD) | 47 (54.1±20.4) | 80 (86.2±30.0) | 77 (94±53.1) | 79 (81.2±25.8) | ||
| Parasite clearance time ⩾48 hours (%) | 6 (38%) | 32 (94%) | 6 (100%) | 13 (93%) | P = 0.001c mhor = 0.08 (0.02, 0.41) | |
| Hypoglycaemia episodeblood gluc⩽3.6 mmol/l (%) | 0 (0%) | 4 (44%) | 0 (0%) | 1 (50%) | P<0.001d mhor = | |
| Median fever clearance<38°C time, hour (mean±SD) | 0 (2.6±5.7) | 40 (50.9±35.2) | 12.5 (16.5±20.1) | 80 (80±39.6) | ||
Note: Numeric outcome measures; categorical measures examined for confounding effect by ethnicity via Mantel–Haenszel odds ratio (mhor) compared to crude (unstratified) odds ratio (OR). mhor is not available for this calculation.
aPhomogeneity of ORs = 0.562.
bPhomogeneity of ORs = 0.750.
cPhomogeneity of ORs = 0.018.
dPhomogeneity of ORs = not avalable for this calculation.
Discussion
This is the first report comparing IV AS to IV Q in severe FM in a non-endemic country. We found IV AS to be safe and effective in the treatment of severe malaria, with shorter parasite and fever clearance times and a shorter length of stay, compared to patients treated with IV Q. Because all our patients receiving AS acquired their malaria in Africa, this case series also provides information on the effectiveness of intravenous AS in adult cases of severe malaria acquired in Africa. These findings are consistent with previous randomised controlled trials in Asia,17,18,20,21 where rapid parasite clearance in cases treated with AS has been reported. Smaller European case series report similar efficacy with AS, but no comparison treatments are given. Zoller et al. reported 25 patients treated with AS in continental Europe,27 Bartolini et al. reported eight patients treated with combined AS and Q in Italy,28 and Mørch et al. reported nine patients treated with AS in Norway.29
We saw no hypoglycaemic episodes or fatalities in the AS group, as well as fewer admissions to ICU, and days in ICU. Also, fever clearance was more rapid with AS than Q. While our patient numbers for these outcome measures are small, similar findings are reported in other studies: Krudsood et al. found less fatalities (2 versus 5 deaths, respectively) and shorter fever clearance time (80 hours versus 107 hours, respectively) in their AS group (n = 77) versus Q (n = 74).18 Newton et al. reported significantly reduced hypoglycaemic episodes in patients treated with AS (59 compared to 54 controls: 6 versus 15 hypoglycaemic episodes, respectively),20 as well as Dondorp et al. who also found a reduction in mortality of 34.7% in the AS group.21 Less need for intensive care, cessation of blood glucose monitoring, and reduced hospital stay are all associated with reductions in costs and manpower. We found a reduced length of stay in patients receiving AS, compared to patients treated with Q, a finding not previously documented.27–29 We consider LOS is an important proxy for faster clinical recovery. The only studies looking at length of stay come from the developing world and found no reduction, which may be related to resource constraints or similar factors.20,22
Our case series has several limitations. These include a relatively small sample size, its retrospective nature, as well as the fact that stable patients had parasitaemia checks only once daily, so that the actual time to parasite clearance will be shorter than we recorded. Another limitation is the higher proportion of Africans in the AS group, compared to the Q group. Africans showed a reduced risk of an unfavourable outcome, as defined by prolonged hospital stay, death, or ICU stay, in a previous study at our centre.2 African ethnicity has a potential confounding effect, with Africans being over-represented in the AS group and also having shorter hospital stays and fewer ICU admissions.
Further, a degree of overestimating differences in outcome measures between AS and Q may have occurred by not using Q loading doses in Q patients. Faster action with Q loading dose compared to normal Q dose was shown by a Cochrane literature review and in a recent large study in Asia. However, the use of a Q loading dose as currently recommended in many national/international guidelines, was found to be associated with a higher risk of toxicity, whereas no mortality benefit was observed.30,31
Our one patient with late-onset, prolonged haemolysis was the only, but highly significant complication we observed in patients treated with AS. Six cases of late-onset haemolysis after AS were reported by Zoller et al. among 25 travellers from seven treatment centres in Europe.27 In those cases, haemolysis was diagnosed <15 days after the first dose of AS; in two cases, Coombs tests were performed, and both were negative. In our patient, G6PD deficiency was excluded, but the Coombs test was positive, indicating immune-mediated haemolysis. Itoda et al. reported a case of late-onset haemolytic anaemia in Tanzania, which developed in an individual on day 11 of successful treatment with AS for severe malaria.32 While late-onset haemolysis was not documented in the larger scale trials, it is conceivable that this might have been missed if routine follow-up was not undertaken post-discharge, warranting further evaluation of this late and potentially serious complication.17,21
In conclusion, our experience of IV AS supports its replacement of IV Q as the first-line drug for severe FM in the UK.
