Abstract
Objectives
Monitoring acute bacterial meningitis in northern Cameroon.
Methods
Health professionals collected cerebrospinal fluid (CSF) specimens from patients presenting with clinical symptoms of meningitis. Specimens were tested using gram stain, latex agglutination test, and culture. A PCR assay completed the diagnostic testing. Multilocus sequence typing (MLST) was performed on some Neisseria meningitidis (Nm) isolates.
Results
From 2007 through 2010, of the 1429 CSF specimens tested, 292 (20.4%) were positive, either for Nm (205), Streptococcus pneumoniae (Sp) (57), or Haemophilus influenzae (Hi) (30). From 2007 through 2009, the serogroup W135 represented 98.8% of 164 case isolates. Until 2008, most serogroup W135 isolates presented the sequence-type ST-2881 usually associated with sporadic cases. Since 2009, the ST-11 (an epidemic-associated clone) became predominant, although no epidemic occurred. Serogroup A ST-7 was observed in 2010 and caused a localized epidemic. Using the detection PCR on turbid CSF, a 2.7-fold increase in cases with etiologic diagnosis was obtained, compared to culture. All tested meningococcal isolates (42) were susceptible to ampicillin, chloramphenicol, and cefotaxim.
Conclusions
Resurgence of serogroup A and recent increase in ST-11 among serogroup W135 isolates were worrying when considered with the epidemic wave of serogroup A meningitis, which affected neighboring countries and the serogroup W135 epidemic in Niger in 2009–2010.
Keywords: Bacterial meningitis, Cerebrospinal fluid, PCR assay, Northern Cameroon, Neisseria meningitidis, Streptococcus pneumoniae, Haemophilus influenzae
Background
Northern Cameroon, an area of 163 500 km2 between the Adamawa plateau and the Lake Chad, comprises three regions: Far North, North, and Adamawa. The first two regions are located in the African meningitis belt and periodically experience meningitis epidemics during the dry season (January–May). The Adamawa region seems to be less affected by epidemics, probably due to its higher altitude, lower latitude, and lower population density. In the Sahelian zone, the epidemics occur at intervals of 8–12 years and mostly affect people aged less than 20 years.1 Classically, they were due to Neisseria meningitidis (Nm) serogroup A (NmA). The last large epidemic occurred in 1992 (8046 cases and 968 deaths, Ministry of Health, unpublished data).
Scant microbiological data on etiological agents of acute bacterial meningitis (ABM) in northern Cameroon before 2007 are available. Pre-2007, only a few CSF specimens were tested in the laboratory and therefore meningococcal meningitis cases were presumed to be NmA, as it was predominant in most regions of the meningitis belt.
The 2002 epidemic of serogroup W135 in Burkina Faso2 led WHO to recommend that enhanced surveillance of meningitis be implemented in all countries in the meningitis belt, in order to improve the detection and response to epidemics. In 2007, the French Ministry of Foreign Affairs initiated a program to improve patient diagnosis and strengthen surveillance – in six countries affected by epidemic meningococcal meningitis, Niger, Mali, Burkina Faso, Ivory Coast, Cameroun, and Central African Republic – including confirmation of bacterial meningitis pathogens by introducing the use of PCR. The results obtained in northern Cameroon during the period 2007–2010 are reported in this paper. This monitoring will measure the effect of the monovalent meningococcal A conjugate vaccine (MenAfricaVac), which will be introduced in Cameroon at the end of 2011 in the North and Far-North regions and at the end of 2012 in the Adamawa region. All children aged less than 1 year will be vaccinated with the new vaccine. The plain vaccine will no longer be used.
Methods
Epidemiological surveillance
The three northern Cameroon regions (Far North, North, and Adamawa) in this analysis had a total (estimated in 2009) population of 6 274 116 inhabitants, 54.7% of which resides in the Far North region, 29.7% in the North region, and 15.6% in the Adamawa region. The public health system in Cameroon is organized into health areas, serving approximately 10 000 inhabitants each, under the responsibility of a nurse. Each district, managed by a doctor, includes approximately 10 health areas and reports to the region.
