Abstract
Objectives
To identify the variables that predict the failure to treat amoebic liver abscesses.
Methods
We prospectively carried out a case–control study on a cohort of patients who had been diagnosed with amoebic liver abscesses using clinical, ultrasonic, and serologic methods. Patients with pyogenic abscesses, negative ELISA tests for amoebiasis, immunosuppression status, or previous abdominal surgery were excluded. All patients received metronidazole, and those who demonstrated 4 days of unfavorable clinical responses received percutaneous or surgical draining of the abscess. Demographic, laboratory, and ultrasonographic characteristics were assessed as prognostic indications of failure.
Results
Of 40 patients with amoebic liver abscess, 24 (mean age: 36.7±11.2 years) responded to medical treatment and 16 (41.8±11.6 years) required drainage, including 14 patients who underwent percutaneous drainage and two patients who required surgery. The albumin level, abscess volume, abscess diameter, and alkaline phosphatase level were all statistically significant (P<0.05) on the bivariate analysis. The highest (>99%) sensitivity and negative predictive value were observed for an abscess volume >500 ml and diameter >10 cm, while the best specificity and positive predictive value were achieved with the combination of low serum albumin level, high alkaline phosphatase level, and large abscess volume or diameter.
Conclusions
The prognostic indications of the failure to treat amoebic liver abscesses include low albumin, high alkaline phosphatase, and large abscess volume or diameter. The combination of these variables is a useful and easy tool for determining appropriate therapy.
Keywords: Amoebic liver abscess, Failure, Prognosis
Introduction
Amoebiasis is an infectious disease caused by Entamoeba histolytic, a protozoan that is transmitted through the fecal–oral route and is associated with poor hygienic conditions.1 It is estimated that 10% of the world population is infected, and 90% of infected individuals are asymptomatic. It is probably the second most common cause of death by a parasitic disease.1,2 In Mexico, the annual incidence of amoebiasis was 43 cases per 10 000 inhabitants and the annual incidence of amoebic liver abscess was 6.7 per 100 000 inhabitants in 2000, with a reported male/female ratio of 12∶1. The south, southeastern, and the central regions (San Luis Potosí and its neighbors) of Mexico are considered endemic zones.3–5
Amoebiasis presents a wide variety of clinical forms that range from intestinal to extraintestinal sickness, in both acute and chronic forms. The most frequent extraintestinal form of amoebiasis is amoebic liver abscess.6–8 For the diagnosis of amoebic liver abscess, serological tests such as enzyme-linked immunesorbent assay (ELISA) and polymerase chain reaction have high rates of sensitivity and specificity (close to 99%);9 ultrasonography allows physicians to determinate the site, number, and volume of the abscesses, while Gallium scan differentiate between amoebic (cold images) and pyogenic abscesses (hot images). Thus, the difference is based on the absence (amoebic) or presence (pyogenic) of white blood cells in the abscess.10–12 The initial treatment of amoebic liver abscess is medical; nevertheless, in refractory cases it is required to drain the abscesses.8,13–15 Drainage treatment consists of percutaneous aspiration and surgical drainage. The indications for percutaneous aspiration are not well defined, but in general it is performed on abscesses >150 ml, when there are less than three abscesses present, when there has been no response to medical treatment for 3–5 days, when the abscess are localized in the left lobe, rupture is imminent, and if puncture accessibility is an issue.15–19
The aim of this study is to identify the variables that would allow physicians to predict the failure of medical treatment in response to amoebic liver abscess.
Experimental Procedures
A case–control study was performed that used a cohort of male and female patients who were older than 15 years and diagnosed with amoebic liver abscesses via clinical, ultrasonographic, and serologic diagnosis (e.g. ELISA) methods. This trial was carried out through the surgery and internal medicine departments of Hospital Central ‘Dr. Ignacio Morones Prieto’ in San Luis Potosí, México between May 1999 and April 2000. Patients with clinically suspected of pyogenic liver abscess, if they presented with the absence of diarrhoea and were older than 50 years, but were excluded for positive hemoculture or negative ELISA for amoebic antigens. We also excluded patients with the following: immunosuppression (e.g. HIV), abdominal or biliar surgical and amoebiasis antecedents, and abdominal tumors. Patients who did not fulfill the study-management protocol were removed. The study was approved by the ethics committee of the hospital, and every patient signed an informed consent form. This trial complies with the principles laid down in the 1975 Declaration of Helsinki, as revised in 2000 (ClinicalTrials.gov identifier: NCT00641992).
