Abstract
Four Amblyomma sabanerae ticks collected from a turtle (Kinosternon sp.) in San Miguel, El Salvador, were found by molecular analysis to be infected by Rickettsia bellii. We provide the first report of Rickettsia bellii in Central America, and the first report of a Rickettsia species in El Salvador.
Keywords: Rickettsia bellii, Amblyomma sabanerae, El Salvador, Central America
A recent review reported 13 Rickettsia species occurring in Latin America and Caribbean.1 Among these species, Rickettsia bellii is the one that has been found in the largest number of tick species, being the following ixodid species in Brazil and Argentina: Amblyomma aureolatum, Amblyomma dubitatum, Amblyomma humerale, Amblyomma incisum, Amblyomma neumanni, Amblyomma nodosum, Amblyomma oblongoguttatum, Amblyomma ovale, Amblyomma scalpturatum, Amblyomma tigrinum, Haemaphysalis juxtakochi, and Ixodes loricatus.1 R. bellii is also known to occur in North America, where it was reported infecting either argasid or ixodid ticks.2 On the other hand, R. bellii has never been reported in Central America.
In July 2010, four tick specimens were collected from a naturally infested turtle (Kinosternon sp.) in San Miguel, El Salvador. The ticks were all females, attached to the turtle carapace (Fig. 1). Ticks were removed, placed in a vial containing absolute ethanol, and sent to the laboratory for analyses. All ticks were identified as Amblyomma sabanerae following Fairchild et al.3 and Jones et al.4
Figure 1.
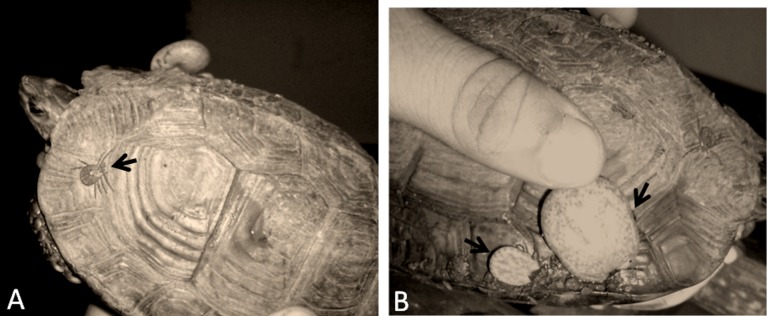
Amblyomma sabanerae female ticks attached to the carapace of a turtle Kinosternon sp. in El Salvador: (A) one non-engorged female indicated by an arrow; (B) two partially engorged females indicated by arrows.
The four ticks were individually submitted to DNA extraction by the guanidine isothiocyanate-phenol technique, as previously described.5 A ‘blank’ tube containing no tick sample was included in the DNA extraction. Samples were tested individually by PCR using primers CS-78 and CS-323 targeting a 401-bp fragment of the rickettsial gene gltA, as previously described.6 One negative control tube containing water was included, and also a positive control tube containing DNA of Rickettsia parkeri strain NOD, previously isolated in Vero cells from Amblyomma nodosum ticks in our laboratory.7 Samples that yielded visible amplicons of the expected size by the gltA PCR were further tested by a second PCR assay using primers Rr190.70p and Rr190.602n targeting a 532-bp fragment of the rickettsial gene ompA, as previously described.8 In addition, to confirm tick taxonomic identification, DNA samples were individually tested by a PCR assay using primers T1B and T2A targeting a 360-bp fragment of the tick 12S rRNA mitochondrial gene, as previously described.9 PCR products were submitted to direct DNA sequencing in an automated ABI Prism 310 genetic analyzer (Applied Biosystems, Foster City, CA, USA). The BLAST program (National Center for Biotechnology Information, Bethesda, MD, USA) was used to compare appropriate similarities of the rickettsial or tick mitochondrial partial sequence generated in the current study.
The four A. sabanerae female ticks yielded amplicons of the expected size by the gltA PCR. Both the DNA extraction blank tube and the negative control tube yielded no visible amplicon. The four ticks were negative by PCR targeting the ompA gene. The products of the gltA PCR were DNA sequenced. Through blast analysis, these four A. sabanerae ticks were found infected with a Rickettsia 100% (320/320) identical to Rickettsia bellii strain AT, previously detected in Amblyomma tigrinum from Argentina (EU826511), and 99.4% (324/326) similar to at least seven corresponding sequences of R. bellii from different tick species in Brazil (EU567181, DQ865204, AY362703, DQ146481), Argentina (DQ517288), and the USA (CP000087, RBU59716). The four ticks were molecularly confirmed to be A. sabanerae; their individual 12S rRNA edited sequences (345-bp) were identical to each other and 98.3% (339/345) similar to GenBank accession number AY766150, which according to a recent study, refers to A. sabanerae.10 The DNA sequences of R. bellii (gltA) and A. sabanerae (12S rRNA) from El Salvador generated in the present study have been deposited in GenBank under the accession numbers JQ664297 and JQ928695, respectively.
