Abstract
Plasma levels of pro- and anti-inflammatory cytokines of Plasmodium falciparum-infected patients with severe malaria (SM; n = 62) and uncomplicated malaria (UM; n = 69) from Sri Lanka were assessed. SM patients had significantly higher levels of TNF-alpha (P<0.01), IL-6 (P<0.01), and IL-10 (P<0.05) compared to the UM patients. Plasma IL-2 levels of these patients were undetectable. TNF-alpha levels of a third group of patients with uncomplicated P. falciparum malaria, who were recruited during their fever episodes (UMF; n = 14) were significantly higher than those of the UM patients (P<0.001) and comparable to SM patients. Plasma IFN-gamma levels of SM patients were higher compared to UM patients, but was not statistically significant. Body temperature in both SM and UMF groups were significantly higher compared to UM group, whereas percentages of parasitemia in all three groups were comparable. Analysis of plasma TNF-alpha levels and the ratio of TNF-alpha/IL-10 in UM (n = 34) and SM (n = 34) patients carrying TNF1 and TNF2 allelic types showed that SM patients carrying TNF2 had significantly higher TNF-alpha levels as well as TNF-alpha/IL-10 ratio compared to UM patients carrying TNF1, UM patients carrying TNF2 and SM patients carrying TNF1 (P<0.05). These results suggest that the high circulating TNF-alpha levels and the inadequate IL-10 response in the SM patients carrying TNF2 allele could have contributed to the development of severe falciparum malarial disease.
Keywords: P. falciparum, Tumor necrosis factor-alpha, Interleukin-10, Severe malaria, Sri Lanka
Introduction
Plasmodium falciparum malaria is a major cause of morbidity and responsible for over one million deaths annually among children under 5 years of age in sub-Saharan Africa.1 The spectrum of disease prevalent differs in geographical areas; cerebral malaria and severe anemia is more common in Africa2 and multiple organ dysfunction is reported to be more prevalent in Asia.3 Both host and parasite determinants were attributed to severe malarial disease.4–6 Several studies have shown the involvement of pro-inflammatory cytokines in pathogenesis of severe falciparum malaria where high plasma TNF-alpha, IL-1beta, IL-6, IL-10, and IFN-gamma levels were associated with severe disease.7–10 Cerebral malaria patients were shown to have high TNF-alpha levels,7 whereas severely anemic children had low TNF-alpha levels comparable to that of mild malaria.11 Even though TNF-alpha and other pro-inflammatory cytokines protect the host against asexual blood stages of malaria parasite, excessive levels of TNF-alpha was associated with cerebral malaria and death.7,12 T cell studies however, have shown that mounting a rapid TNF-alpha and IL-2 response may protect against severe disease and reinfection.13
Parasite strain variation14 as well as allelic polymorphism of TNF promoter region through its transcriptional activation15 contributes to induction of high TNF response.16 Further, associations have been shown with the homozygocity for TNF-308A (TNF2) allele with cerebral malaria,17 TNF-238A allele with severe malarial anemia,12 and TNF-308GA heterozygous (TNF1/2) state with severe falciparum malaria.18 Polymorphisms in other cytokine genes such as IFN-gamma, IL-1, IL-10, and IL-12 have been associated with disease severity.4 IL-10 is an anti-inflammatory cytokine with immunosuppressive properties that inhibits production of pro-inflammatory cytokines such as TNF-alpha, IL-1beta, IL-6, IFN-gamma, and IL-12; both in vivo and in vitro studies have shown that IL-10 counter regulates the pro-inflammatory responses, to P. falciparum19,20 and in sepsis.21 The overall magnitude of the cytokine response and the eventual balance between pro- and anti- inflammatory cytokines was shown to be critical for determining severity of disease and its outcome.8,22
In this study, we measured plasma TNF-alpha, IL-2, IL-6, IFN-gamma, and IL-10 levels in severe (SM) and uncomplicated (UM) P. falciparum malaria patients in Sri Lanka. TNF-alpha and IL-10 levels were also assessed in a separate group of uncomplicated P. falciparum malaria patients with fever (UMF) at the time of diagnosis and recruitment. In addition, TNF-alpha/IL-10 ratio of the above three groups of patients were compared to examine the balance between pro- and anti-inflammatory cytokines. TNF allelic types of a subgroup of UM and SM patients were analyzed to investigate the association of TNF allelic types on TNF-alpha levels and TNF-alpha/IL-10 ratios of each disease category.
Patients and Methods
Patients
The present study was a part of a research programme on pathogenesis of malaria conducted at the Malaria Research Unit, Faculty of Medicine, University of Colombo.18,23–25 Of the P. falciparum-infected patients admitted to the National Hospital of Sri Lanka in Colombo, the Lady Ridgeway Hospital for children in Colombo, and the General Hospital of Anuradhapura in the North Central Province of Sri Lanka over a 3-year period,24 145 patients were selected for this study on plasma cytokine levels. Clinical categorization was performed according to the criteria described by Warrell et al.26 The inclusion and exclusion criteria described in Pathirana et al.24 were applicable to this study, i.e. patients with confirmed diagnosis of P. falciparum malaria were included and those who had anti-malarial treatment 12 hours before microscopic confirmation of P. falciparum malaria infection and co-infection with P. vivax were excluded. In addition, plasma samples from patients who had more than 15 days of symptoms were excluded and age-sex matched samples were selected for the two study groups. This included 69 uncomplicated malaria (UM) and 62 severe malaria (SM) patients. During this study period, plasma samples were obtained from 14 uncomplicated P. falciparum malaria patients having a fever episode (body temperature >37.7°C) at the time of diagnosis and sample collection. These patients were clinically characterized and included as a third patient group, referred to as UMF patients. The SM group of patients in this study included 10% with cerebral malaria, 6% with severe anemia, and others (84%) with hepatic, renal, and/or pulmonary dysfunction. All SM patients recovered from the disease without any sequellae except for one patient in whom it was fatal. In all UM patients, the intensity of clinical symptoms was evaluated using a previously validated scoring system.27 A complete past history was obtained from all patients and the body temperature was measured with a digital thermometer. The patients in the three study groups (UM, UMF, and SM) had comparable parasitemias (percentage of erythrocytes infected) and duration of symptoms (Table 1). All three study groups comprised both children (1–15 years) and adults. The parasitemias and body temperatures of children and adults within each of these three groups were comparable and therefore, they were considered together for comparison of plasma cytokine levels in this study.