Acknowledgments
We expressed our sincere thanks to Geoffrey Pasvol, Professor of Infection & Tropical Medicine at Imperial College London, for his encouragement and our helpful discussions on the subject. We extend our thanks also to the junior doctors who contributed to the maintenance of our departmental malaria database over the years.
References
- 1.Health Protection Agency. Health Prot. Rep. 2011;5:17–28. Available from: http://www.hpa.org.uk/hpr/archives/2011/hpr1711.pdf(accessed 28/03/2012) [Google Scholar]
- 2.Phillips A, Bassett P, Zeki S, Newman S, Pasvol G. Risk factors for severe disease in adults with falciparum malaria. Clin Infect Dis. 2009;48(7):871–8. doi: 10.1086/597258. [DOI] [PubMed] [Google Scholar]
- 3.Severe falciparum malaria. World Health Organization, Communicable Diseases Cluster. Trans R Soc Trop Med Hyg. 2000;94(Suppl 1):S1–90. [PubMed] [Google Scholar]
- 4.Lalloo DG, Shingadia D, Pasvol G, Chiodini PL, Whitty CJ, Beeching NJ, et al. UK malaria treatment guidelines. J Infect. 2007;54(2):111–21. doi: 10.1016/j.jinf.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 5.British Infection Society. Malaria — Algorithm for Initial Assessment and Management in Adults. Princes Risborough: BIS; 2007. Available from: http://www.britishinfection.org/drupal/sites/default/files/MalariaAlgorithm07.pdf (accessed 28/03/2012) [Google Scholar]
- 6.Severe and complicated malaria. World Health Organization, Division of Control of Tropical Diseases. Trans R Soc Trop Med Hyg. 1990;84(Suppl 2):1–65. [PubMed] [Google Scholar]
- 7.Day N, Dondorp AM. The management of patients with severe malaria. Am J Trop Med Hyg. 2007;77(6 Suppl):29–35. [PubMed] [Google Scholar]
- 8.White NJ, Warrell DA, Chanthavanich P, Looareesuwan S, Warrell MJ, Krishna S, et al. Severe hypoglycemia and hyperinsulinemia in falciparum malaria. N Engl J Med. 1983;309(2):61–6. doi: 10.1056/NEJM198307143090201. [DOI] [PubMed] [Google Scholar]
- 9.Bonington A, Davidson RN, Winstanley PA, Pasvol G. Fatal quinine cardiotoxicity in the treatment of falciparum malaria. Trans R Soc Trop Med Hyg. 1996;90(3):305–7. doi: 10.1016/s0035-9203(96)90264-3. [DOI] [PubMed] [Google Scholar]
- 10.White NJ. Cardiotoxicity of antimalarial drugs. Lancet Infect Dis. 2007;7(8):549–58. doi: 10.1016/S1473-3099(07)70187-1. [DOI] [PubMed] [Google Scholar]
- 11.Cumming JN, Ploypradith P, Posner GH. Antimalarial activity of artemisinin (qinghaosu) and related trioxanes: mechanism(s) of action. Adv Pharmacol. 1997;37:253–97. doi: 10.1016/s1054-3589(08)60952-7. [DOI] [PubMed] [Google Scholar]
- 12.Wu Y. How might qinghaosu (artemisinin) and related compounds kill the intraerythrocytic malaria parasite? A chemist’s view. Acc Chem Res. 2002;35(5):255–9. doi: 10.1021/ar000080b. [DOI] [PubMed] [Google Scholar]
- 13.Li W, Mo W, Shen D, Sun L, Wang J, Lu S, et al. Yeast model uncovers dual roles of mitochondria in action of artemisinin. PLoS Genet. 2005;1(3):e36. doi: 10.1371/journal.pgen.0010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.ter Kuile F, White NJ, Holloway P, Pasvol G, Krishna S. Plasmodium falciparum: in vitro studies of the pharmacodynamic properties of drugs used for the treatment of severe malaria. Exp Parasitol. 1993;76(1):85–95. doi: 10.1006/expr.1993.1010. [DOI] [PubMed] [Google Scholar]
- 15.Hess KM, Goad JA, Arguin PM. Intravenous artesunate for the treatment of severe malaria. Ann Pharmacother. 2010;44(7–8):1250–8. doi: 10.1345/aph.1M732. [DOI] [PubMed] [Google Scholar]
- 16.Newton PN, Barnes KI, Smith PJ, Evans AC, Chierakul W, Ruangveerayuth R, et al. The pharmacokinetics of intravenous artesunate in adults with severe falciparum malaria. Eur J Clin Pharmacol. 2006;62(12):1003–9. doi: 10.1007/s00228-006-0203-2. [DOI] [PubMed] [Google Scholar]
- 17.Jones KL, Donegan S, Lalloo DG. Artesunate versus quinine for treating severe malaria. Cochrane Database Syst Rev. 2007;(4):CD005967. doi: 10.1002/14651858.CD005967.pub2. [DOI] [PubMed] [Google Scholar]
