Abstract
Objectives
We examined the prevalence of Strongyloides stercoralis (Ss) infection in a cohort of AIDS patients from a US urban centre. We monitored our cohort for possible cases of dissemination or immune reconstitution inflammatory syndrome after antiretroviral therapy (ART) initiation.
Methods
One hundred and three HIV-infected participants were prospectively sampled from a cohort observational study of ART-naive HIV-1-infected patients with CD4 ⩽100 T cells/μl. Clinical symptoms, corticosteroid therapy, eosinophilia, CD4 count, and plasma HIV-RNA were reviewed. Sera were tested by an enzyme-linked immunosorbent assay (CrAg-ELISA) to crude Ss extract or to an Ss-specific recombinant protein (NIE) and by luciferase immunoprecipitation system assay (LIPS) for Ss-specific antibodies.
Results
Twenty-five per cent of study participants were Strongyloides seropositive by CrAg-ELISA and 62% had emigrated from Strongyloides-endemic areas. The remaining 38% of the seropositives were US born and tested negative by NIE and LIPS. CrAg-ELISA-positive participants had a median CD4 count of 22 T cells/μl and a median HIV-RNA of 4.87 log10 copies/ml. They presented with diarrhea (27%), abdominal pain (23%), and skin manifestations (35%) that did not differ from seronegative patients. Peripheral blood eosinophilia was common among seropositive patients (prevalence of 62% compared to 29% in seronegatives, P = 0.004). Seropositive patients were treated with ivermectin. There were no cases of hyperinfection syndrome.
Discussion
Strongyloidiasis may be prevalent in AIDS patients in the USA who emigrated from Ss-endemic countries, but serology can be inconclusive, suggesting that empiric ivermectin therapy is a reasonable approach in AIDS patients originating from Strongyloides endemic areas.
Keywords: AIDS, Strongyloidiasis, Antiretroviral therapy
Introduction
Strongyloides stercoralis (Ss) is an intestinal nematode that affects up to an estimated 100 million people residing in tropical and subtropical regions worldwide.1 Areas of low Ss endemicity have been observed in the USA, specifically in the Appalachians and parts of the Southeast.2,3 An increase in international travel and immigration has led to a rise in imported Ss cases in industrialized countries.4–6 Despite the heightened risk for Ss infections in the USA, limited data exist regarding Ss prevalence, and diagnosis may be missed when not specifically sought.
Chronic S. stercoralis infection can last a lifetime in the host, with clinical illness sometimes appearing as late as 60 years after the infection is first acquired.4,7 Most infected patients are asymptomatic or present with unexplained eosinophilia. Symptoms associated with chronic infection may include rash (larva currens or chronic urticaria), abdominal pain and diarrhea.8,9 Altered cell-mediated immunity can predispose patients to hyperinfection syndrome and dissemination, particularly those receiving corticosteroid therapy and those with certain malignancies or HTLV-1 infection.8–11 Although HIV infection is not a risk factor for disseminated Ss and hyperinfection syndrome, co-infection with HIV and Ss is common in persons who have resided in endemic areas.12–15 Several case reports have also identified a possible increased risk of immune reconstitution inflammatory syndrome (IRIS) phenomenon leading to hyperinfection after initiation of antiretroviral therapy (ART) in co-infected subjects.16–20
Despite the widespread occurrence of Strongyloides, timely and accurate diagnosis remains difficult. Early parasite detection in the stool is challenging, as larvae are excreted intermittently by the adult worms, thereby requiring multiple stool sample tests for accurate diagnosis.21,22 Serologic approaches to determine infection have improved Ss detection rates, but the specificity and sensitivity of the tests remain unknown in AIDS patients who may have altered immune responses to the parasite.23 Thus, it is clear that the prevalence, evaluation, and management of Ss infection in HIV should be better established.
In this study, we sought to determine the prevalence of Strongyloides infection in an urban AIDS cohort in the USA. Given that HIV patients may exhibit complex symptomatology due to several co-morbidities, we also sought to establish potential clinical and laboratory characteristics useful for detecting and managing Ss/HIV co-infection. Lastly, we compared existing Ss serodetection methods, to investigate their potential utility in an AIDS cohort.
Materials and Methods
Participants
HIV-infected adult persons participating in a prospective observational study of HIV-1 infected patients with CD4 ⩽100 T cells/μl who are ART-naive at the National Institutes of Health in Bethesda, Maryland (NCT #00286767) were selected. The Institutional Review Board at the National Institute of Allergies and Infectious Diseases approved this research and all patients signed informed consent. All individuals at the National Institutes of Health who enrolled in the study between December 2006 and March 2011 were included in this analysis. All patients initiated and remained on ART.