Table 1. Characterization of the P. falciparum malaria patient categories.
| Uncomplicated malaria (UM) | Uncomplicated malaria with fever (UMF) | Severe malaria (SM) | |
| No. of patients | 69 | 14 | 62 |
| Mean age, years, (range) | 28.8 (1–68) | 27.0 (9–47) | 26.3 (1–81) |
| Days of symptoms, median (range) | 7 (2–15) | 8 (4–12) | 6 (2–15) |
| Geometric mean parasitemia (*/SE) (% of erythrocytes infected) | 0.139 (*/0.123) | 0.043 (*/0.216) | 0.049 (*/0.639) |
| Temperature (°C), median, (range) | 37.0 (36.2–37.7) | 38.2* (37.8–39.2) | 38.9* (36.7–40.8) |
Note: *Temperature in SM and UMF groups were significantly higher compared to UM group (P<0.001, Mann–Whitney U test). All other parameters in the three categories were comparable.
Informed consent was obtained from all the adult patients enrolled and from the parents/guardians of the children. The study protocol was approved by the Ethics Review Committee of the Faculty of Medicine, University of Colombo.
Cytokine assay
For cytokine measurement, plasma samples collected from UM and SM P. falciparum patients during their acute infections and in UMF patients, during fever episodes were used. Blood (5 ml) was drawn by venepuncture into tubes containing 100 μl of 100 mM EDTA (Sigma, St Louis, MO, USA) as an anticoagulant, and 100 μl of Aprotinin (Sigma) as a protease inhibitor, which gave final concentrations of 2 mM and 0.5 TIU respectively. Blood was centrifuged at 800 g for 10 minutes, and plasma was separated and were frozen in aliquots of 100 μl at −20°C until they were used for cytokine assays. Plasma samples were tested for cytokine levels within 3 months of the sample collection.
Plasma TNF-alpha, IL-2, IL-6, IL-10, and IFN-gamma levels were determined using Enzyme-linked immunosorbant assay (ELISA) kits (Cytoscreen™; Biosource International, Camarillo, CA, USA) following manufacturer’s instructions. Briefly, the plasma samples were thawed immediately before the experiment and specified volume of plasma, 100 μl for TNF-alpha and IL-10, and 200 μl for IL-2, IL-6, and IFN-gamma were added to the wells in the ELISA plate. Recombinant cytokine standards provided with the kit were diluted using the dilution buffer provided, to make standard cytokine solutions of 1000 pg/ml and two-fold dilutions up to 1.9 pg/ml. The ELISA was carried out in duplicate for both cytokine standard solutions and plasma samples. Absorbance was read at 450 nm using a microplate reader (Bio-Tek, Winooski, VT, USA). Each plate included a standard curve of recombinant cytokine and known positive and negative controls. The levels of cytokines in plasma samples were determined using the standard curves plotted for each cytokine. The lowest detection levels were 15, 2, 5, 1, and 4 pg/ml for TNF-alpha, IL-2, IL-6, IL-10, and IFN-gamma, respectively.
Allelic typing of TNF-alpha gene
Patient blood samples were collected into microvette tubes (Sarsted, Numbrecht, Germany) and frozen at −20°C until they were used for extraction of host DNA. DNA was extracted from patient’s blood using standard phenol choloroform extraction method28 and typed for TNF-alpha gene alleles as described previously.18 Briefly, a fragment of 519 bp from the promoter region of the human TNF-alpha gene which includes a single-base polymorphism at −308, was amplified from the DNA samples by PCR with oligonucleotide primers T1 and T2. The TNF-alpha primer sequences were: T1, 5′ CAA ACA CAG GCC TCA GGA CTC 3′; T2, 5′ AGG GAG CGT CTG CTG GCT G 3′. The PCR amplified products were probed with allele-specific oligonucleotide probes by the Southern hybridization technique. The sequences of these allele-specific oligonucleotide probes were end-labeled with gamma32P, for TNF-1 allelic type, 5′ AGG GGC ATG GGG ACG GG 3′ and for TNF-2 allelic type, 5′ AGG GGC ATG AGG ACG GG 3′.
Data analysis
Statistical analysis was done using SPSS Statistical Software Package version 17 for Windows (SPSS Inc., Chicago, IL, USA). Values are given as mean±SEM if normally distributed. Non-parametric data are represented either as geometric mean*/ SEM or median and range/25th–75th percentiles. Compaforisons between cytokine levels of different patient categories and the TNF-alpha/IL-10 ratio were made by the linear univariate analysis with age correction. Temperature was compared using Mann–Whitney U test. Correlation analyses were performed using Pearson and Spearman correlations for parametric and non-parametric data, respectively. A P value of <0.05 was considered statistically significant.
Results and Discussion
Plasma cytokine levels in uncomplicated and severe P. falciparum malaria patients
Plasma TNF-alpha levels were significantly higher in the SM group (12-fold) compared to that of the UM group (median: 334.7 and 27.2 pg/ml, respectively; P<0.001) (Fig. 1A). The third category of patients (UMF) had much higher TNF-alpha levels (median value: 414.5 pg/ml) which were significantly higher (15-fold) than the UM group (P<0.001); however, they were comparable to those of the SM group. TNF-alpha levels in UM patients (median: 27.2 pg/ml) were comparable to that of a non-malarial healthy control groups of a previous study conducted in Sri Lanka (median: 27.0 pg/ml)27 and in India (mean: 13.1 pg/ml).29
Figure 1.
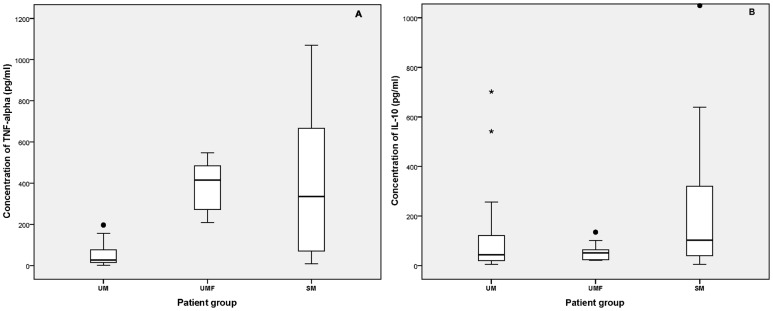
Plasma TNF-alpha (A) and IL-10 (B) levels (pg/ml) in three P. falciparum malaria patient categories, uncomplicated (UM), uncomplicated fever (UMF), and severe (SM). Boxes represent 25th–75th percentiles, solid bar within boxes represent median values, values within the bars represent non-outlier maximum and minimum, mild outlier (•), and extreme outlier (*). Plasma TNF-alpha levels of both SM and UMF categories are significantly high compared to UM (P<0.001).