Studied variables
The possible factors that were studied to determine how they predict failure of treatment included the following: age, gender, level of schooling (defined as the number of years attended since first grade), alcoholism (defined as an average ingestion of 90 ml of pure alcohol, or its equivalent, per day for 4 or more days per week), malnutrition [diagnosed as a body mass index (BMI) ⩽18.4 kg/m2; the degree of malnutrition was classified as slight (BMI: 17–18.4 kg/m2), moderate (BMI: 16–16.9 kg/m2) or severe (BMI: <16 kg/m2)], and duration of illness.
The lab parameters assessed on admission included: hemoglobin (g/dl), leucocytes (cells×103/μl), lymphocytes (cells×103/μl), albumin (g/dl), total bilirubin (mg/dl), and alkaline phosphatase (UI). Liver ultrasonography, for obtaining measurements as well as performing the ultrasonography-guided punctures, was carried out using a video camera (Matrix Instruments model 1010, Sterling Philips) with 3.5–7.5 Hz transductors. The abscess volume was measured in terms of milliliters, its largest diameter in terms of centimeters, and the number and sites of the lesions were also confirmed (left lobe, right lobe, or both). Finally, the hospital stay was recorded in days.
Management protocol
The protocol for managing the patients at the hospital is shown in Fig. 1. On admission, all patients examined as follows: hematic cytometry to determine glucose, creatinine, glutamic oxaloacetic transaminase, glutamic pyruvic transaminase, and albumin levels; urine analysis; determination of prothrombin time and partial thromboplastin time; serology to determine the presence of amoebic pathogens using ELISA; and thoracic and abdominal X-rays. In all cases, diagnosis was verified by liver and biliar path ultrasonography. ELISA studies to determine serum amoebic antigens were performed at the Immunological Laboratory for Experimental Medicine Unit at the Universidad Nacional Autónoma de México medical school located at the ‘Hospital General’ in Mexico City. IgG antibodies against total amoeba anti-antigen were determined in the control group, which consisted of patients attending the Hospital Central for other reasons, at an optic density of 490 nm. The tests were carried out in duplicate, and the average of both tests was reported. The test was considered positive if it was more than two SD greater than the control average.
Figure 1.
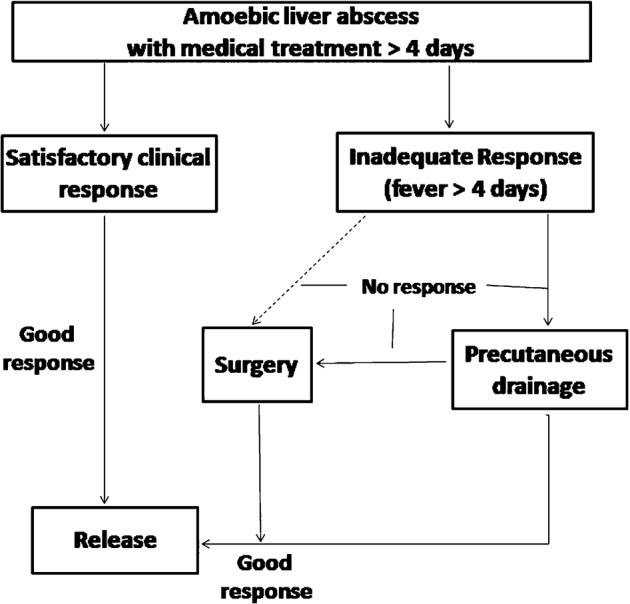
Management protocol in patients with amebic liver abscess. Medical treatment in all cases was metronidazole 750 mg every 8 hours.