Laboratory testing
White blood cell count (total cells/μl), total eosinophils (K/μl), and per cent eosinophils were determined by automated technique from blood collected at designated study time points. The normal range of absolute eosinophil counts was 0.04–0.54 K/μl for males and 0.04–0.36 K/μl for females. An ultrasensitive bDNA assay was used to determine plasma HIV-RNA (Versant HIV-1 version 3.0; Siemens, New York City, NY, USA).
Antibody testing for Strongyloides
Crude antigen enzyme-linked immunosorbent assay
Serum samples were tested using the crude antigen enzyme-linked immunosorbent assay (CrAg-ELISA) at the Centers for Disease Control and Prevention (CDC) in Atlanta, Georgia. This quantitative validated assay has a sensitivity of 96% and a specificity of 98%. Sensitivity was obtained by testing 68 Strongyloides proven cases and specificity was obtained by testing 84 Strongyloides uninfected individuals from the USA. The specificity was reduced to 72% when samples from patients with other infections were included in the calculation; undectected/unreported Strongyloides infection could not be ruled out in these cases.24,25 Microtiter plates (Immulon II HB; Thermo, Milford, MA, USA) were sensitized with the purified crude antigen at a concentration of 0.9 μg/ml in sensitization buffer (0.1 M NaHCO3/Na2CO3, pH 9.6), sealed and incubated at 4°C overnight. Unknown sera, controls, and standard curve points were diluted 1∶100 in phosphate-buffered saline (PBS)/0.3% Tween 20 and delivered to the appropriate wells after the previously sensitized plate was washed three times with PBS/0.3% Tween 20 using a microplate washer (BioTek EL ×405, Winooskii, VT, USA). The diluted samples were incubated at 37°C for 1 hour. After a second wash with PBS/0.3% Tween 20, Goat anti-human IgG alkaline phosphatase conjugate (Sigma, St Louis, MO, USA) was added to all wells and incubated at 37°C for 1 hour. After the third wash with PBS/0.3% Tween 20, the substrate mixture (0.05 M NaHCO3/Na2CO3, pH 9.6, 1 mM MgCl2, 1 mg/ml p-nitrophenyl phosphatase (Sigma) was added to all wells and incubated at 37°C for 15 minutes. The reaction was stopped with 3 M NaOH. The plate was read with a microplate reader at a wavelength of 405 nm. Results from individual specimens were generated by using a four-parameter logistic–log curve fitting model using SoftMax Pro software (Molecular Devices, Sunnyvale, CA, USA) for microplate data acquisition and analysis. All reactions of ⩽1.7 units/μl were considered negative and all reactions of >1.7 units/μl were considered positive, indicative of infection with Ss at some indeterminate point of time.
One specimen tested at CDC was assayed in 2007 when the CDC assay had a different format. The previous CDC assay also used a purified crude antigen. All reactions of <8% were considered negative and all reactions ⩾8% were considered positive, indicative of infection with Ss at some indeterminate point of time. The sensitivity of the assay was 95% and specificity was 100% in controls from the USA, but was 82% in patients with other parasitic diseases.4
NIE enzyme-linked immunosorbent assay
Ninety-six-well plates (Immulon 4HBX; Thermo Scientific) were coated with 0.125 μg/ml of NIE antigen in coating buffer (45 mM NaHCO3, 18 mM Na2H CO3). Plates were incubated and washed as previously described.26 Patient sera were diluted 1:200 in diluent buffer (PBS, 1% BSA, 0.05% Tween 20), added in triplicate and incubated for 1 hour at 37°C. Plates were washed four times in wash buffer. Goat anti-human IgG (Fc-specific) alkaline phosphatase (1∶2500; Jackson ImmunoResearch) in diluent buffer was added and plates were incubated for 1 hour at 37°C. After four washes, a pNPP phosphatase substrate (Sigma) was added and plates were read at 405 nm in an ELISA reader (SpectraMax Plus; Molecular Devices, Sunnyvale, CA, USA). All data were corrected for background reactivity (sera not added). Cutoffs for negative values were below 96 units/ml and positive values were above 100 units/ml. Indeterminate values were between 96 and 100 units/ml; these were repeated and further classified depending on results. Cutoff values were determined by previously described similar methodology.26 For further validation, subgroups of samples from Strongyloides-infected patients and normal healthy controls were run concurrently. Standard curves were used and values from patient samples were interpolated from the curve and units of NIE-specific IgG were determined.