The levels of IL-10 observed in SM patients were two times higher than that of UM patients (median: 102.2 and 43.8 pg/ml, respectively; P = 0.022) (Fig. 1B). Unlike the increase in TNF-alpha, the IL-10 levels in UMF patient group (median value: 51.2 pg/ml) was comparable to that of the UM group. Both plasma IL-6 and IFN-gamma levels were higher in the SM group (mean±SE: 272.0±24.5 and 74.2±41.9 pg/ml, respectively) compared to the UM group (mean±SE: 106.1±16.1 and 12.9±3.2 pg/ml, respectively); however, the difference was statistically significant only for IL-6 (P<0.001) (Fig. 2) but not for IFN-gamma (data not shown). It is noteworthy that IFN-gamma levels were measured for a subset of samples (11 and 12 plasma samples for SM and UM groups, respectively) and the lack of significance may be partly due to the small sample size. Both IL-10 and IFN-gamma levels were shown to be elevated in P. falciparum-infected patients compared to the normal controls as reported previously where normal healthy controls had undetectable levels of plasma IL-10 and IFN-gamma (<4 and <1 pg/ml, respectively).30 There were no significant correlation between different cytokine levels (IL-6, IL-10, TNF-alpha, and IFN-gamma) within either UM or SM group. IL-2 levels were not detectable in both SM and UM groups of patients (<2 pg/ml). Although there were both children (age: 1–15 years) and adults in all three study groups, their cytokine levels within each group were comparable.
Figure 2.
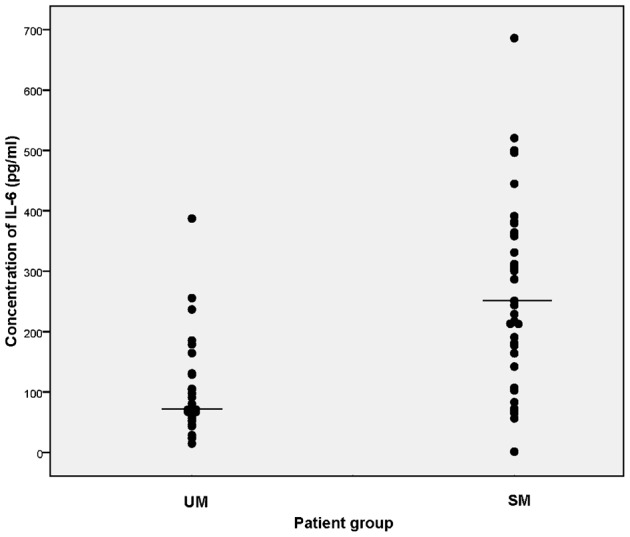
Plasma IL-6 levels (pg/ml) in two P. falciparum malaria patient categories, uncomplicated (UM) and severe (SM). Horizontal bars represent the mean values. Plasma IL-6 levels of SM category are significantly high compared to those of UM (P<0.001).
Percentage of parasitemia (asexual stages) at the time of diagnosis in SM patients was lower than that of the UM patients; however, it was not statistically significant (geometric mean*/SE: 0.049*/0.639 and 0.139*/0.123, respectively; P = 0.062) (Table 1). Significantly higher body temperatures were found in both SM and UMF groups of patients compared to those of the UM group (median: 38.9, 38.2, and 37.0°C, respectively; P<0.001) (Table 1). There was no correlation between temperature and TNF-alpha levels within the three patients groups (UM, SM, and UMF) (P>0.05, Spearman correlation). However, significant positive correlations between body temperature and plasma TNF-alpha, IL-6, and IL-10 levels were observed considering all P. falciparum patients in these three groups (r = 0.247, 0.288, and 0.307, respectively; P<0.05) and a marginal association between IFN-gamma levels and body temperature. Further, within the UM group, the IL-6, IL-10, and TNF-alpha levels of those who had no previous malaria infections and those who had 1–10 past infections were comparable.
Since the balance between pro- and anti-inflammatory cytokine responses is more important than a single cytokine level, we analyzed the TNF-alpha/IL-10 ratio of the SM and UM groups. As shown in Fig. 3, the geometric mean of TNF-alpha/IL-10 ratio in the SM patients was significantly higher compared to the UM patients (P = 0.009). UMF patients also showed a significantly higher TNF-alpha/IL-10 ratio compared to the UM patients (P<0.001). No such relationship was found in the IL-6/IL-10 ratios of the three different patient groups.
Figure 3.
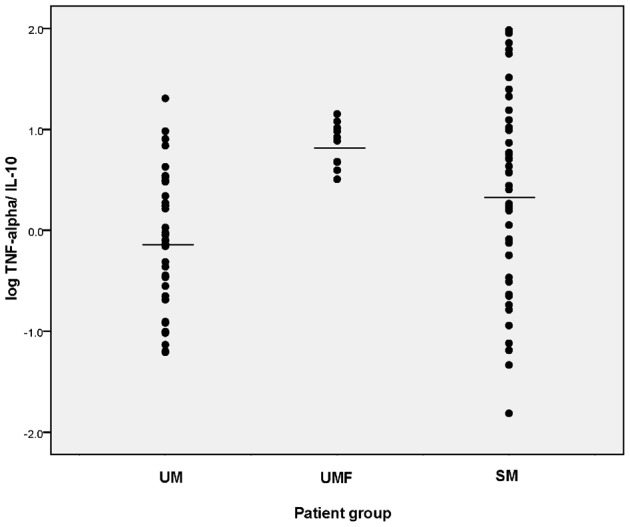
Log TNF-alpha/IL-10 ratio in UM and SM P. falciparum malaria patient categories. Horizontal bars represent the mean values. TNF-alpha/IL-10 ratios of both SM and UMF categories are significantly high compared to UM (P = 0.009 and P<0.001).