All patients diagnosed with an amoebic liver abscess were treated with metronidazole at an oral dose of 750 mg administered every 8 hours. In the case of intolerance, it was intravenously (IV) applied. Patients weighing <50 kg and those who were strongly intolerant to medication due to secondary side effects received 500 mg metronidazole that was administrated orally or IV every 8 hours. They were kept in observation for at least 4 days. In the case of a satisfactory clinical response, which was defined as the absence of symptoms (e.g. fever, pain, and intolerance to oral intake) or a significant reduction in symptoms within the first 4 days of treatment, patients were discharged under the discretion of if the treating physician. If a fever persisted for more than 4 days, 1 g cefotaxime was delivered IV every 12 hours. In the case of an allergic reaction, amikacin was administered at a dose of 500 mg delivered IV every 12 hours. The observers of these chemical variables and ultrasonograms were blind to this study.
Failure of medical treatment and further management
Failure of medical treatment was considered when abdominal pain increased and/or persisted and if a fever (>37.5°C) persisted, did not decrease, or increased by at least 0.3°C by the fourth day. Percutaneous drainage was performed on these patients after the fourth day of treatment. Puncture was performed using a simple needle for both single and multiple aspirations. In cases of intense pain, imminent rupture, or the presence of peritoneal irritation, surgical drainage by means of an exploratory laparotomy was performed followed by treatment with 750 mg metronidazole every 8 hours and 1 g IV cefotaxime every 12 hours. The decision to proceed with intervention (percutaneous puncture or exploratory laparotomy) was made by a surgeon other than the main investigator.
All patients received at least 4 days of in-patient hospital treatment with metronidazole and they continued afterwards under ambulatory conditions until completing 15 days. The duration of treatment was based on the management guidelines for treating amoebic liver abscess in endemic regions.20,21
Statistical analysis
The sample size was calculated based on a retrospective pilot study.22 For albumin, the SD was 0.65 with a delta of 0.95, resulting in a necessary sample size of 8.4. For abscess diameter, the SD was 2.24 with a delta of 4.05, resulting in a sample size of 5.9. Finally, for alkaline phosphatase, the SD was 90.3 with a delta of 123, resulting in a sample size of 9.5. All of the calculations were considered statistically significant at P<0.05 and 80% power. Central tendency, dispersion, and normality measures of all variables were estimated. Bivariate analysis was also performed on all of the variables. The Student’s t-test or the Mann–Whitney U-test was used to analyze the continuous variables, depending on their distribution, and the Chi-squared or the Fisher’s exact test was used to analyze the categorical variables.
Ranges of reference for maximum sensitivity and the specificity of the significant variables were obtained by constructing receiver operating characteristic (ROC) curves. Cross-tabs on the presence/absence of the variables in different estimated ranges were generated using diagnostic tests for the purpose of establishing sensitivity, specificity, positive predictive values, and negative predictive values for isolated variables, as well as combination values, as a way of forecasting the lack of response to medical treatment of amoebic liver abscess. The odds ratios (ORs) were also calculated for the estimated ranges. The variables that yielded statistical significance P<0.05 on the bivariate analysis were included in the logistic regression model.
All statistical analyses were carried out using the JMP IN 4 program (SAS Institute Inc., Cary, NC, USA).
Results
Forty-three patients with clinical and ultrasonographic findings and positive for anti-amoebic antibodies were identified. Three patients were removed from the study: two patients did not receive medical treatment for at least 4 days and one patient underwent surgery for acute appendicitis. Forty patients were included for analysis, and of these 24 patients demonstrated a good response to medical treatment, 16 patients did not respond to medical treatment, and, of these, two patients underwent surgical drainage due to pain and persistent fever and 14 required external percutaneous drainage, of which two patients had abdominal pain and the remaining 12 had persistent fever. No patients died during this study. The flow of the analyzed patients is shown in Fig. 2. Table 1 shows the results of the variables studied in both treatment groups.
Figure 2.

Flow of patients assessed in the study.