Luciferase immunoprecipitation system assay (LIPS)
The LIPS assay for Ss was performed as described previously.25 Sera samples were diluted 1∶10 in assay buffer (20 mM Tris, pH 7.5, 150 mM NaCl, 5 mM MgCl2, 1% Triton X-100) and then added to 100 μl containing 1×106 LU of Renilla luciferase-fused antigen for 5 minutes at room temperature in 96-well filter plates. Seven microlitres of a 30% suspension of protein A/G beads (Pierce, Rockford, IL, USA) in PBS was added next and incubated for 5 minutes at room temperature. The plates were washed in PBS through a vacuum manifold. After the final wash, all plates were processed on a Berthold LB 960 Centro microplate luminometer using a colenterazine substrate mix (Promega). All data were corrected for background reactivity (sera not added). Cut offs for negative and positive values were determined at 600 LU using similar previously described methodology.25
Stool ova and parasites
The agar plate culture method was used to detect Ss larvae in select participants, as clinically indicated for diarrhea work up. One gram of stool was placed in the centre of a blood agar plate and incubated for 24–48 hours at 37°C. The plate was examined for Ss larval tracks. The presence of ova was determined using a modified Ritchie formalin–ether method.27 Parasites were concentrated using the fecal parasite concentrator where stool was preserved in 5% or 10% formalin and spun at 1900 rev/min for 10 minutes (FCP; Evergreen Scientific, Los Angeles, CA, USA).28 Ova of other parasites and larvae from isolated layers were analysed by wet mount microscopy.
Statistical methods
Median values were used as the measurement of central tendency. Groups were compared with Kruskal–Wallis and Mann–Whitney U tests and paired values were assessed using the Wilcoxon matched-paired signed rank tests. Analyses were performed using Prism v5.0 (GraphPad Software, La Jolla, CA, USA).
Results
Participant characteristics
Twenty-six of 103 subjects were Strongyloides CrAg-ELISA seropositive, with an overall prevalence of 25% within this cohort. Among the 54 participants originating from known Strongyloides-endemic areas, 30% were tested positive for Strongyloides-specific antibodies by CrAg-ELISA. The majority of participants immigrated to the USA from Central America, Mexico, or East Africa (Table 1). There were no significant differences in gender, CD4 count, or plasma HIV-RNA between the two groups. Most of the study subjects were male and 30–48 years of age with a median baseline CD4 count of 19–23 T cells/μl and a median plasma HIV-RNA level of 4.87–5.16 log10 copies/ml (Table 1).
Table 1. Baseline (pre-antiretroviral therapy) characteristics and region of origin of Strongyloides CrAg-ELISA-positive and -negative participants.
| Characteristic | Strongyloides CrAg-ELISA-positive | Strongyloides CrAg-ELISA-negative | P value | |
| n = 26 | n = 77 | |||
| Male no. (%) | 20 (77) | 59 (77) | 0.98 | |
| Age (IQR) | 40 (30–47) | 40 (33–48) | 0.77 | |
| CD4 T cells/μl (IQR) | 23 (8–43) | 19 (7–48) | 0.81 | |
| HIV-RNA Log10 copies/ml (IQR) | 4.87 (4.50–5.24) | 5.16 (4.7–5.5) | 0.06 | |
| Corticosteroid treatment no. (%) | 3 (11) | 14 (18) | 0.45 | |
| Region of origin | ||||
| No. (%) | ||||
| Central Africa | 1 (4) | 1 (1) | ||
| East Africa | 5 (19) | 8 (10) | ||
| North Africa | … | … | ||
| South Africa | … | … | ||
| West Africa | 1 (4) | 2 (3) | ||
| Central America/Mexico | 9 (35) | 25 (33) | ||
| North America | 10 (38) | 37 (48) | ||
| South America | … | 2 (3) | ||
| Eastern Europe | … | 1 (1) | ||
| Western Europe | … | 1 (1) | ||
| Clinical signs and symptoms no. (%) | ||||
| Abdominal pain | 6 (23) | 19 (24) | 0.89 | |
| Diarrhea | 7 (27) | 17 (22) | 0.60 | |
| Dermatitis | 9 (35) | 21 (27) | 0.46 | |
| Eosinophilia (%)* | 16 (62) | 23 (29) | 0.004 | |
| Eosinophilia (no.)† | 10 (38) | 10 (13) | 0.004 | |
Note: *Defined as >7.0% for males and >5.8% for females.
†Defined as >0.54 K/μl for males >0.36 K/μl for females.