Association between TNF-alpha allelic types and plasma TNF-alpha levels in UM and SM P. falciparum patients
DNA samples from a subgroup of age–gender matched UM and SM patients (n = 34 from each group) were used for TNF-alpha allelic typing to investigate the possible association of TNF1 and TNF2 allelic types with TNF-alpha levels of each disease group. As shown for the total sample of this study, SM subgroup of patients showed significantly higher plasma TNF-alpha levels (mean±SE: 404.29±65.48 pg/ml) compared to UM subgroup (mean±SE: 45.10±9.19 pg/ml; P<0.001). Further, among these 68 patients, there was no significant difference in the plasma TNF-alpha levels of the homozygous TNF1 (−308GG; n = 31) individuals (mean±SE: 149.35±44.09 pg/ml) compared to those carrying the −308A allele (including −308GA and −308AA; referred to as TNF2; n = 37) (mean±SE: 287.83±61.06 pg/ml; P = 0.580) showing the lack of any direct relationship between TNF-alpha levels and the host TNF allelic type. Further, the present study did not show an association between the heterozygocity for TNF2 allele with severe disease (P = 0.81) as reported previously;18 however, these data were consistent with the findings of a subsequent study including a larger sample size.31
TNF-alpha levels of the UM and SM subgroups of patients categorized according to their TNF1 and TNF2 types allelic types are depicted in Fig. 4. Individuals carrying TNF1 and TNF2 allele who had UM infections had comparable plasma TNF-alpha levels (mean±SE: 63.0±17.6 and 26.9±6.2 pg/ml, respectively; P = 0.051). However, within the SM group, individuals carrying TNF2 allele had significantly higher circulating TNF-alpha levels compared to individuals carrying TNF1 (mean±SE: 533.7±87.1 and 240.4 ± 84.4 pg/ml, respectively; P = 0.024), reflefcting a significant association between TNF2 and higher circulating TNF-alpha levels in patients who developed severe falciparum malaria. Further, the two subgroups of SM patients carrying TNF1 and TNF2 showed significantly higher plasma TNF-alpha levels compared to the UM patients carrying TNF1 (P = 0.043) and TNF2 (P = 0.001), respectively, which suggest the possible influence of other factors such as parasite strain variation in the SM group that may contribute higher circulating TNF-alpha levels. This was further corroborated by the significantly high circulating TNF-alpha levels in TNF2-SM patients compared to TNF2-UM patients (P<0.001).
Figure 4.
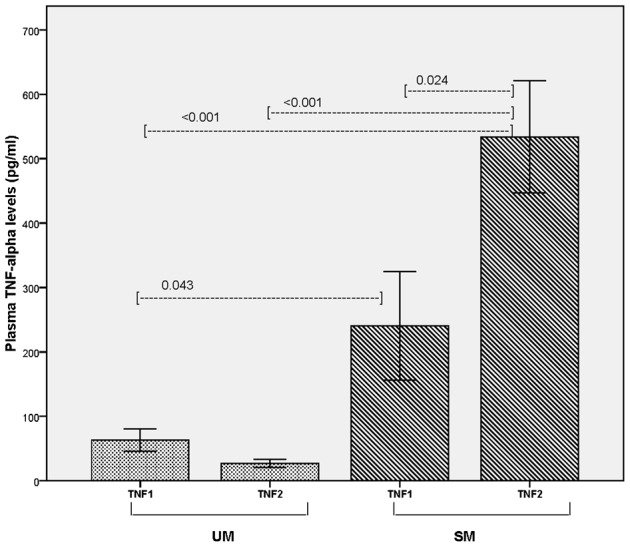
Plasma TNF-alpha levels in UM (n = 34) and SM (n = 34) P. falciparum malaria patient categories carrying TNF1 and TNF2 allelic types. TNF1: patients who are homozygous for TNF1 allele (−308GG; n = 31) and TNF2: patients carrying the TNF2 (−308A) allele including heterozygous (−308GA) and homozygous (−308AA), n = 37. The four subgroups included 16, 18, 15, and 19 patients for TNF1-UM, TNF2-UM, TNF1-SM, and TNF2-SM groups, respectively.
Association between TNF-alpha allelic types and ratio of TNF-alpha/IL-10 in UM and SM P. falciparum patients
The significant differences observed for circulating TNF-alpha levels among the four subgroups (TNF1-UM, TNF2-UM, TNF1-SM, and TNF2-SM) were also reflected in the comparison of TNF-alpha/IL-10 ratio in these four subgroups. SM patients carrying TNF2 showed a significantly higher TNF-alpha/IL-10 ratio compared to the first two subgroups, UM patients carrying TNF1 (P = 0.026) and UM patients carrying TNF2 (P = 0.005), and only marginally significant compared to SM patients carrying TNF1 (P = 0.039) (Table 2). These results show that the SM patients carrying TNF2 had not only the highest circulating TNF-alpha levels but also inadequate IL-10 responses which could have contributed to the disease severity.
Table 2. Comparison of TNF-alpha and IL-10 ratios of UM and SM P. falciparum malaria patients carrying TNF1 and TNF2 alleles.
| Patient category | TNF allelic type (n) | TNF-alpha/IL-10 ratio, median (interquartile range) | Significance (P)* |
| UM | TNF1 (16) | 0.59 (0.295–0.938) | 0.026 |
| UM | TNF2 (18) | 0.28 (0.098–1.669) | 0.005 |
| SM | TNF1 (15) | 0.31 (0.138–3.075) | 0.039 |
| SM | TNF2 (19) | 4.39 (0.628–19.756) | … |
Note: *Compared to SM patients carrying TNF2 allele (Mann–Whitney U test).
General Discussion
We have found significantly higher plasma levels of TNF-alpha, IL-6, and IL-10 in P. falciparum-infected patients who had severe malaria compared to those of who had uncomplicated malaria infections. Cytokine levels (TNF-alpha, IL-6, IFN-gamma, and IL-10) of P. falciparum-infected patients in Sri Lanka have not been reported previously. These findings are consistent with the previous reports on higher cytokine levels in severe/cerebral malaria patients, from studies conducted in other malaria endemic regions, i.e. the Gambia and Mali in Africa, Madagascar, Gulf of Guinea, and Vietnam.7–9,32–34 Most of these studies had cerebral malaria patients as well as severe, non-cerebral patients, who had comparable cytokine levels, whereas the present study had a majority of severe, non-cerebral patients. In Gambia, the cerebral patients who had fatal outcomes had higher TNF-alpha levels (269 pg/ml)7 which were comparable to those observed in SM patients in the present study. The cerebral malaria survivors had intermediate levels of TNF-alpha (51 pg/ml). The mild malaria patients had the lowest TNF-alpha levels (24 pg/ml), which were in the same range as observed in the UM P. falciparum infection in the present study.