Table 1. Comparison of the variables of interest between groups.
| Failure to medical treatment (n = 16) | Response to medical treatment (n = 24) | P value | |
| Age† (years) | 41.8±11.6 | 36.5±11.2 | 0.15§ |
| Sex (male/female) | 13∶3 | 21∶3 | 0.66 |
| Education‡ (years) | 6 (0–12) | 4 (0–9) | 0.16* |
| Alcoholism | 12 | 19 | 1 |
| Alcohol consumption‡ (ml/day) | 200 (0–510) | 150 (0–574) | 0.49* |
| Evolution time‡ (days) | 8 (3–25) | 6 (2–60) | 0.53* |
| Malnutrition | 1 | 2 | 1 |
| BMI† (kg/m2) | 23.93±3.51 | 23.01±3.33 | 0.45§ |
| Hemoglobin† (g/dl) | 11.82±1.96 | 12.38±1.80 | 0.37§ |
| Leucocytes† (×103 μl) | 20.65±9.56 | 16.61±6.14 | 0.87§ |
| Lymphocytes† (×103 μl) | 1904.5±819.93 | 1645.43±780.27 | 0.33§ |
| Albumin† (g/dl) | 2.2±0.74 | 2.95±0.71 | 0.004§ |
| Total bilirubin‡ (mg/dl) | 1.49 (0.4–6.1) | 1.1 (0.3–4.5) | 0.92* |
| Alkaline phosphatase‡ (UI) | 354 (161–754) | 181 (112–488) | 0.003* |
| Number of abscesses‡ | 1 (1–3) | 1 (1–3) | 0.16* |
| Location of the abscesses | |||
| Left | 0 | 0 | – |
| Right | 9 | 17 | 0.31 |
| Both sides | 7 | 6 | 0.31 |
| Largest diameter† (cm) | 12.63±2.54 | 9.56±2.64 | <0.001§ |
| Volume‡ (ml) | 768 (364–2543) | 410 (68–1077) | <0.001* |
| Days in hospital‡ | 11 (5–19) | 6.5 (4–16) | 0.003* |
Note: †Mean and SD.
‡Median (minimum–maximum) values.
The Student’s t-test§ or Wilcox Ranges* was used to calculate the P values; all others were calculated using Fisher exact F test.
The significant variables were albumin, alkaline phosphatase, and large abscess diameter and volume. With the use of the ROC curves, ranges of reference were the following: 2.5 g/dl albumin, 300 UI alkaline phosphatase, diameter >10 cm, and abscess volume >500 ml. Using diagnostic tests, several models were tested using these variables, either alone or in combination, and the obtained results were employed in the ROC curves. The sensitivity, specificity, and predictive values of each model are shown in Table 2. We observed higher specificity and positive predictive values in models 13–16, and, of these, model 16 had the highest sensitivity and negative predictive values.
Table 2. Sensitivity, specificity, PPV, and NPV of the different predictive models used to determine failure to medical treatment.
| Model | Sensitivity | Specificity | PPV | NPV |
| 1. Albumin <3 g/dl | 0.88 | 0.59 | 0.61 | 0.87 |
| 2. Albumin <2.5 g/dl | 0.75 | 0.64 | 0.60 | 0.78 |
| 3. Alkaline phosphatase >200 UI | 0.81 | 0.55 | 0.59 | 0.79 |
| 4. Alkaline phosphatase >300 UI | 0.56 | 0.85 | 0.75 | 0.71 |
| 5. Volume >500 ml | 0.94 | 0.75 | 0.71 | 0.95 |
| 6. Larger diameter >10 cm | 0.94 | 0.62 | 0.63 | 0.94 |
| 7. Volume >250 ml | 1.00 | 0.25 | 0.47 | 1.00 |
| 8. Larger diameter >8 cm | 1.00 | 0.38 | 0.52 | 1.00 |
| 9. Albumin <2.5 g/dl, volume >500 ml | 0.62 | 0.83 | 0.71 | 0.75 |
| 10. Albumin <2.5 g/dl, diameter >10 cm | 0.75 | 0.77 | 0.71 | 0.81 |
| 11. Albumin <3 g/dl, volume >500 ml | 0.81 | 0.82 | 0.76 | 0.86 |
| 12. Albumin <3 g/dl, diameter >10 cm | 0.81 | 0.73 | 0.68 | 0.84 |
| 13. Albumin <2.5 g/dl, volume >500 ml, alkaline phosphatase >200 UI | 0.5 | 1.00 | 1.00 | 0.71 |
| 14. Albumin <3 g/dl, volume >500 ml, alkaline phosphatase >200 UI | 0.69 | 1.00 | 1.00 | 0.80 |
| 15. Albumin <2.5 g/dl, diameter >10 cm, alkaline phosphatase >200 UI | 0.62 | 1.00 | 1.00 | 0.77 |
| 16. Albumin <3 g/dl, diameter >10 cm, alkaline phosphatase >300 UI | 0.75 | 1.00 | 1.00 | 0.83 |
Note: PPV, positive predictive value; NPV, negative predictive value.