Throughout the analysis period between January 2007 and December 2010, counts of meningitis cases and deaths were reported to the Cameroon National Service of Epidemiology. According to the surveillance standard operating procedure, health agents were supposed to complete and submit a weekly report with the age, gender, and disease outcome. Cases were reported on the basis of clinical presentation, combining sudden onset of fever and stiff neck. Laboratory confirmation of meningitis was not required for reporting. Doctors as well as nurses can perform spinal tap, and laboratory testing of CSF specimens was free of charge. The technical skills are heterogeneous between physicians and nurses, and between nurses who usually manage alone the most part of meningitis cases in basic health facilities. Compliance with recommended clinical definition was never assessed. However, meetings were held periodically with health agents involved in the microbiologic surveillance, in order to share the results and to consolidate the network.
Microbiological surveillance
The microbiological surveillance that had been abandoned for several years was reintroduced in 2007, spurred on by the Centre Pasteur of Cameroon-Annexe Garoua (CPC-AG) team with the approval of health region directors. Physicians from districts and nurses from health areas permitted to perform lumbar punctures were invited to collect CSF specimen from patients presenting with a clinical picture meeting the definition of acute meningitis, and to send these samples to the CPC-AG as soon as possible. The samples were not transported on Trans Isolate (TI) media. When clinical specimens could not reach the laboratory within a satisfactory time, they were kept frozen until dispatch. A short epidemiological and clinical questionnaire was completed and accompanied each sample. No incentive was provided for submitting spinal fluid samples.
According to the current surveillance procedures in Cameroon, reporting of suspected meningitis cases consists of weekly numbers of cases and deaths, so it was not possible to match a given CSF specimen received for laboratory testing to one of the notified cases. It is likely that some laboratory-confirmed cases were not notified and it is certain that many notified cases did not undergo laboratory testing.
In northern Cameroon, the CPC-AG is the only laboratory having the capability of carrying out a complete analysis of CSF specimens using classical bacteriological methods (Gram stain, latex agglutination test, and culture). When a pathogen agent was identified in a CSF, the case was a ‘confirmed case’. When no pathogen agent was identified in a turbid CSF, the case was a ‘probable case’. Antibiotic susceptibility testing was performed according to the recommendations of the French Society for Microbiology.
Some CSF specimens, whose volumes were sufficient to perform the test, were also tested at CPC-AG using a multiplex PCR assay for the three main causative agents of ABM: Nm,3 Streptococcus pneumoniae (Sp),4 and Haemophilus influenzae (Hi).5 A second PCR was performed on specimens found positive for Nm in order to identify NmA, B, C, X, and Y/W135. In addition, isolates of Y/W135 were differentiated to Y or W135 by a confirmative PCR. Specimens positive for Hi were further tested to confirm type b.
Each year, Nm isolates collected were sent to the WHO Collaborating Centre for Reference and Research on Meningococci (CCRRM) in Marseille (France), or, from 2010, to the WHO CCRRM in Oslo (Norway) for serogroup confirmation, serotyping, and multilocus sequence typing (MLST).6 Multilocus sequence typing directly identifies alleles from the nucleotide sequences of the internal fragments of seven housekeeping genes and can characterize each strain by its sequence type (ST).
Ethical aspects
Collection and laboratory testing of CSF specimens were part of the routine clinical management of patients suspected of having meningitis. Thus informed patient consent or approval from the Cameroon national ethics committee was not required.
Results
Between January 2007 and December 2010, the numbers of suspected meningitis cases reported yearly remained consistent and were of the same order of magnitude as those reported since 2002: approximately 200–1000 cases per year (74 cases for 100 000 inhabitants on average). Cameroon did not experience any large outbreaks during the studied period.
Throughout the study period, 1429 CSF specimens were sent to the CPC-AG, of which 1143 CSF (80%) were from the North, 236 (16%) were from the Far North, and 50 (4%) were from the Adamawa region. Forty-seven health facilities or hospitals in 36 districts were involved in the microbiological surveillance.