The present study supports the hypothesis that excessive pro-inflammatory cytokines such as TNF-alpha and IL-6 production may contribute to the pathogenesis of severe malaria. Parasite-derived ‘toxins’ induce cytokine production resulting in increased cytokine levels locally, in tissues of vital organs which would upregulate the expression of endothelial receptors.35 This in turn leads to increased parasite adherence and subsequent microvascular obstruction, decreasing oxygen delivery, and/or possibly the release of NO from the endothelium which may ultimately contribute to the pathogenesis. However, recent study has shown that low nitric oxide bioavailability in fact contributes to the genesis of cerebral malaria in mice36 and the exact role of cytokine network in malaria pathogenesis remains to be further elucidated.
One important observation in this study was the high TNF-alpha levels in uncomplicated P. falciparum patients experiencing fever, chills, and rigors (UMF) during the time of sample collection compared to the samples collected from other UM patients during acute infection, in between paroxysms. The TNF-alpha levels in these UMF group of patients were comparable to the high TNF-alpha levels observed in SM patients. Since there is a positive correlation between TNF-alpha levels and body temperature as shown in the present study, the TNF-alpha levels of UMF patients can be expected to decline to lower levels with the decrease in body temperature. A rapid decrease in the high levels of cytokines from the blood, almost immediately after the paroxysm, has been reported earlier in P. vivax malaria.37 During fever paroxysms in P. vivax patients, the absolute levels of TNF-alpha were highly variable among the patients ranging at peak from 100 to 3000 pg/ml37 and were comparable to TNF-alpha concentrations observed in P. falciparum patients from 172 to 1526 pg/ml.38,39 It is questionable that why these higher levels of cytokines observed in P. vivax patients, do not induce severe disease as in P. falciparum patients. This may be explained by several biological differences between the two species. In particular, P. falciparum mature forms of parasitized red blood cells are not present in the peripheral circulation as they are sequestered in vessels of internal organs and thus, giving rise to much increased cytokine levels locally causing a cytokine imbalance, in the internal organs, as opposed to the cytokine levels detected in peripheral circulation, as in P. vivax infections.
Several studies have indicated that TNF-alpha is a critical mediator of malarial fever both in P. vivax and P. falciparum.37,40 TNF-alpha is released in intermittent bursts that coincide with the schizont rupture37,41 and in addition, IL-6 is shown to have pyrogenic properties.40 The significantly high body temperatures observed in the SM group compared to UM group is thus consistent with the higher levels of pyrogenic cytokines (IL-6 and TNF-alpha) in SM patients. Although a direct association between body temperature and cytokine levels was not apparent in either SM or UM group, there were significant positive correlations between body temperature and plasma TNF-alpha, IL-6, and IL-10 levels, considering both SM and UM P. falciparum patients and a marginal association between IFN-gamma levels and body temperature.
High levels of IL-10 observed in the present study and others8 may suggest either a beneficial role of IL-10 by reducing the parasite-induced inflammatory response, or a detrimental one by decreasing cellular immune responses. As reported earlier, IL-10 is an inhibitor of activated macrophages; this involves the homeostatic control of the innate immune reaction and cell-mediated immunity.42 Moreover, exogenous IL-10 had been shown to inhibit malarial antigen induced production of cytokines by peripheral blood mononuclear cells (PBMC), such as TNF-alpha, IL-1beta, and IL-6.43 Further, addition of anti-IL-10 antibodies markedly enhanced the production of these cytokines and this effect was significantly greater on PBMC from patients with uncomplicated P. falciparum infections than PBMC from patients with severe disease.43 These findings suggested that IL-10 counter-regulates the pro-inflammatory response to P. falciparum and that severe falciparum malaria may be associated with an inadequate negative feedback response by IL-10.43 The lack of negative feedback was apparent from the IL-10 and TNF-alpha data from the present study, i.e. though the circulating IL-10 levels in SM group were two-fold higher compared to UM group, a proportionate decrease in TNF-alpha levels was not apparent in SM group; instead, the circulating TNF-alpha levels were 12-fold higher compared to UM group. This was further evident by the higher ratio of TNF-alpha/IL-10 in SM group compared to UM group in the present study. The UMF group had even higher ratio of TNF-alpha/IL-10 compared to both SM and UM groups. However, the underlying mechanisms operating in UMF group may differ from that of the SM group as SM patients had persistent fever for >3 days during their infections.
Similar unbalanced pro-inflammatory responses have been shown in other forms of severe falciparum malaria. Studies conducted in Africa have shown significant associations of low concentrations of IL-1044,45 and higher ratio of TNF-alpha/IL-1044,46 in severe malarial anemia compared to other malarial disease groups, suggesting that insufficient IL-10 response to high TNF concentrations may have a role in development of severe malaria anemia. Children with respiratory distress, a symptom underlying metabolic acidosis which identified as a major risk factor for mortality in severe malaria in Africa, have also been associated with significantly high ratio of TNF-alpha/IL-10 than those without respiratory distress.45 High ratios of pro- and anti-inflammatory cytokines were also associated with increased risk of fever in previous studies,47 which is consistent with the finding of the present study where high ratio of TNF-alpha/IL-10 was observed in UM patients with fever at the time of recruitment.