Table 3 shows the OR of the different cuts of the statistically significant variables in the bivariate analysis where a biological gradient can be observed. The sensitivity is higher for the largest diameter and the highest alkaline phosphatase level. The discrepancies between levels 2 and 3 of the albumin (values between 2.01 and 3.00 g/dl) and abscess volume (251–750 ml) could be attributed to the small number of individuals.
Table 3. OR with 95% confidence intervals in groups of treatment concerning levels of albumin, volume, larger diameter of abscess and alkaline phosphatase in different cuttings cuts.
| Failure to medical treatment | Response to medical treatment | |
| Albumin (g/dl) | ||
| <2 | 1.93 (0.97–3.82) | 0.50 (0.19–1.33) |
| 2.01–2.5 | 1.47 (0.71–3.05) | 0.72 (0.35–1.46) |
| 2.51–3 | 1.66 (0.68–4.08) | 0.55 (0.11–2.81) |
| >3 | 0.21 (0.05–0.82) | 2.21 (1.28–3.82) |
| Volume (ml) | ||
| 0–250 | * | 1.88 (1.37–2.59) |
| 251–500 | 0.13 (0.02–0.93) | 2.07 (1.32–3.25) |
| 501–750 | 2.06 (1.03–4.11) | 0.49 (0.18–1.27) |
| 751–1000 | 1.28 (0.44–3.17) | 0.81 (0.29–2.25) |
| >1000 | 3.11 (1.68–5.74) | 0.17 (0.02–1.10) |
| Larger diameter (cm) | ||
| 0–8 | * | 2.06 (1.43–2.97) |
| 8.1–10 | 0.34 (0.04–2.00) | 1.57 (1.01–2.47) |
| 10.1–15 | 2.71 (1.05–6.99) | 0.54 (0.31–0.93) |
| >15 | 2.84 (1.83–4.41) | * |
| Alkaline phosphatase (UI) | ||
| 0–200 | 0.36 (0.12–1.04) | 1.92 (1.08–3.40) |
| 201–300 | 0.86 (0.36–2.05) | 1.11 (0.60–2.06) |
| 301–400 | 1.88 (0.97–3.64) | 0.46 (0.13–1.53) |
| >400 | 2.06 (1.10–3.85) | 0.32 (0.05–1.92) |
Note: *Zero cases.
Logistic regression models were fitted to include statistically significant variables into the bivariate analysis. The best-fitted models were: (1) volume (P = 0.01) and albumin (P = 0.02) with a determination coefficient of 0.46 (P = 0.03); and (2) largest diameter (P = 0.006), albumin (P = 0.02), and alkaline phosphatase (P = 0.04) with a determination coefficient of 0.51 (P<0.01). Abscess volume and largest diameter represent similar measurements of the same phenomenon. These values showed 97% correlation. The duration of time spent in the hospital were also significantly different, with patients whose medical treatments failed requiring longer stays in the hospital.
