Abstract
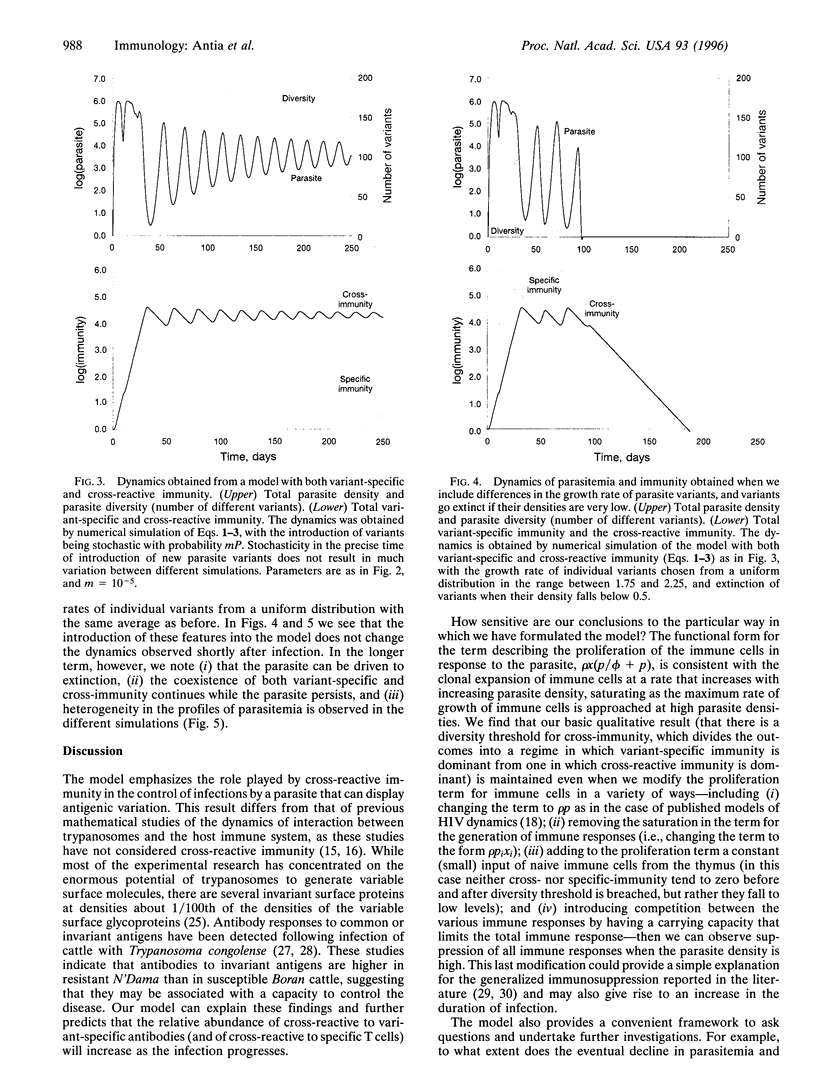
Many parasites exhibit antigenic variation within their hosts. We use mathematical models to investigate the dynamical interaction between an antigenically varying parasite and the host's immune system. The models incorporate antigenic variation in the parasite population and the generation of immune responses directed against (i) antigens specific to individual parasite variants and (ii) antigens common to all the parasite variants. Analysis of the models allows us to evaluate the relative importance of variant-specific and cross-reactive immune responses in controlling the parasite. Early in the course of infection within the host, when parasite diversity is below a defined threshold value (the value is determined by the biological properties of the parasite and of the host's immune response), the variant-specific immune responses are predominant. Later, when the parasite diversity is high, the cross-reactive immune response is largely responsible for controlling the parasitemia. It is argued that increasing antigenic diversity leads to a switch from variant-specific to cross-reactive immune responses. These simple models mimic various features of observed infections recorded in the experimental literature, including an initial peak in parasitemia, a long and variable duration of infection with fluctuating parasitemia that ends with either the clearance of the parasite or persistent infection.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agur Z., Abiri D., Van der Ploeg L. H. Ordered appearance of antigenic variants of African trypanosomes explained in a mathematical model based on a stochastic switch process and immune-selection against putative switch intermediates. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9626–9630. doi: 10.1073/pnas.86.23.9626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R. M., May R. M., Gupta S. Non-linear phenomena in host-parasite interactions. Parasitology. 1989;99 (Suppl):S59–S79. doi: 10.1017/s0031182000083426. [DOI] [PubMed] [Google Scholar]
- Authié E., Muteti D. K., Williams D. J. Antibody responses to invariant antigens of Trypanosoma congolense in cattle of differing susceptibility to trypanosomiasis. Parasite Immunol. 1993 Feb;15(2):101–111. doi: 10.1111/j.1365-3024.1993.tb00589.x. [DOI] [PubMed] [Google Scholar]
- Barry J. D. Antigenic variation during Trypanosoma vivax infections of different host species. Parasitology. 1986 Feb;92(Pt 1):51–65. doi: 10.1017/s0031182000063447. [DOI] [PubMed] [Google Scholar]
- Barry J. D., Turner C. M. The dynamics of antigenic variation and growth of African trypanosomes. Parasitol Today. 1991 Aug;7(8):207–211. doi: 10.1016/0169-4758(91)90143-c. [DOI] [PubMed] [Google Scholar]