Higher levels of parasitemia were observed in uncomplicated P. falciparum infections compared to the severe malaria infections. This is mainly because 32% of the SM patients had very low levels of parasite densities in peripheral blood. One reason for this could be that the parasites were sequestered in deep vasculature having low or undetectable parasite densities in the thick blood smears (negative thick smears). P. falciparum infections in these patients were diagnosed by dipstick test, which indicated having P. falciparum HRP2 antigen in circulation. It is unlikely that delay in receiving anti-malarial treatment could have contributed to the difference in parasite densities since both UM and SM groups had comparable days of symptoms. Previous studies have shown the anti-parasitic effects of TNF-alpha.7 However, no correlation was found with TNF-alpha levels and parasitemia in patients of either UM or SM groups in the present study. This may be due to the fact that parasitemias were lower in P. falciparum patients included and that an association was not apparent (it may also be due to the fact that realistic estimation of parasitemia is not possible in P. falciparum due to sequestration). In the African study, where much higher parasitemias were prevalent in mild and cerebral malaria patients, a significant association between TNF-alpha levels on patient’s parasitemia was observed.7 However, the significant, negative correlation found between parasitemia with IL-6 and IFN-gamma levels in this study, may suggest that these cytokines had anti-parasitic effects (data not shown). This may occur through a variety of different pathways: (1) macrophages and neutrophils stimulated in vitro with TNF-alpha or IL-1, particularly in the presence of IFN-gamma, show increased phagocytic ingestion of P. falciparum-infected erythrocytes;48 and (2) these pro-inflammatory cytokines stimulate macrophages and neutrophils to produce free oxygen radicals which are inhibitory for growth of P. falciparum in vitro.49
Recent studies have shown that both parasite and host genetic factors influence the ability to induce TNF production by human monocytes. Apart from the P. falciparum strain variation in TNF-inducing activity,14 genetic factors were shown to influence TNF-alpha levels and disease severity. The presence of TNF2 allele (−308A) of the TNF-alpha promotor gene which is known to increase TNF-alpha transcription,50 either in its homologous status17 or heterologous status,18 was shown to be associated in cerebral and severe malaria, whereas −238A was shown to be associated with severe anemia.12 The present study was a part of an overall study on parasite and host genetic determinants of severe falciparum malaria which included studies on human host genetic factors and their association with pathogenesis of P. falciparum malaria.31 Thus, the analysis of TNF-alpha levels in relation to their host genetic background was carried out considering both the TNF allelic type and the disease category. Interestingly, the two UM subgroups carrying TNF1 and TNF2 alleles did not show a difference in their circulating TNF-alpha levels nor the ratio of TNF-alpha/IL-10. The two SM groups carrying TNF1 and TNF2 alleles had significantly high circulating TNF-alpha levels; however, the ratio of TNF-alpha/IL-10 was higher in SM TNF2 patient group. The SM patients carrying either TNF1 or TNF2 alleles having high circulating levels of TNF-alpha may reflect distinct parasite strain variants that induce TNF-alpha production regardless of the host genetic background. However, the highest circulating TNF-alpha levels and TNF-alpha/IL-10 ratio observed in SM patients carrying TNF2 reflects that the combination of the presence of TNF2 allele as well as an inadequate IL-10 response could have contributed to the development of severe falciparum malaria. Findings of the present study on high TNF-alpha/IL-10 ratio in severe patients are consistent with those conducted in central Africa which has shown associations with cerebral malaria and severe anemia.39 As previously described for Sri Lanka,3 these complications are not that common and the severe malaria group in the present study included a mixture of severe malarial complications. In addition to the findings associated with mutation at −308 position (TNF2), other associations were observed with mutations at position −238 of the TNF promoter regions.12 In contrast, a recent study has shown lack of association between −308 TNF polymorphism and susceptibility to CM in Central Sudanese children.51 As TNF gene is located in the MHC class III region, flanked by the MHC class I and class II regions, certain linkages such as TNF −238A allele and HLA B53 and HLA DR1*1302 have also been reported.12 It is possible that observed genetic associations arise from functional variations in neighboring genes. These findings thus emphasize the importance of TNF as a mediator, the inadequate IL-10 response, and the genetic predispositions in the pathogenesis of severe falciparum malaria.
Author Contributions
SMH and SP suggested the idea and obtained funding for the study. MKP, NPH, SLP, KNM, SP, and SMH developed the study protocol. MKP and NPH collected samples, and did the laboratory work. MP-K and HKA performed the clinical characterization of patients. MKP, SMH, and SLP did the data analysis. MKP wrote the first draft, and SMH reviewed and finalized the manuscript. All authors went through and approved the final manuscript.
Acknowledgments
We gratefully acknowledge the cooperation of the staff of the National Hospital of the Sri Lanka and the General Hospital of Anuradhapura. We wish to thank S. Bandara, K. L. R. L. Perera, G. M. Kapilananda, N. Fernando, S. D. Abeynayeka, K. Ekanayaka, C. Wimalasena, J. Rajakaruna, and Priyankara Perera for technical assistance. This investigation received support from the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Disease (TDR), grant ID 930807, ID 94304, and ID 960603.
References
- 1.Langhorne J, Ndungu FM, Sponaas AM, Marsh K. Immunity to malaria: more questions than answers. Nat Immunol. 2008;9(7):725–32. doi: 10.1038/ni.f.205. [DOI] [PubMed] [Google Scholar]