Discussion
The literature describes several factors that may influence the mortality of amoebic liver abscess.13,23 There is evidence that the presence of encephalopathy, serum bilirubin levels >3.5 mg/dl, hypoalbuminemia (<2 g/dl), hemoglobin <8 g/dl, pleural effusion, abscess volume, and the number of abscesses are all independently related to death.24 Moreover, the presence of discrete focal liver injury, pleural disease, distorted or absent venous flow, or venous thrombosis in ultrasonography requires complementary image studies.25 The factors that determine a lack of response to treatment and indicate early drainage are not very clear, although some studies suggest the diameter of the abscess, low albumin, and increased alkaline phosphatase as possible contributing factors.22 Although some studies have pointed out the benefit of percutaneous and laparoscopic drainage for the treatment of amoebic liver abscess,14,15,17,18,26 there are no defined criteria that are typically used to decide its application.
Up to now, there are two imaging classifications according to N’Gbesso and Nari that could be used to help determine the required treatment. N’Gbesso classification focuses on the features of the abscess: non-collected, collected, and healed, (type I, II, and III respectively), and Nari classification complements it with two additional types for this classification: one of these considers the abscesses in an intermediate evolutionary stage between non-collected and collected types, where a partial degree of confluence is seen; the second type are abscesses that shown complications such as rupture, communication to thorax, or intrahepatic complications, or those with ecographic alarm signs including a size greater that 10 cm, a superficial location, and thin walled abscesses. In this way, this classification proposed the division into: non-collected, intermediate, collected, complicated or warning signs, and ways of healing.3,24 N’Gbesso et al.27 state percutaneous drainage should be carried out in non-collected type, while Nari et al.3 defend that the non-collected and intermediate types should be drained.
Based on our results, it seems that abscess volume and largest diameter together with the serum levels of albumin and alkaline phosphatase are a useful indicator that could be applied to predict response or failure of medical treatment for amoebic liver abscess. The relationship between size and alkaline phosphatase could be associated with the extrinsic compression of the extrahepatic conducts by large abscesses, in such a way that a pathophysiological explanation supports our proposed predictors.22,23
The accuracies of models 13–16 (Table 3) demonstrate specificity and positive predictive values equal to 1, and, models 14 and 16 demonstrate higher sensitivity and negative predictive values. Patients with albumin levels <3 g/dl, an abscess volume >500 ml, and an alkaline phosphatase level >200 UI or an albumin level <3 g/dl, an abscess diameter >10 cm, and an alkaline phosphatase >300 UI will not respond (zero patient in our series) to medical treatment and should be strongly considered as candidates for primary percutaneous drainage, such suggestion has been previously in the literature.28 On the other hand, in models 7 and 8, which assessed isolated variables, demonstrate sensitivity and negative predictive values equal to 1. Patients with an abscess volume <250 ml or diameter <8 cm demonstrated a 100% response to medical treatment. The critical values required to make a decision regarding intermediate patients varies between a volume of 250–500 ml and a largest diameter of 8–10 cm. Patients with abscesses <500 ml in volume and <10 cm in diameter demonstrated 94% probability of responding to medical treatment. In these patients, we recommend careful observation of the albumin and alkaline phosphatase levels, especially if they stray from the higher sensitivity and specificity values, in order to make a therapeutic decision, which must always be personalized after considering demographic factors such as other comorbidities, abscess accessibility, and clinical course. The clinical applications of logistic regression analysis to therapeutic decision-making is, however, limited to our models.
The application of the studied criteria could allow selected patients who are at a high risk of failure to avoid delaying the drainage of lesions, which could help reduce the development of complications. By assigning patients without these medical factors, the risks could be reduced by handling complications that stem from unnecessary puncturing.
Given the location of our centre within an endemic zone, and the added use of rigorous methodological and statistical strategies, we believe that this primary theoretical approach can be used to develop an initial management guide. Studies should be performed to evaluate additional models, such as those that include N’Gbesso and Nari classifications, together with the prognostics variables identified in this study.