Baseline signs and symptoms
No significant difference in clinical symptoms such as diarrhea, rash, and abdominal pain was found between Ss-seropositive and -seronegative patients (Table 1). There was no significant difference in the incidence of non-localized urticaria or pruritic papular rash between the CrAg-ELISA-seronegative and -seropositive groups. None of the patients in either group presented with any of the characteristic skin manifestations of Ss infection.29,30
Prior to ART initiation, 10 Ss-seropositive patients presented with peripheral blood eosinophilia defined as an absolute eosinophil count (AEC) >0.54 K/μl for males and >0.36 K/μl for females (Table 1 and Fig. 1). The median eosinophil count of the Strongyloides CrAg-ELISA-positive patients was 0.27 K/μl (IQR: 0.06–0.66) and was higher than the median count of 0.12 K/μl (IQR: 0.05–0.22) in the Strongyloides CrAg-ELISA-negative patients (P = 0.06) (Figure 1a). When patients receiving corticosteroids at baseline examination were excluded from the analysis, the difference in the eosinophil count was statistically significant (P = 0.02). In addition, the median proportion of eosinophils was significantly higher in the seropositive compared to seronegative patients [11.8 (IQR: 3.1–18.9) versus 3.1 (IQR: 1.0–7.6), P = 0.002)]. Sixteen out of 26 seropositive patients had stool analysed for ova and parasites (Table 2). Among the 16 tested, only three had Ss parasites in stool analysis.
Figure 1.
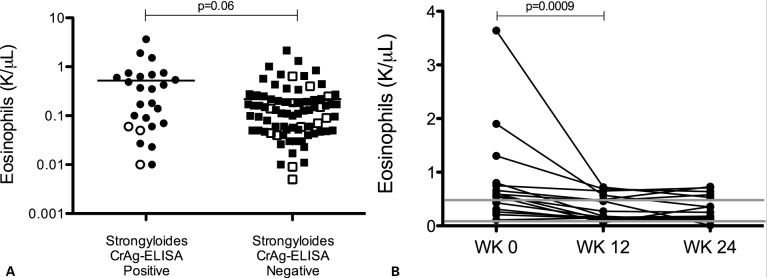
Comparison of eosinophil count between Strongyloides CrAg-ELISA-seropositive and -seronegative participants pre-antiretroviral therapy (pre-ART). Number of eosinophils (A) was assessed from 103 patients at their baseline visit (pre-ART). Significance determined by Mann–Whitney U test. Open shapes indicate patients on corticosteroid therapy at the time of blood sampling. When patients on corticosteroid therapy were excluded from the analysis, there was a statistically significant difference in eosinophil count (P = 0.02). (B) Absolute eosinophil counts from 16 Strongyloides CrAg-ELISA-positive patients treated with ivermectin with longitudinal follow-up for 24 weeks after ivermectin administration. WK 0 indicates treatment with a standard dose of ivermectin (200 μg/kg for 1–2 days). Grey lines denote the range of normal absolute eosinophil counts (0.04–0.54 K/μl for males and 0.04–0.36 K/μl for females).
Table 2. Comparison of Strongyloides testing methodology at baseline, pre-antiretroviral therapy.
| Patient no. | CrAg-ELISA | LIPS | NIE | Stool O&P | Country of origin |
| 1 | + | − | − | − | Ethiopia |
| 2 | + | + | − | Not done | Ethiopia |
| 3 | + | − | − | Not done | Guatemala |
| 4 | + | − | − | − | Ethiopia |
| 5 | + | − | − | − | USA |
| 6 | + | − | − | Not done | USA |
| 7 | + | − | − | Not done | Cameroon |
| 8 | + | − | − | Not done | USA |
| 9 | + | − | − | − | USA |
| 10 | + | − | − | Not done | USA |
| 11 | + | + | − | + | Honduras |
| 12 | + | − | − | Not done | USA |
| 13 | + | + | − | + | Honduras |
| 14 | + | − | − | − | USA |
| 15 | + | − | − | − | Honduras |
| 16 | + | + | − | Not done | Honduras |
| 17 | + | − | − | Not done | USA |
| 18 | − | − | +/− | Not done | El Salvador |
| 19 | − | − | +/− | − | Ethiopia |
| 20 | + | − | + | Not done | Sierra Leone |
| 21 | − | + | − | − | El Salvador |
| 22 | + | − | − | + | Mexico |
| 23 | − | + | − | − | El Salvador |
| 24 | + | − | − | Not done | Uganda |
| 25 | + | − | − | − | Guatemala |
| 26 | + | − | − | Not done | USA |
| 27 | + | + | − | Not done | El Salvador |
| 28 | + | − | − | − | USA |
| 29 | + | − | − | − | Guatemala |
| 30 | + | − | − | − | Ethiopia |
Note: At baseline, pre-ART negative results were seen in all four Strongyloides detection methods in 73 other patients, when tested. Stool studies were sent prospectively for evaluation of diarrhea in select patients, as clinically indicated.