- 2.Marsh K, Froster D, Waruiru C, Mwangi I, Winstanley M, Marsh V, et al. Indicators of life threatening malaria in African children. N Engl J Med. 1995;332(21):1399–404. doi: 10.1056/NEJM199505253322102. [DOI] [PubMed] [Google Scholar]
- 3.Alles HK, Mendis KN, Carter R. Malaria mortality rates in South Asia and in Africa: implications for malaria control. Parasitol Today. 1998;14(9):369–75. doi: 10.1016/s0169-4758(98)01296-4. [DOI] [PubMed] [Google Scholar]
- 4.Kwiatkowski DP. How malaria has affected the human genome and what human genetics can teach us about malaria. Am J Hum Genet. 2005;77:171–90. doi: 10.1086/432519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Normark J, Nilsson D, Ribacke U, Winter G, Moll K, Wheelock CE, et al. PfEMP1-DBL 1α amino acid motifs in severe disease states of Plasmodium falciparum malaria. Proc Natl Acad Sci USA. 2007;104(40):15835–40. doi: 10.1073/pnas.0610485104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kyes SA, Kraemer SM, Smith JD. Antigenic variation in Plasmodium falciparum: gene organization and regulation of the var multigene family. Eukaryot Cell. 2007;6(9):1511–20. doi: 10.1128/EC.00173-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kwiatkowski D, Hill AV, Sambaou I, Twumasi P, Castracane J, Manogue KR, et al. TNF concentration in fatal cerebral, non-fatal cerebral and uncomplicated Plasmodium falciparum malaria. Lancet. 1990;336:1201–4. doi: 10.1016/0140-6736(90)92827-5. [DOI] [PubMed] [Google Scholar]
- 8.Day PJ, Hien TT, Schollaardt T, Loc PP, Chuong LV, Chau TT, et al. The prognostic and pathophysiologic role of pro- and anti-inflammatory cytokines in severe malaria. J Infect Dis. 1999;180:1288–97. doi: 10.1086/315016. [DOI] [PubMed] [Google Scholar]
- 9.Lyke KE, Burges R, Cissoko Y, Sangare L, Dao M, Diarra I, et al. Serum level of the proinflammatory cytokines interleukin-1 beta (IL-1β), IL-6, IL-8, IL-10, tumor necrosis factor alpha, and IL-12 (p70) in Malian children with severe Plasmodium falciparum malaria and matched uncomplicated malaria or healthy controls. Infect Immun. 2004;72(10):5630–7. doi: 10.1128/IAI.72.10.5630-5637.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark IA, Budd AC, Alleva LM, Cowden WB. Human malarial disease: a consequence of inflammatory cytokine release. Malar J. 2006;5:85. doi: 10.1186/1475-2875-5-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGuire W, Knight JC, Hill AV, Allsopp CE, Greenwood BM, Kwiatkowski D. Severe malarial anemia and cerebral malaria are associated with different tumor necrosis factor promoter alleles. J Infect Dis. 1999;179:287–90. doi: 10.1086/314533. [DOI] [PubMed] [Google Scholar]
- 12.Kwiatkowski D, Perlmann P.Inflammatory processes in the pathogenesis of malaria Wahlgren M, Perlmann P.editors. Malaria: molecular and clinical aspects. 1st edAmsterdam: Harwood Academic Publishers; 1999329–361. [Google Scholar]
- 13.Ramharter M, Kremsner PG, Willheim M, Winkler H, Graninger W, Winkler S. Plasmodium falciparum-specific interleukin-2 and tumor necrosis factor-alpha expressing-T cells are associated with resistance to reinfection and severe malaria in healthy African children. Eur Cytokine Netw. 2004;15(3):189–96. [PubMed] [Google Scholar]
- 14.Allan RJ, Beattie P, Bate C, Hensbroek MB, Morris-Jones S, Greenwood BM, et al. Strain variation in tumor necrosis factor induction by parasites from children with acute falciparum malaria. Infect Immun. 1995;63(4):1173–5. doi: 10.1128/iai.63.4.1173-1175.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson AG, di Giovine FS, Blackmore AI, Duff GW. Single base polymorphism in the human tumor necrosis factor alpha (TNF-α) gene detectable by Ncol restriction of PCR product. Hum Mol Genet. 1992;1(5):353. doi: 10.1093/hmg/1.5.353. [DOI] [PubMed] [Google Scholar]
- 16.Bouma G, Crusius JB, Oudkerk Pool M, Kolkman JJ, von Blomberg BM, Kostense PJ, et al. Secretion of tumor necrosis factor α and lymphotoxin α in relation to polymorphisms in the TNF genes and HLA-DR alleles. Relevance for inflammatory bowel disease. Scand J Immunol. 1996;43:456–63. doi: 10.1046/j.1365-3083.1996.d01-65.x. [DOI] [PubMed] [Google Scholar]
- 17.McGuire W, Hill AV, Allsopp CE, Greenwood BM, Kwiatkowski D. Variation in the TNF-α promoter region associated with susceptibility to cerebral malaria. Nature. 1994;371:508–11. doi: 10.1038/371508a0. [DOI] [PubMed] [Google Scholar]
- 18.Wattawidanage J, Carter R, Perera KL, Munasingha A, Bandara S, McGuinness D, et al. TNFα*2 marks high risk of severe disease during Plasmodium falciparum malaria and other infections in Sri Lankans. Clin Exp Immunol. 1999;115(2):350–5. doi: 10.1046/j.1365-2249.1999.00804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li C, Corraliza I, Langhorne J. A defect in interleukin-10 leads to enhanced malarial disease in Plasmodium chabaudi chabaudi infection in mice. Infect Immun. 1999;67(9):4435–42. doi: 10.1128/iai.67.9.4435-4442.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho M, White NJ. Molecular mechanisms of cytoadherence in malaria. Am J Physiol Cell Physiol. 1999;276:C1231–42. doi: 10.1152/ajpcell.1999.276.6.C1231. [DOI] [PubMed] [Google Scholar]
- 21.Grutz G. New insights into the molecular mechanism of interleukin-10-mediated immunosuppression. J Leukoc Biol. 2005;77:3–15. doi: 10.1189/jlb.0904484. [DOI] [PubMed] [Google Scholar]
- 22.Penman B, Gupta S. Evolution of virulence in malaria. J Biol. 2008;7:22. doi: 10.1186/jbiol83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alles HK. Colombo: University of Colombo; 1997. Study of clinical aspects and pathophysiology of malaria and epidemiology of severe and complicated malaria [PhD thesis]. [Google Scholar]
- 24.Pathirana SL, Alles HK, Bandara S, Phone-Kyaw M, Perera MK, Wickramasinghe AR, et al. ABO-blood-group types and protection against severe, Plasmodium falciparum malaria. Ann Trop Med Parasitol. 2005;99(2):119–24. doi: 10.1179/136485905X19946. [DOI] [PubMed] [Google Scholar]
- 25.Pathirana SL, Ekanayeke K, Howard RJ, Mendis KN, Handunnetti SM. Cytoadherence and its association with clinical disease in Plasmodium falciparum infections in Sri Lanka. J Natl Sci Found Sri Lanka. 2010;38(4):233–40. [Google Scholar]
- 26.Warrell DA, Molyneux ME, Beales PF. Severe and complicated malaria. Trans Roy Soc Trop Med Hyg. 1990;84(Suppl. 2):1–65. [PubMed] [Google Scholar]