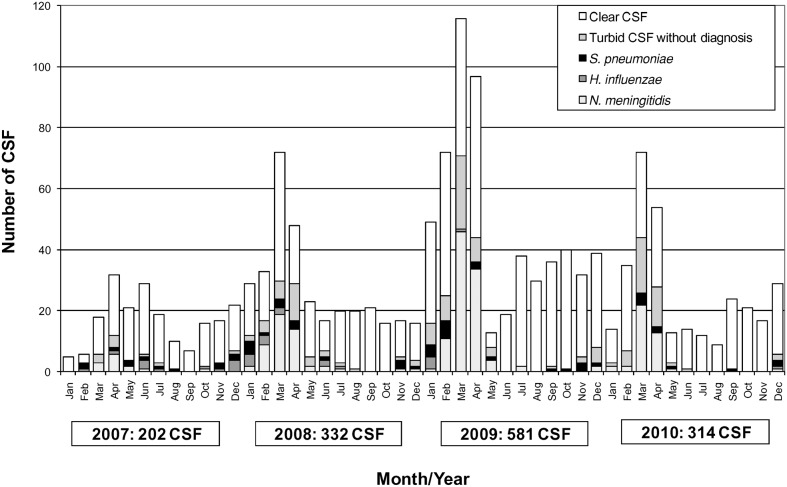
Overall, 70% of the CSF specimens were clear and had ≤5 white blood cells (WBC) (Table 1). All 1429 CSF specimens were cultured unless they had been kept frozen or refrigerated. The monthly distribution of results obtained from the analyses of the CSF specimens is presented in Fig. 1. The distribution of cases by province can be seen in Fig. 2.
Table 1. Cerebrospinal fluid specimens from suspected cases of meningitis received yearly at the CPC-AG distributed according to final result (January 2007–December 2010).
| Year | |||||
| Laboratory result | 2007 | 2008 | 2009 | 2010 | Total |
| Clear CSF | 155 | 219 | 399 | 221 | 994 |
| Turbid CSF without diagnosis | 10 | 34 | 58 | 41 | 143 |
| Etiological agent identified | 37 | 79 | 124 | 52 | 292 |
| Total | 202 | 332 | 581 | 314 | 1429 |
Figure 1.
Monthly distribution of cerebrospinal fluid (CSF) specimens tested at Centre Pasteur laboratory in Garoua and results, January 2007–December 2010. Red bars: Neisseria Meningitidis A (NmA); blue bars: Neisseria meningitidis (Nm) W135; yellow bars: Haemophilus Influenzae (Hi); green bars: Streptococcus Pneumoniae (Sp); black bars: turbid CSF without diagnosis; white bars: clear CSF without diagnosis.
Figure 2.
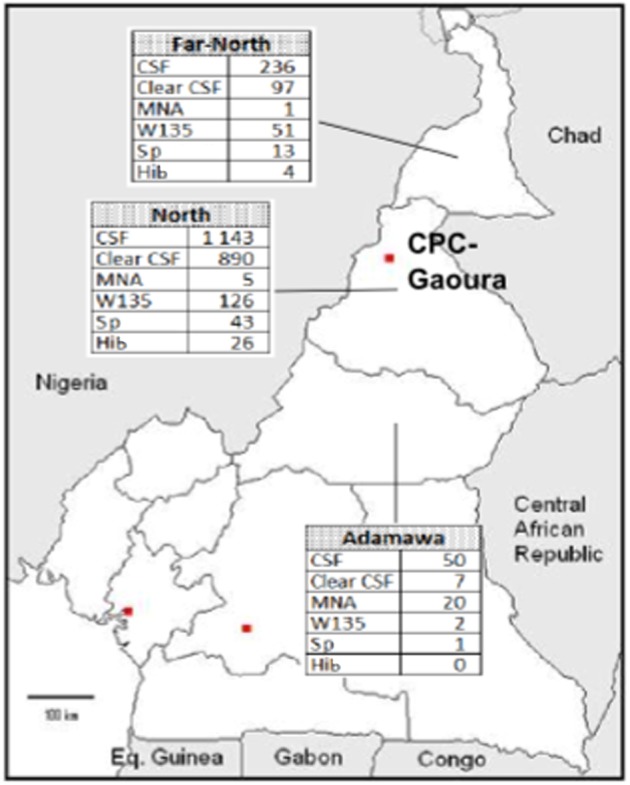
Map of Cameroon with results of cerebrospinal fluid examined from 2007 to 2010.