References
- 1.Walsh JA. Problems in recognition and diagnosis of Ameobiasis: estimation of the global magnitude of morbidity and mortality. Rev Infect Dis. 1986;8:228–38. doi: 10.1093/clinids/8.2.228. [DOI] [PubMed] [Google Scholar]
- 2.Petri WA, Jr, Singh U. Diagnosis and management of Ameobiasis. Clin Infect Dis. 1999;29:1117–25. doi: 10.1086/313493. [DOI] [PubMed] [Google Scholar]
- 3.Nari GA, Ceballos Espinosa R, Carrera Ladrón de Guevara S, Preciado Vargas J, Cruz Valenciano JL, Briones Rivas JL, et al. Amebic liver abscess. Three years experience. Rev Esp Enferm Dig. 2008;100(5):268–72. doi: 10.4321/s1130-01082008000500004. [DOI] [PubMed] [Google Scholar]
- 4.Kuri-Morales P, Mancha-Moctezuma C, Álvarez-Lucas C, Revuelta-Herrera A, Sandoval-Cázarez C, Cruz-Cruz V, et al. 2001. Epidemiological information of morbidity 2000. D.F; Secretaría de Salud; ago. [Google Scholar]
- 5.Escandón Romero C, Treviño García Manzo N, Escobedo de la Peña J, Hernández Ramos JM, Olvera Alvarez J, Cabral Soto J. La amibiasis y el absceso hepático amebiano en México, un problema de salud pública de actualidad. Rev Gastroenterol Méx. 1996;61:378–86. [PubMed] [Google Scholar]
- 6.Kucik CJ, Martin GL, Sortor BV. Common intestinal parasites. Am Fam Physician. 2004;69:1161–8. [PubMed] [Google Scholar]
- 7.Sharma MP, Dasarathy S, Sushma S, Verma N. Long term follow-up of amebic liver abscess: clinical and ultrasound patterns of resolution. Trop Gastroenterol. 1995;16:24–8. [PubMed] [Google Scholar]
- 8.Mohan S, Talwar N, Chaudhary A, Andley M, Ravi B, Kumar A. Liver abscess: a clinicopathological analysis of 82 cases. Int Surg. 2006;91:228–33. [PubMed] [Google Scholar]
- 9.Visser LG, Verweij JJ, van Esbroeck M, Edeling WM, Clerinx J, Polderman AM. Diagnostic methods for differentiation of Entamoeba histolytic and Entamoeba dispar in carriers: performance and clinical implications in a non-endemic setting. Int J Med Microbiol. 2006;296:397–403. doi: 10.1016/j.ijmm.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Pinilla AE, López MC, Castillo B, Murcia MI, Nicholls RS, Duque S, et al. A diagnostic approach to hepatic abscess. Rev Med Chil. 2003;131:1411–20. [PubMed] [Google Scholar]
- 11.Benedetti NJ, Desser TS, Jeffrey RB. Imaging of hepatic infections. Ultrasound Q. 2008;24(4):267–78. doi: 10.1097/RUQ.0b013e31818e5981. [DOI] [PubMed] [Google Scholar]
- 12.Ximénez C, Morán P, Rojas L, Valadez A, Gómez A, Ramiro M, et al. Novelties on amoebiasis: a neglected tropical disease. J Glob Infect Dis. 2011;3(2):166–174. doi: 10.4103/0974-777X.81695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoffner RJ, Kilaghbian T, Esekogwu VI, Henderson SO. Common presentations of amebic liver abscess. Ann Emerg Med. 1999;34:351–5. doi: 10.1016/s0196-0644(99)70130-7. [DOI] [PubMed] [Google Scholar]
- 14.Kurland JE, Brann OS. Pyogenic and amebic liver abscesses. Curr Gastroenterol Rep. 2004;6:273–9. doi: 10.1007/s11894-004-0078-2. [DOI] [PubMed] [Google Scholar]
- 15.Avendaño-Arredondo AA, Gil-Galindo G, García-Solís M de J, Pulido-Rodríguez J. Clinical experience of early percutaneous drainage of amebic hepatic abscess. Cir Cir. 2007;75:157–62. [PubMed] [Google Scholar]