- 27.Karunaweera ND, Carter R, Grau GE, Mendis KN. Demonstration of anti-disease immunity to Plasmodium vivax malaria in Sri Lanka using a quantitative method to assess clinical disease. Am J Trop Med. 1998;58(2):204–10. doi: 10.4269/ajtmh.1998.58.204. [DOI] [PubMed] [Google Scholar]
- 28.Sambrook J, Fritsch EF, Maniatis T. New York: Cold Spring Harbor Laboratory Press; 1989. Molecular cloning: a laboratory manual. 2nd ed. [Google Scholar]
- 29.Sohail M, Kaul A, Bali P, Raziuddin M, Singh MP, Singh OP, et al. Alleles −308A and −1031C in the TNF-α gene promoter do not increase the risk but associated with circulating levels of TNF-α and clinical features of vivax malaria in Indian patients. Mol Immunol. 2008;45:1682–92. doi: 10.1016/j.molimm.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Perera RA. Colombo: University of Colombo; 2003. Peripheral blood mononuclear cells and cytokine Profile in severe and uncomplicated malaria patients in Sri- Lanka [PhD thesis]. [Google Scholar]
- 31.Herath NP. Colombo: University of Colombo; 2003. Human host genetic factors and cytokine responses associated with the pathogenesis of Plasmodium falciparum malarial disease [PhD thesis]. [Google Scholar]
- 32.Grau GE, Taylor TE, Molyneux ME, Wirima JJ, Vassalli P, Hommel M, et al. Tumor necrosis factor and disease severity in children with falciparum malaria. N Engl J Med. 1989;320:1586–91. doi: 10.1056/NEJM198906153202404. [DOI] [PubMed] [Google Scholar]
- 33.Peyron F, Burdin N, Ringwald JP, Vuillez F, Rousset F, Banchereau J. High levels of circulating IL-10 in human malaria. Clin Exp Immunol. 1994;95:300–3. doi: 10.1111/j.1365-2249.1994.tb06527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baptista JL, Vanham G, Wery M, Marck EV. Cytokine levels during mild and cerebral malaria in children living in a mesoendemic area. Trop Med Int Health. 1997;2(7):673–9. doi: 10.1046/j.1365-3156.1997.d01-355.x. [DOI] [PubMed] [Google Scholar]
- 35.Hunt NH, Grau GE. Cytokines: accelerators and brakes in the pathogenesis of cerebral malaria. Trends Immunol. 2003;24(9):491–9. doi: 10.1016/s1471-4906(03)00229-1. [DOI] [PubMed] [Google Scholar]
- 36.Gramaglia I, Sobolewski P, Meays D, Contreras R, Nolan JP, Frangos JA, et al. Low nitric oxide bioavailability contributes to the genesis of experimental cerebral malaria. Nat Med. 2006;12(12):1417–22. doi: 10.1038/nm1499. [DOI] [PubMed] [Google Scholar]
- 37.Karunaweera ND, Grau GE, Gamage P, Carter R, Mendis KN. Dynamics of fever and serum TNF levels are closely associated during clinical paroxysms in P. vivax malaria. Proc Natl Acad Sci USA. 1992;89:3200–3. doi: 10.1073/pnas.89.8.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kern P, Hemmer CJ, van Damme J, Gruss HJ, Dietrich M. Elevated tumor necrosis factor alpha and interleukin-6 serum levels as markers for complicated Plasmodium falciparum malaria. Am J Med. 1989;87(2):139–43. doi: 10.1016/s0002-9343(89)80688-6. [DOI] [PubMed] [Google Scholar]
- 39.May J, Lell B, Luty AJ, Meyer CG, Kremsner PG. Plasma interleukin-10: tumor necrosis factor (TNF)-α ratio is associated with TNF promoter variants and predicts malarial complications. J Infect Dis. 2000;182:1570–3. doi: 10.1086/315857. [DOI] [PubMed] [Google Scholar]
- 40.Kwiatkowski D. Malarial toxins and the regulation of parasite density. Parasitol Today. 1995;11(6):206–12. doi: 10.1016/0169-4758(95)80079-4. [DOI] [PubMed] [Google Scholar]
- 41.Kwiatkowski D, Cannon JG, Manogue KR, Cerami A, Dinarello CA, Greenwood BM. Tumour necrosis factor production in falciparum malaria and its association with schizont rupture. Clin Exp Immunol. 1989;77:361–6. [PMC free article] [PubMed] [Google Scholar]
- 42.Moore KW, de Waal MR, Coffman RL, O’Garra A. Interleukin-10 and interleukin-10 receptor. Ann Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 43.Ho M, Schollaardt T, Snape S, Looareesuwan S, Suntharasamai P, White NJ. Endogenous interleukin-10 modulates pro-inflammatory response in Plasmodium falciparum malaria. J Infect Dis. 1998;178:520–5. doi: 10.1086/515640. [DOI] [PubMed] [Google Scholar]
- 44.Kurtzhals JA, Adabayeri V, Goka BQ, Akanmori DB, Oliver-Commey J, Nkrumah K, et al. Low plasma concentrations of interleukin-10 in severe malarial anemia compared with cerebral and uncomplicated malaria. Lancet. 1998;351(9118):1768–72. doi: 10.1016/S0140-6736(97)09439-7. [DOI] [PubMed] [Google Scholar]
- 45.Awandare GA, Goka B, Boeuf P, Tetteh JK, Kurtzhals JA, Behr C, et al. Increased levels of inflammatory mediators in children with severe Plasmodium falciparum malaria with respiratory distress. J Infect Dis. 2006;194:1438–46. doi: 10.1086/508547. [DOI] [PubMed] [Google Scholar]
- 46.Othoro C, Lal AA, Nahlen B, Koech D, Orago AS, Udhayakumara V. A low interleukin-10 tumor necrosis factor-α ratio is associated with malaria anemia in children residing in a holoendemic malaria region in Western Kenya. J Infect Dis. 1999;179:279–82. doi: 10.1086/314548. [DOI] [PubMed] [Google Scholar]
- 47.Dodoo D, Omer FM, Todd J, Akanmori BD, Koram KA, Riley EM. Absolute levels and ratios of proinflammatory and anti-inflammatory cytokine production in vitro predict clinical immunity to Plasmodium falciparum malaria. J Infect Dis. 2002;185:971–9. doi: 10.1086/339408. [DOI] [PubMed] [Google Scholar]
- 48.Kumaratilleke LM, Ferrante A, Rzepczyk CM. The role of T lymphocytes in immunity to Plasmodium falciparum. Enhancement of neutrophil-mediated parasite killing by lymphotoxin and IFN: comparisons with tumor necrosis factor effects. J Immunol. 1991;146(2):762–7. [PubMed] [Google Scholar]
- 49.Wozencraft AO, Dockrell HM, Taverne J, Targett GA, Playfare JH. Killing of human malaria parasites by macrophage secretory products. Infect Immun. 1984;43(2):664–9. doi: 10.1128/iai.43.2.664-669.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilson AG, Gordon C, Di Giovine FS, de Vries N, van der Putte LB, Emery P, et al. A genetic association between systemic lupus erythematosus and tumor necrosis factor alpha. Eur J Immunol. 1994;24(1):191–5. doi: 10.1002/eji.1830240130. [DOI] [PubMed] [Google Scholar]
- 51.Mergani A, Khamis AH, Haboor AB, Hashim E, Gumma M, Awadelseed B, et al. Lack of association between −308 tumor necrosis factor polymorphism and susceptibility to cerebral malaria among Central Sudanese children. Int J Genetics and Mol Biol. 2010;2(5):67–71. [Google Scholar]