Overall, 69.5% of CSF specimens were clear and had ≤5 WBC. All received CSFs were cultured, unless they had been kept frozen.
Most confirmed cases of ABM occurred during the dry season, between January and May. An etiology was identified for 292 cases (20.4%), with Nm, Sp, and Hi representing, respectively, 70.2%, 19.5%, and 10.3% of all identifications. Of 1137 CSF specimens testing negative for Nm, Sp, and Hi, 143 (12.6%) had a WBC count >5 and 994 (87.4%) had a WBC count ≤5. Overall, during the 4 years, the serogroup W135 represented 86.3% of 205 confirmed Nm cases. The serogroup W135 was the only serogroup identified in 2007 (13 positive CSF) and 2008 (51 positive CSF). In 2010, serogroup A represented 24 of 41 confirmed Nm cases (58.5%) and 17 of 41 NmW135 cases (41.5%) (Table 2). NmW135 cases were observed in many places in the North and Far North regions. NmA cases were identified in Ngaoundere, the main city of the Adamawa region, where a localized outbreak of meningitis was reported in February and March 2010.7 According to the 2009 estimated population, the yearly incidence of confirmed Nm meningitis cases varied between 0 per 100 000 (Adamaoua 2007) and 2.7 per 100 000 (North region 2009), and the yearly incidence of probable cases reached 3.9 in North region in 2009. Sp meningitis cases were observed as sporadic cases, but a cluster of five confirmed cases was observed in Ngong (North region) in 2008. A case of Hi meningitis was confirmed in December 2010, but none have been observed since March 2009.
Table 2. Distribution of the Neisseria Meningitidis (Nm) serogroups identified in the cerebrospinal fluid specimens received at the CPC-AG between January 2007 and December 2010.
| Year | |||||
| Serogroup | 2007 | 2008 | 2009 | 2010 | Total |
| NmA | 0 | 0 | 2 | 24 | 26 |
| NmW135 | 13 | 51 | 98 | 17 | 179 |
| Total Nm | 13 | 51 | 100 | 41 | 205 |
Only 135 CSF specimens were sent with a valid questionnaire form. The male to female ratio among the suspected cases was 1.5:1 and 86% of patients had fever over 38°C. The median ages were 10, 12, and 1.2 years, respectively, for 49 Nm, 13 Sp, and four Hi meningitis patients. Information on disease outcome was rare and could not be used.
A comparison of PCR and culture was carried out on a sample of 150 turbid CSF specimens. While 21 tested positive for Nm, Sp, or Hi using culture, 54 more specimens were positive using PCR. Overall, 2 strains of NmA, 40 of NmW135, 14 of Hi, and 18 of Sp were obtained from culture and were tested for susceptibility to antibiotics commonly used in ABM. All Nm isolates were susceptible to ampicillin, chloramphenicol, and cefotaxim. Nine of 14 (64.3%) Hi isolates had a reduced susceptibility to ampicillin, four (28.6%) had a reduced susceptibility to cefotaxim, and eight (57.1%) a reduced susceptibility to chloramphenicol. The 18 Sp isolates were susceptible to cefotaxim and to chloramphenicol, but all had a reduced susceptibility to gentamycin. Reduced susceptibility to penicillin of Sp isolates could not be studied at the CPC-AG.
Multilocus sequence typing investigation of 12 NmW135 isolates from the 2008 season determined that 10 (83.3%) were ST-2881 and two (16.7%) were ST-11. Conversely, of 15 NmW135 isolates obtained in 2009, 12 (80%) were ST-11 and three (20%) were ST-2881. The two NmW135 isolates from the 2010 season were also ST-11. The two NmA isolates (one from 2009 and one from 2010) were both ST-7.