CrAg-ELISA, crude antigen enzyme-linked immunosorbent assay; LIPS, luciferase immunoprecipitation system assay; NIE, recombinant-based immunoassay; stool O&P, stool ova and parasites.
Suspected Strongyloides-related IRIS events
Two patients presented with suspected Strongyloides-related IRIS events in the first several weeks after starting ART. One patient presented with peripheral eosinophilia (peak AEC 1.31 K/μl), and new onset diffuse urticarial rash resistant to topical steroids. Both the rash and eosinophilia responded clinically to ivermectin. A skin biopsy in this patient revealed perivascular lymphocytic infiltrates with eosinophils consistent with urticarial reaction. The patient received no corticosteroids before or during the suspected Strongyloides-related IRIS event and stool exams showed no ova or parasites. The other patient had been treated for Strongyloides based on positive serology pre-ART and then presented with fever, thickening of the small bowel, a peak AEC of 1.52 K/μl and extremely elevated C-reactive protein 10 days after ART initiation with a decrease in the Strongyloides antibody titre from pre-ART. A jejunum biopsy revealed mixed inflammation with mainly plasma cells and eosinophils and stool exams were negative for ova and parasites. The patient did not receive corticosteroid treatment prior to or during symptom onset. He responded clinically to broad spectrum antibiotics and repeat ivermectin administration.
Evolution of eosinophilia
All patients in the Strongyloides CrAg-ELISA positive group received a standard dose of oral ivermectin (200 mcg/kg for 1–2 days). Some patients received ivermectin before starting corticosteroids if Ss infection was suspected based on endemic country of origin. At 24 and 48 weeks after ART initiation, there was no statistically significant difference in the AEC between the seropositive and negative groups (P = 0.97 and P = 0.46). Among the patients with positive baseline Ss CrAg-ELISA serology treated with ivermectin, 16 returned to the clinic for at least 24 weeks of follow-up after ivermectin administration. These patients’ AEC values decreased by week 12 compared to pre-therapy levels (P = 0.0009) and had normalized by 24 weeks after ivermectin therapy (Fig. 1B).
Strongyloides antibody detection
Both the LIPS assay and the NIE EIA have specificities that exceed that of the ELISA using crude Strongyloides extract, in non-HIV-infected individuals.25 In our study, testing by the CrAg-ELISA revealed a total of 26 Strongyloides-seropositive patients. Among these, 10 were suspected to be possible false positives, as they emanated from individuals from the Mid-Atlantic states of the USA, with no travel history to Ss-endemic regions within the USA or internationally (Table 2). In addition, these subjects had no history of risk factors that have been previously associated with Ss infection, such as pica, institutionalisation, or occupations that increase contact with contaminated soil.8 The LIPS assay detected no suspected false-positive tests at baseline and showed seven Ss seropositives among the patients from endemic areas. All three patients with stool positive for ova and parasites were also positive by CrAg-ELISA and two of them were positive by LIPS. NIE EIA results were discordant with all but one of the seropositive patients by CrAg-ELISA or LIPS.
Discussion
In this study, we showed that even in a non-endemic, urban area of the USA, 25% of patients of AIDS patients were Strongyloides-seropositive by ELISA testing using crude Ss extract. This high rate of Strongyloides seropositivity likely reflects the large number of recent immigrants to the USA, and a high frequency of international travel in the surrounding urban area and probably includes false-positive testing, as a high proportion of cases were USA born and tested negative by NIE and LIPS assays. In addition, there were no specific clinical or laboratory findings that could raise the suspicion of Ss other than eosinophilia which was absent when patients were treated with corticosteroids, a situation that poses the highest risk for hyperinfection in this setting.