- 18.Krudsood S, Wilairatana P, Vannaphan S, Treeprasertsuk S, Silachamroon U, Phomrattanaprapin W, et al. Clinical experience with intravenous quinine, intramuscular artemether and intravenous artesunate for the treatment of severe malaria in Thailand. Southeast Asian J Trop Med Public Health. 2003;34(1):54–61. [PMC free article] [PubMed] [Google Scholar]
- 19.Maude RJ, Plewes K, Faiz MA, Hanson J, Charunwatthana P, Lee SJ, et al. Does artesunate prolong the electrocardiograph QT interval in patients with severe malaria? Am J Trop Med Hyg. 2009;80:126–32. doi: 10.4269/ajtmh.2009.08-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Newton PN, Angus BJ, Chierakul W, Dondorp A, Ruangveerayuth R, Silamut K, et al. Randomized comparison of artesunate and quinine in the treatment of severe falciparum malaria. Clin Infect Dis. 2003;37(1):7–16. doi: 10.1086/375059. [DOI] [PubMed] [Google Scholar]
- 21.Dondorp A, Nosten F, Stepniewska K, Day N, White N. South East Asian Quinine Artesunate Malaria Trial (SEAQUAMAT) group. Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial. Lancet. 2005;366(9487):717–25. doi: 10.1016/S0140-6736(05)67176-0. [DOI] [PubMed] [Google Scholar]
- 22.Dondorp AM, Fanello CI, Hendriksen IC, Gomes E, Seni A, Chhaganlal KD, et al. Artesunate versus quinine in the treatment of severe falciparum malaria in African children (AQUAMAT): an open-label, randomised trial. Lancet. 2010;376(9753):1647–57. doi: 10.1016/S0140-6736(10)61924-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lubell Y, Yeung S, Dondorp AM, Day NP, Nosten F, Tjitra E, et al. Cost-effectiveness of artesunate for the treatment of severe malaria. Trop Med Int Health. 2009;14(3):332–7. doi: 10.1111/j.1365-3156.2009.02227.x. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization. Guidelines for the treatment of malaria 2nd edition. Geneva: WHO; 2010. Available from: http://whqlibdoc.who.int/publications/2010/9789241547925_eng.pdf (accessed 28/03/2012) [Google Scholar]
- 25.World Health Organization. Guidelines for the treatment of malaria 2nd edition — Rev. 1. Geneva: WHO; 2011. Available from: http://www.who.int/malaria/publications/atoz/mal_treatchild_revised.pdf (accessed 28/03/2012) [Google Scholar]
- 26.Centers for Disease Control and Prevention. Artesunate is available to treat severe malaria in the United States, 8 February 2010. Available from: http://www.cdc.gov/malaria/diagnosis_treatment/artesunate.html (accessed 28/03/2012) [Google Scholar]
- 27.Zoller T, Junghanss T, Kapaun A, Gjorup I, Richter J, Hugo-Persson M, et al. Intravenous artesunate for severe malaria in travelers, Europe. Emerg Infect Dis. 2011;17(5):771–7. doi: 10.3201/eid1705.101229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bartoloni A, Tomasoni L, Bartalesi F, Galli L, Sani S, Veloci S, et al. Combined intravenous treatment with artesunate and quinine for severe malaria in Italy. Am J Trop Med Hyg. 2010;83(2):274–6. doi: 10.4269/ajtmh.2010.10-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mørch K, Strand Ø, Dunlop O, Berg A, Langeland N, Leiva RA, et al. Severe malaria and artesunate treatment, Norway. Emerg Infect Dis. 2008;14(11):1816–8. doi: 10.3201/eid1411.080636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lesi AF, Meremikwu MM.High first dose quinine regimen for treating severe malaria. Cochrane Database Syst Rev. 20043CD003341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tariq M, et al. Efficacy and safety of quinine loading dose in patients with severe falciparum malaria at a tertiary care hospital in Pakistan. J Pak Med Assoc. 2011;61(1):27–31. [PubMed] [Google Scholar]
- 32.Itoda I, Yasunami T, Kikuchi K, Yamaura H, Totsuka K, Yoshinaga K, et al. [Severe falciparum malaria with prolonged hemolytic anemia after successful treatment with intravenous artesunate]. Kansenshogaku Zasshi. 2002;76(8):600–3. doi: 10.11150/kansenshogakuzasshi1970.76.600. Japanese. [DOI] [PubMed] [Google Scholar]