The present report adds the tick species A. sabanerae to the growing list of American ticks that have been found infected by R. bellii, which now includes 13 Amblyomma species in the Neotropical region. In addition, our findings represent the first report of R. bellii in Central America, and the first report of a Rickettsia species in El Salvador. Previous reports related to Rickettsia in El Salvador has been restricted to seroepidemiological studies, indicating that humans in this country were exposed to either a spotted fever group (SFG) or a typhus group (TG) agent.11,12 Recent genetic studies have indicated that R. bellii is not either a SFG or a TG agent; it has been classified in a basal group together with other genotypes associated mostly with insects.13 Therefore, our negative results of the ompA PCR are expected, since this PCR protocol has been shown to work only for some members of the SFG rickettsiae.8
Vertical transmission of R. bellii in ticks is also known to occur.14 Although we tested a small sample size, all four ticks were shown to be infected. Previously, a 100% infection rate by R. bellii was reported in Amblyomma rotundatum ticks in Brazil.6 Interestingly, both A. sabanerae and A. rotundatum are commonly found feeding on reptiles.3,4,15 Despite a wide distribution of R. bellii in the New World,1,2 the role of this organism as human or animal pathogen remains unknown.
Acknowledgments
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). Parts if this work has been facilitated through the ‘Programa Iberoamericano de Ciencias y Tecnologia para el Desarrollo’ (CYTED) to ‘Red Iberoamerivana para la Investigacion y Control de las Enfermedades Rickettsiales’ (RIICER).
References
- 1.Labruna MB, Mattar S, Nava S, Bermudez S, Venzal JM, Dolz G, et al. Rickettsioses in Latin America, Caribbean, Spain and Portugal. Rev MVZ Córdoba. 2011;16:2435–57. [Google Scholar]
- 2.Philip RN, Casper EA, Anacker RL, Cory J, Hayes SF, Burgdorfer W, et al. Rickettsia belli sp. nov.: a tick-borne rickettsia, widely distributed in the USA, that is distinct from the spotted fever and typhus biogroups. Int J Syst Bacteriol. 1983;33:94–106. [Google Scholar]
- 3.Fairchild GB, Kohls GM, Tipton VJ.The ticks of Panama (Acarina: Ixodoidea) Wenzel W R, Tipton V J.editorsEctoparasites of Panama Chicago (IL)Field Museum of Natural History; 1966. p167–219. [Google Scholar]
- 4.Jones EK, Clifford CM, Keirans JE, Kohls GM. The ticks of Venezuela (Acarina: Ixodoidea) with a key to the species of Amblyomma in the Western Hemisphere. Brigham Young Univ Sci Bull, Biol Ser. 1972;17:1–40. [Google Scholar]
- 5.Sangioni LA, Horta MC, Vianna MC, Gennari SM, Soares RM, Galvão MA, et al. Rickettsial infection in animals and Brazilian spotted fever endemicity. Emerg Infect Dis. 2005;11:265–70. doi: 10.3201/eid1102.040656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Labruna MB, Whitworth T, Bouyer DH, McBride JW, Camargo LM, Camargo EP, et al. Rickettsia bellii and Rickettsia amblyommii in Amblyomma ticks from the state of Rondônia, Western Amazon, Brazil. J Med Entomol. 2004;41:1073–81. doi: 10.1603/0022-2585-41.6.1073. [DOI] [PubMed] [Google Scholar]
- 7.Ogrzewalska M, Pacheco RC, Uezu A, Richtzenhain LJ, Ferreira F, Labruna MB. Rickettsial infection in Amblyomma nodosum ticks (Acari: Ixodidae) from Brazil. Ann Trop Med Parasitol. 2009;103:413–25. doi: 10.1179/136485909X451744. [DOI] [PubMed] [Google Scholar]
- 8.Regnery RL, Spruill CL, Plikaytis BD. Genotypic identification of rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. J Bacteriol. 1991;173:1576–89. doi: 10.1128/jb.173.5.1576-1589.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beati L, Keirans JE. Analysis of the systematic relationships among ticks of the genera Rhipicephalus and Boophilus (Acari: Ixodidae) based on mitochondrial 12S ribosomal DNA gene sequences and morphological characters. J Parasitol. 2001;87:32–48. doi: 10.1645/0022-3395(2001)087[0032:AOTSRA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 10.Ogrzewalska M, Uezu A, Labruna MB. Ticks (Acari: Ixodidae) infesting wild birds in the eastern Amazon, northern Brazil, with notes on rickettsial infection in ticks. Parasitol Res. 2010;106:809–16. doi: 10.1007/s00436-010-1733-1. [DOI] [PubMed] [Google Scholar]
- 11.WHO. Global surveillance of rickettsial diseases: memorandum from a WHO meeting. Bull World Health Organ. 1993;71:293–6. [PMC free article] [PubMed] [Google Scholar]
- 12.Kovácová E, Sixl W, Stünzner D, Urvölgyi J, Kazár J. Serological examination of human and animal sera from six countries of three continents for the presence of rickettsial antibodies. Eur J Epidemiol. 1996;12:85–9. doi: 10.1007/BF00144434. [DOI] [PubMed] [Google Scholar]
- 13.Weinert LA, Werren JH, Aebi A, Stone GN, Jiggins FM. Evolution and diversity of Rickettsia bacteria. BMC Biol. 2009;7:6. doi: 10.1186/1741-7007-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horta MC, Pinter A, Schumaker TT, Labruna MB. Natural infection, transovarial transmission, and transstadial survival of Rickettsia bellii in the tick Ixodes loricatus (Acari: Ixodidae) from Brazil. Ann NY Acad Sci. 2006;1078:285–90. doi: 10.1196/annals.1374.053. [DOI] [PubMed] [Google Scholar]
- 15.Guglielmone AA, Estrada-Pena A, Keirans JE, Robbins RG. 2003. Ticks (Acari: Ixodida) of the Neotropical zoogeographic region. International Consortium on Ticks and Tick-borne Diseases (ICTTD-2). Houten: Atalanta. [Google Scholar]