In this study, it was impossible to determine whether any of our patients would have gone on to develop hyperinfection syndrome in the absence of treatment. Although no cases of hyperinfection syndrome occurred, two patients did present with signs and symptoms suspicious of Strongyloides-IRIS following ART initiation which appeared to resolve with ivermectin treatment. Both Strongyloides CrAg-ELISA-seropositive and -seronegative patients frequently presented with diarrhea, abdominal pain, and skin manifestations, suggesting that these symptoms alone are not useful predictors of Strongyloides infection in persons with AIDS. Nine of 26 Ss CrAg-ELISA serology-positive patients presented also with skin manifestations that included pruritic rash, eosinophilic folliculitis, and generalized rash, but none had the characteristic cutaneous manifestations of Strongyloides. Eosinophilia was the only laboratory difference between Ss CrAg-ELISA-seropositive and -seronegative patients; however, treatment with corticosteroid therapy as part of the standard of care for opportunistic infections frequently masked eosinophilia.
Diagnosis of chronic Strongyloides infection was challenging in this cohort. Owing to the technical difficulties of detecting Ss larvae in the stool, serology tests using crude parasite antigens have been increasingly used to diagnose strongyloidiasis. However, significant cross-reactions in patients with other tissue-invasive helminths (e.g. filarial infections) make this type of test less specific for Ss infection.31 NIE-based EIA and LIPS testing represent more specific approaches to Strongyloides testing and have demonstrated improved specificity compared with CrAg-ELISA in other (immunocompetent) cohorts.25,26 LIPS assays have also been successfully applied to diagnosis of multiple infections including Pneumocystis, HIV, and hepatitis.32 Before this study, none of these three serologic tests evaluated had been specifically examined in patients with HIV infection and CD4⩽100 cells/μl. In our study, a significant proportion of the CrAg-ELISA-seropositive patients were suspected to be false positives and were subsequently negative by LIPS testing. False positivity and diverse test performance have previously been seen with crude antigen ELISA serology tests in immunosuppressed patients with haematological malignancies and in AIDS patients co-infected with other pathogens such as Leishmania.23,33 LIPS testing on the other hand detected at baseline fewer positive patients from endemic areas and missed one patient who was stool positive. HIV-induced B-cell hyperactivity is associated with inappropriate antibody production in untreated HIV patients34 and severe CD4+ T-cell depletion is associated with both impaired humoral immunity and loss of serological memory,35 which could account for discordant serological testing in patients with AIDS and severe lymphopenia that render the available tests less reliable. To fully characterize and compare the sensitivity and specificity of these tests in a CD4 ⩽100 cells/μl AIDS population, future studies comparing serological testing in a group of parasitologically proven Strongyloides cases and a group of bona fide negatives would be required.
Based on the potential high-prevalence, the non-specific symptomatology, the discordance of Strongyloides serology results in severely immunosuppressed HIV+ patients and the insensitivity of stool testing, empiric treatment for Ss may be justified in patients with AIDS originating from Ss-endemic areas. This could negate the need for serological testing that can be costly, time consuming, and suboptimal in AIDS patients. History of travel to an endemic area in conjunction with eosinophila should also prompt suspicion of Ss infection. Our data thus suggest that empiric treatment with ivermectin, which has a low side effect profile, may be the optimal approach in patients with AIDS originating from Ss-endemic areas, particularly in those receiving corticosteroid therapy, to help mitigate the risk of dissemination.
Acknowledgments
The authors thank the study participants at the National Institutes of Health and the staff of the Outpatient Clinic 8 at NIAID.
References
- 1.Genta RM. Global prevalence of strongyloidiasis: critical review with epidemiologic insights into the prevention of disseminated disease. Rev Infect Dis. 1989;11(5):755–67. doi: 10.1093/clinids/11.5.755. [DOI] [PubMed] [Google Scholar]