Discussion
In 2007, a microbiological surveillance of ABM was reintroduced in northern Cameroon, a vast territory located within the African meningitis belt. From 1995 through 2006, 10–60 CSF specimens were tested each year at CPC-AG, all collected at Garoua hospital. Since 2007, specimens were sent from many distant health facilities and their number increased markedly. Free laboratory testing along with scientific interest probably accounted for a large part of health agents’ adherence. Unfortunately, the epidemiological report forms were poorly completed.
As usual in areas in the meningitis belt, Nm was the most frequently identified cause of ABM, but only 20.4% of CSF enabled confirmation of a bacterial etiology. With 69.5% of all received CSF specimens being clear and testing negative for Nm, Sp, and Hi, one must keep in mind that cases of bacterial meningitis that are reported can greatly overestimate the true number of ABM cases. Health staff must be made aware of the importance of adherence to the clinical case definition, in order to lessen the number of patients wrongly diagnosed with meningitis while they may have diseases requiring specific treatment. Since the reintroduction of the microbiological surveillance in northern Cameroon, the proportion of probable cases and confirmed cases among notified meningitis cases has increased due to a drop in notification of clinically suspected cases with negative CSF. The absence of confirmation of Hi meningitis cases during 20 months after the introduction of Hi type b immunization in Cameroon was reported.8 Streptococcus pneumoniae is still a frequent cause of meningitis. Conjugate vaccines against Sp infections are rarely used in Cameroon and making them available would represent an important advance in meningitis control.
In northern Cameroon, the last large meningococcal epidemic dates back to 1992 and, more than 15 years later, the risk of new epidemic must be considered seriously because of the decrease in naturally acquired immunity. In March 2007, WHO experts warned about the likelihood of a new meningitis epidemic wave in Africa following several years of low incidence and considering the increasing spread of a new strain of NmA belonging to ST-2859.9 In fact, a large epidemic of meningococcal meningitis, the largest since 1996, struck Niger, Nigeria, and Chad in 2009. It was mainly due to NmA, with some NmW135 cases in Chad.10 Although northern Cameroon has long common borders with Nigeria and Chad and cross-border population movements are important, Cameroon has so far been spared. However, the localized NmA meningitis outbreak observed in early 2010 in Ngaoundere may herald an impending epidemic of greater importance.
The exact emergence of serogroup W135 in northern Cameroon is difficult to identify. A hospital-based survey in Garoua (North region) showed that in 1993, of 77 Nm isolates recovered from CSF specimens, 28 were serogroup A, one was serogroup C, and 48 could not be serogrouped using anti-A, anti-B, and anti-C antisera.11 We cannot rule out the hypothesis that a number of the isolates belonged to serogroup W135. An increase of meningitis cases due to NmW135 was observed in 1999–2000 in Yaounde, in the southern part of Cameroon,12 an area characterized by a sub-equatorial Guinean climate, with 1600 mm rainfall and a mean annual temperature of 25°C, conditions that are different from those of the meningitis belt. Serogroup W135 was a great cause of concern in Africa in 2002–2003, and then appeared to recede.13 Yet, it was the cause of a new large epidemic in Niger in 2010.14 According to MLST investigation, most isolates recovered in 2008 in northern Cameroon were ST-2881,15 a ST that was first identified in 2002 and that was only recently associated with invasive, not epidemic, meningococcal disease.16 On the other hand, in 2009 and 2010, the ST-11 became the most frequently confirmed. ST-11 was involved in the epidemic in Burkina Faso in 2002–200313 and in Niger in 2010.14 The two recent observations made in Cameroon, the return of NmA and the predominance of ST-11 among NmW135 isolates, are both worrying in the context of the recent epidemic wave due to NmA in neighboring countries and of the NmW135 epidemic in Niger.
As a consequence of the surveillance, recommendations on anti-meningococcal immunization in northern Cameroon were updated and tetravalent meningococcal A/C/W15/Y is now more frequently requested by private individuals or by companies implementing staff immunization. However, due to the high price, the number of administered doses of tetravalent vaccine for preventive immunization remains low.