- 16.Thomas J, Turner SR, Nelson RC, Paulson EK. Postprocedure sepsis in imaging-guided percutaneous hepatic abscess drainage: how often does it occur? Am J. Roentgenol. 2006;186:1419–22. doi: 10.2214/AJR.04.1914. [DOI] [PubMed] [Google Scholar]
- 17.Liztradi MJ. Percutaneous drainage of liver abscess complicated by hepato-venous fistula. Singapore Med J. 2003;44:299–301. [PubMed] [Google Scholar]
- 18.Mogollón Prado A, Molina Sánchez G, Martínez Macías F, Sánchez Villanueva P, Sánchez Trejo S, Dávila Fernández BC, et al. Percutaneous drainage of amebic hepatic abscess by guided ultrasound. Preliminary results. Rev Gastroenterol Mex. 1999;64:134–8. [PubMed] [Google Scholar]
- 19.Saraswat VA, Agarwal DK, Baijal SS, Roy S, Choudhuri G, Dhiman RK, et al. Percutaneous catheter drainage of amoebic liver abscess. Clin Radiol. 1992;45:187–9. doi: 10.1016/s0009-9260(05)80639-7. [DOI] [PubMed] [Google Scholar]
- 20.van Hal SJ, Stark DJ, Fotedar R, Marriott D, Ellis JT, Harkness JL. Amoebiasis: current status in Australia. Med J Aust. 2007;186(8):412–6. doi: 10.5694/j.1326-5377.2007.tb00975.x. [DOI] [PubMed] [Google Scholar]
- 21.Goessling W, Chung RT. Amebic liver abscess. Curr Treat Options Gastroenterol. 2002;5(6):443–9. doi: 10.1007/s11938-002-0032-z. [DOI] [PubMed] [Google Scholar]
- 22.Graillet R, Sánchez-Aguilar M, Morán-Mendoza AO, Hernández-Sierra JF, Gordillo-Moscoso A, Tapia-Pérez JH. Analysis of factors associated to failure of medical treatment of amoebic liver abscess. Cir Esp. 2008;84(2):83–6. doi: 10.1016/s0009-739x(08)72139-0. [DOI] [PubMed] [Google Scholar]
- 23.Sharma MP, Dasarathy S, Verma N, Saksena S, Shukla DK. Prognostic markers in amebic liver abscess: a prospective study. Am J Gastroenterol. 1996;91:2584–8. [PubMed] [Google Scholar]
- 24.Ortiz Sanjuán FM, Devesa Jordà F, Ferrando Ginestar J, Ferrando I, Borghol A, Gutiérrez J. Amebic liver abscess: medical treatment or percutaneous aspiration? Gastroenterol Hepatol. 2007;30(7):399–401. doi: 10.1157/13108815. [DOI] [PubMed] [Google Scholar]
- 25.Secretaría de Salud. Guía de Práctica Clínica para el Diagnóstico y tratamiento del absceso hepático amebiano no complicado. México, DF, 2010. Available from: http://www.cenetec.salud.gob.mx/descargas/gpc/CatalogoMaestro/432_GPC__Absceso_hepatico/GER_Absceso_hepxtico_amebiano.pdf. [Google Scholar]
- 26.Domínguez-Guzmán DJ, Moreno-Portillo M, García-Flores C, Blas-Franco M. Laparoscopic drainage of liver abscess. Initial experience. Cir Cir. 2006;74(3):189–94. [PubMed] [Google Scholar]
- 27.N'Gbesso RD, Kéita AK. Ultrasonography of amebic liver abscesses. Proposal of a new classification. J Radiol. 1997;78(8):569–76. [PubMed] [Google Scholar]
- 28.Blessmann J, Binh HD, Hung DM, Tannich E, Burchard G. Treatment of amoebic liver abscess with metronidazole alone or in combination with ultrasound-guided needle aspiration: a comparative, prospective and randomized study. Trop Med Int Health. 2003;8:1030–4. doi: 10.1046/j.1360-2276.2003.01130.x. [DOI] [PubMed] [Google Scholar]