- 2.Walzer PD, Milder JE, Banwell JG, Kilgore G, Klein M, Parker R, et al. Epidemiologic features of Strongyloides stercoralis infection in an endemic area of the United States. Am J Trop Med Hyg. 1982;31(2):313–9. doi: 10.4269/ajtmh.1982.31.313. [DOI] [PubMed] [Google Scholar]
- 3.Berk SL, Verghese A, Alvarez S, Hall K, Smith B. Clinical and epidemiologic features of strongyloidiasis. A prospective study in rural Tennessee. Arch Intern Med. 1987;147(7):1257–61. [PubMed] [Google Scholar]
- 4.Loutfy MR, Wilson M, Keystone JS, Kain KC. Serology and eosinophil count in the diagnosis and management of strongyloidiasis in a non-endemic area. Am J Trop Med Hyg. 2002;66(6):749–52. doi: 10.4269/ajtmh.2002.66.749. [DOI] [PubMed] [Google Scholar]
- 5.Nuesch R, Zimmerli L, Stockli R, Gyr N, Christoph Hatz FR. Imported strongyloidosis: a longitudinal analysis of 31 cases. J Travel Med. 2005;12(2):80–4. doi: 10.2310/7060.2005.12204. [DOI] [PubMed] [Google Scholar]
- 6.Posey DL, Blackburn BG, Weinberg M, Flagg EW, Ortega L, Wilson M, et al. High prevalence and presumptive treatment of schistosomiasis and strongyloidiasis among African refugees. Clin Infect Dis. 2007;45(10):1310–5. doi: 10.1086/522529. [DOI] [PubMed] [Google Scholar]
- 7.Hauber HP, Galle J, Chiodini PL, Rupp J, Birke R, Vollmer E, et al. Fatal outcome of a hyperinfection syndrome despite successful eradication of Strongyloides with subcutaneous ivermectin. Infection. 2005;33(5–6):383–6. doi: 10.1007/s15010-005-5060-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keiser PB, Nutman TB. Strongyloides stercoralis in the immunocompromised population. Clin Microbiol Rev. 2004;17(1):208–17. doi: 10.1128/CMR.17.1.208-217.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Segarra-Newnham M. Manifestations, diagnosis, and treatment of Strongyloides stercoralis infection. Ann Pharmacother. 2007;41(12):1992–2001. doi: 10.1345/aph.1K302. [DOI] [PubMed] [Google Scholar]
- 10.Croker C, Reporter R, Redelings M, Mascola L. Strongyloidiasis-related deaths in the United States, 1991–2006. Am J Trop Med Hyg. 2010;83(2):422–6. doi: 10.4269/ajtmh.2010.09-0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benvenuti F, Carrascosa JM, Boada A, Ferrandiz C. Disseminated Strongyloides stercoralis infection with a cutaneous presentation in an immunosuppressed patient. Eur J Dermatol. 2009;19(4):404–5. doi: 10.1684/ejd.2009.0701. [DOI] [PubMed] [Google Scholar]
- 12.Viney ME, Brown M, Omoding NE, Bailey JW, Gardner MP, Roberts E, et al. Why does HIV infection not lead to disseminated strongyloidiasis? J Infect Dis. 2004;190(12):2175–80. doi: 10.1086/425935. [DOI] [PubMed] [Google Scholar]
- 13.Ramanathan R, Nutman T. Strongyloides stercoralis infection in the immunocompromised host. Curr Infect Dis Rep. 2008;10(2):105–10. doi: 10.1007/s11908-008-0019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hochberg NS, Moro RN, Sheth AN, Montgomery SP, Steurer F, McAuliffe IT, et al. High prevalence of persistent parasitic infections in foreign-born, HIV-infected persons in the United States. PLoS Negl Trop Dis. 2011;5(4):e1034. doi: 10.1371/journal.pntd.0001034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karp CL, Auwaerter PG. Coinfection with HIV and tropical infectious diseases. II. Helminthic, fungal, bacterial, and viral pathogens. Clin Infect Dis. 2007;45(9):1214–20. doi: 10.1086/522180. [DOI] [PubMed] [Google Scholar]
- 16.Haddow LJ, Mahlakwane MS, Ramdial PK, Moosa MY. Histopathology of Strongyloides stercoralis hyperinfection during immune reconstitution in an HIV-infected patient. AIDS. 2009;23(12):1609–11. doi: 10.1097/QAD.0b013e32832c41f4. [DOI] [PubMed] [Google Scholar]
- 17.Brown M, Cartledge JD, Miller RF. Dissemination of Strongyloides stercoralis as an immune restoration phenomenon in an HIV-1-infected man on antiretroviral therapy. Int J STD AIDS. 2006;17(8):560–1. doi: 10.1258/095646206778145712. [DOI] [PubMed] [Google Scholar]
- 18.Kim AC, Lupatkin HC. Strongyloides stercoralis infection as a manifestation of immune restoration syndrome. Clin Infect Dis. 2004;39(3):439–40. doi: 10.1086/422522. [DOI] [PubMed] [Google Scholar]