The absence of NmA for several years is unexplained. It cannot be related to immunization coverage with the bivalent A+C polysaccharide vaccine because it was not used in mass campaigns for years and routine use (30 000–40 000 doses of plain vaccine per year for approximately six million) was not important enough.
Following the recent extensive use of MenAfriVac vaccine in West-Africa since 2011 and in Cameroon since 2012, one can speculate which serogroup (if any) will replace serogroup A as a cause of epidemics. Serogroup W135, widely distributed, may be the most probable candidate. Comprehensive and improved surveillance of ABM will remain necessary to ascertain the efficacy of the new vaccine and to closely monitor the changing epidemiology of Nm.
The NmA epidemic that occurred in the city of Ngaoundere in 2010 was unusual.7 Climatic and environmental conditions in this area do not correspond to those classically characterizing the African meningitis belt. A previous unusual epidemic, attributed to NmA, had been observed in southwest Cameroon in 2000.17 In countries like Cameroon with a north–south climate gradient, the classical geographic definition of the meningitis belt should not be interpreted to the letter. That should prompt health authorities to implement a close monitoring of Nm that is not restricted to areas of the northern part of the country.
Similar to Niger and Burkina Faso, the multiplex PCR proved valuable for the improved microbiologic surveillance of meningitis due to Nm, Sp, and Hi.18,19 This was particularly true when CSF specimens were collected in remote areas and could not be delivered to the laboratory within a few hours of collection, because the probability of positive culture decreases rapidly due to the fragility of the three bacterial pathogens. In the context of surveillance in Cameroon when proper transport media is unavailable, the PCR enabled a 2.7-fold increase in the confirmation rate, particularly with CSF collected in distant health facilities. Most often, the results of PCR was not timely for supporting case management, but in the current context of limited laboratory capability, the PCR represents a very suitable tool for extending surveillance coverage.
Conclusions
The recently reintroduced microbiological surveillance of ABM in northern Cameroon obtained interesting results. This work was supported by a grant that will end in December 2011 and the long-term funding of the system is not yet established.
The factors driving specific Nm serogroup spread are unknown and the epidemiology of Nm remains largely unpredictable.
Competing Interests
The authors declare that they have no competing interests.
Authors’ Contributions
DM implemented and coordinated the network, supervised laboratory diagnosis, and contributed to draft the manuscript. JB, FA, and CE coordinated field work. BG was involved in laboratory testing. PB participated in the design of the study and contributed to draft the manuscript. All authors read and approved the final manuscript.
Acknowledgments
Authors are indebted to the doctors and nurses who have sent CSF specimens and epidemiological forms to the CPC-AG.
They gratefully acknowledge P Nicolas (WHO CCRRM, IMTSSA-Le Pharo, Marseille, France) and D Caugant (WHOCCRR, Norwegian Institute of Public Health, Oslo, Norway).
Funding was provided by the French Ministry of Foreign Affairs (FSP 2005-14).
References
- 1.Lapeyssonnie L. La méningite cérébro-spinale en Afrique. Bull World Health Org Suppl. 1963;28:3–114. [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Meningococcal disease, serogroup W135, Burkina Faso: preliminary report, 2002. Wkly Epidemiol Rec. 2002;77:152–5. [PubMed] [Google Scholar]
- 3.Taha MK. Simultaneous approach for nonculture PCR-based identification and serogroup prediction of Neisseria meningitidis. J Clin Microbiol. 2000;38:855–7. doi: 10.1128/jcm.38.2.855-857.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garcia P, Garcia JL, Garcia E, Lopez R. Nucleotide sequence and expression of the pneumococcal autolysin gene from its own promoter in Escherichia coli. Gene. 1986;43:265–72. doi: 10.1016/0378-1119(86)90215-5. [DOI] [PubMed] [Google Scholar]
- 5.Falla TJ, Crook DW, Brophy LN, Maskell D, Kroll JS, Moxon ER. PCR for capsular typing of Haemophilus influenzae. J Clin Microbiol. 1994;32:2382–6. doi: 10.1128/jcm.32.10.2382-2386.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maiden MC, Bygraves JA, Feil E, Morelli G, Russell JE, Urwin R, et al. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc Natl Acad Sci USA. 1998;95:3140–5. doi: 10.1073/pnas.95.6.3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Massenet D, Vohod D, Hamadicko H, Caugant DA. Epidemic meningococcal meningitis, Cameroon. Emerg Infect Dis. 2011;17:2070–2. doi: 10.3201/eid1711.110468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Massenet D, Tapindjin-Gake M. Positive effect of the introduction of Haemophilus influenzae type b vaccination in the expanded program on immunization in Cameroon. Vaccine. 2010;28:6404–5. doi: 10.1016/j.vaccine.2010.07.033. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization. Risk of epidemic meningitis in Africa: a cause for concern. Wkly Epidemiol Rec. 2007;82:79–85. [PubMed] [Google Scholar]
- 10.World Health Organization. Meningitis in Chad, Niger and Nigeria: 2009 epidemic season. Wkly Epidemiol Rec. 2010;85:57–63. [PubMed] [Google Scholar]
- 11.Sile Mefo H, Sile H, Mbonda E, Fezeu R, Fonkoua M. Les méningites purulentes de l’enfant au nord Cameroun: aspects épidémiologiques, diagnostiques et évolutifs. Med Afr Noire. 1999;46:83–8. [Google Scholar]
- 12.Fonkoua MC, Taha MK, Nicolas P, Cunin P, Alonso JM, Bercion R, et al. Recent increase in meningitis caused by Neisseria meningitidis serogroups A and W135, Yaounde, Cameroon. Emerg Infect Dis. 2002;8:327–9. doi: 10.3201/eid0803.010308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Traore Y, Njanpop-Lafourcade BM, Adjogble KL, Lourd M, Yaro S, Nacro B, et al. The rise and fall of epidemic Neisseria meningitidis serogroup W135 meningitis in Burkina Faso, 2002–2005. Clin Infect Dis. 2006;43:817–22. doi: 10.1086/507339. [DOI] [PubMed] [Google Scholar]
- 14.Collard JM, Maman Z, Yacouba H, Djibo S, Nicolas P, Jusot JF, et al. Increase in Neisseria meningitidis serogroup W135, Niger, 2010. Emerg Infect Dis. 2010;16:1496–8. doi: 10.3201/eid1609.100510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Massenet D, Inrombe J, Mevoula DE, Nicolas P. Serogroup W135 meningococcal meningitis, northern Cameroon, 2007–2008. Emerg Infect Dis. 2009;15:340–2. doi: 10.3201/eid1502.080988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nicolas P, Djibo S, Moussa A, Tenebray B, Boisier P, Chanteau S. Molecular epidemiology of meningococci isolated in Niger in 2003 shows serogroup A sequence type (ST)-7 and serogroup W135 ST-11 or ST-2881 strains. J Clin Microbiol. 2005;43:1437–8. doi: 10.1128/JCM.43.3.1437-1438.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cunin P, Fonkoua MC, Kollo B, Bedifeh BA, Bayanak P, Martin PM. Serogroup A Neisseria meningitidis outside meningitis belt in southwest Cameroon. Emerg Infect Dis. 2003;9:1351–3. doi: 10.3201/eid0910.030170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sidikou F, Djibo S, Taha MK, Alonso JM, Djibo A, Kairo KK, et al. Polymerase chain reaction assay and bacterial meningitis surveillance in remote areas, Niger. Emerg Infect Dis. 2003;9:1486–8. doi: 10.3201/eid0911.030462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parent du Chatelet I, Traore Y, Gessner BD Antignac A, Naccro B, Njanpop-Lafourcade BM, et al. Bacterial meningitis in Burkina Faso: surveillance using field-based polymerase chain reaction testing. Clin Infect Dis. 2005;40:17–25. doi: 10.1086/426436. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization. Detecting meningococcal meningitis epidemics in highly-endemic African countries. WHO recommendation. Wkly Epidemiol Rec. 2000;75:306–9. [PubMed] [Google Scholar]