- 19.Lanzafame M, Faggian F, Lattuada E, Antolini D, Vento S. Strongyloidiasis in an HIV-1-infected patient after highly active antiretroviral therapy-induced immune restoration. J Infect Dis. 2005;191(6):1027. doi: 10.1086/428099. [DOI] [PubMed] [Google Scholar]
- 20.Lawn SD, Wilkinson RJ. Immune reconstitution disease associated with parasitic infections following antiretroviral treatment. Parasite Immunol. 2006;28(11):625–33. doi: 10.1111/j.1365-3024.2006.00900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dreyer G, Fernandes-Silva E, Alves S, Rocha A, Albuquerque R, Addiss D. Patterns of detection of Strongyloides stercoralis in stool specimens: implications for diagnosis and clinical trials. J Clin Microbiol. 1996;34(10):2569–71. doi: 10.1128/jcm.34.10.2569-2571.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sato Y, Kobayashi J, Toma H, Shiroma Y. Efficacy of stool examination for detection of Strongyloides infection. Am J Trop Med Hyg. 1995;53(3):248–50. doi: 10.4269/ajtmh.1995.53.248. [DOI] [PubMed] [Google Scholar]
- 23.Schaffel R, Nucci M, Carvalho E, Braga M, Almeida L, Portugal R, et al. The value of an immunoenzymatic test (enzyme-linked immunosorbent assay) for the diagnosis of strongyloidiasis in patients immunosuppressed by hematologic malignancies. Am J Trop Med Hyg. 2001;65(4):346–50. doi: 10.4269/ajtmh.2001.65.346. [DOI] [PubMed] [Google Scholar]
- 24.McAuliffe I, Handali S, Lee Y-M, Noh J, Gleim E and Wilkins P. Optimization of an ELISA assay for the detection of S. stercoralis infection in humans. In: Proceedings of the 57th Annual Meeting of the American Society of Tropical Medicine and Hygiene 2008; 2008 Dec 7–11; New Orleans, LA, USA. Washington (DC): ASTMH; 2008. Abstract 532. [Google Scholar]
- 25.Krolewiecki AJ, Ramanathan R, Fink V, McAuliffe I, Cajal SP, Won K, et al. Improved diagnosis of Strongyloides stercoralis using recombinant antigen-based serologies in a community-wide study in northern Argentina. Clin Vaccine Immunol. 2010;17(10):1624–30. doi: 10.1128/CVI.00259-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ravi V, Ramachandran S, Thompson RW, Andersen JF, Neva FA. Characterization of a recombinant immunodiagnostic antigen (NIE) from Strongyloides stercoralis L3-stage larvae. Mol Biochem Parasitol. 2002;125(1–2):73–81. doi: 10.1016/s0166-6851(02)00214-1. [DOI] [PubMed] [Google Scholar]
- 27.Ritchie LS. An ether sedimentation technique for routine stool examinations. Bull U S Army Med Dep. 1948;8(4):326. [PubMed] [Google Scholar]
- 28.Perry JL, Matthews JS, Miller GR. Parasite detection efficiencies of five stool concentration systems. J Clin Microbiol. 1990;28(6):1094–7. doi: 10.1128/jcm.28.6.1094-1097.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arch EL, Schaefer JT, Dahiya A. Cutaneous manifestation of disseminated strongyloidiasis in a patient coinfected with HTLV-I. Dermatol Online J. 2008;14(12):6. [PubMed] [Google Scholar]
- 30.Kalb RE, Grossman ME. Periumbilical purpura in disseminated strongyloidiasis. JAMA. 1986;256(9):1170–1. [PubMed] [Google Scholar]
- 31.Ramanathan R, Burbelo PD, Groot S, Iadarola MJ, Neva FA, Nutman TB. A luciferase immunoprecipitation systems assay enhances the sensitivity and specificity of diagnosis of Strongyloides stercoralis infection. J Infect Dis. 2008;198(3):444–51. doi: 10.1086/589718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burbelo PD, Ching KH, Mattson TL, Esper F, Iadarola MJ, Delwart E, et al. Rapid antibody quantification and generation of whole proteome antibody response profiles using LIPS (luciferase immunoprecipitation systems). Biochem Biophys Res Commun. 2007;352(4):889–95. doi: 10.1016/j.bbrc.2006.11.140. [DOI] [PubMed] [Google Scholar]
- 33.Deniau M, Canavate C, Faraut-Gambarelli F, Marty P. The biological diagnosis of leishmaniasis in HIV-infected patients. Ann Trop Med Parasitol. 2003;97(Suppl 1):115–33. doi: 10.1179/000349803225002598. [DOI] [PubMed] [Google Scholar]
- 34.Moir S, Fauci AS. B cells in HIV infection and disease. Nat Rev Immunol. 2009;9(4):235–45. doi: 10.1038/nri2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cagigi A, Nilsson A, Pensieroso S, Chiodi F. Dysfunctional B-cell responses during HIV-1 infection: implication for influenza vaccination and highly active antiretroviral therapy. Lancet Infect Dis. 2010;10(7):499–503. doi: 10.1016/S1473-3099(10)70117-1. [DOI] [PubMed] [Google Scholar]