- Borst P. Molecular genetics of antigenic variation. Immunol Today. 1991 Mar;12(3):A29–A33. doi: 10.1016/S0167-5699(05)80009-X. [DOI] [PubMed] [Google Scholar]
- Gray D., Skarvall H. B-cell memory is short-lived in the absence of antigen. Nature. 1988 Nov 3;336(6194):70–73. doi: 10.1038/336070a0. [DOI] [PubMed] [Google Scholar]
- Gupta S., Trenholme K., Anderson R. M., Day K. P. Antigenic diversity and the transmission dynamics of Plasmodium falciparum. Science. 1994 Feb 18;263(5149):961–963. doi: 10.1126/science.8310293. [DOI] [PubMed] [Google Scholar]
- Hellriegel B. Modelling the immune response to malaria with ecological concepts: short-term behaviour against long-term equilibrium. Proc Biol Sci. 1992 Dec 22;250(1329):249–256. doi: 10.1098/rspb.1992.0156. [DOI] [PubMed] [Google Scholar]
- Kemp D. J., Cowman A. F., Walliker D. Genetic diversity in Plasmodium falciparum. Adv Parasitol. 1990;29:75–149. doi: 10.1016/s0065-308x(08)60105-0. [DOI] [PubMed] [Google Scholar]
- Kosinski R. J. Antigenic variation in trypanosomes: a computer analysis of variant order. Parasitology. 1980 Apr;80(2):343–357. doi: 10.1017/s0031182000000809. [DOI] [PubMed] [Google Scholar]
- Meyerhans A., Cheynier R., Albert J., Seth M., Kwok S., Sninsky J., Morfeldt-Månson L., Asjö B., Wain-Hobson S. Temporal fluctuations in HIV quasispecies in vivo are not reflected by sequential HIV isolations. Cell. 1989 Sep 8;58(5):901–910. doi: 10.1016/0092-8674(89)90942-2. [DOI] [PubMed] [Google Scholar]
- Moxon E. R., Rainey P. B., Nowak M. A., Lenski R. E. Adaptive evolution of highly mutable loci in pathogenic bacteria. Curr Biol. 1994 Jan 1;4(1):24–33. doi: 10.1016/s0960-9822(00)00005-1. [DOI] [PubMed] [Google Scholar]
- Nash T. Surface antigen variability and variation in Giardia lamblia. Parasitol Today. 1992 Jul;8(7):229–234. doi: 10.1016/0169-4758(92)90119-m. [DOI] [PubMed] [Google Scholar]
- Nowak M. A., Anderson R. M., McLean A. R., Wolfs T. F., Goudsmit J., May R. M. Antigenic diversity thresholds and the development of AIDS. Science. 1991 Nov 15;254(5034):963–969. doi: 10.1126/science.1683006. [DOI] [PubMed] [Google Scholar]
- Nowak M. A., May R. M., Anderson R. M. The evolutionary dynamics of HIV-1 quasispecies and the development of immunodeficiency disease. AIDS. 1990 Nov;4(11):1095–1103. doi: 10.1097/00002030-199011000-00007. [DOI] [PubMed] [Google Scholar]
- Overath P., Chaudhri M., Steverding D., Ziegelbauer K. Invariant surface proteins in bloodstream forms of Trypanosoma brucei. Parasitol Today. 1994 Feb;10(2):53–58. doi: 10.1016/0169-4758(94)90393-x. [DOI] [PubMed] [Google Scholar]
- Roberts D. J., Craig A. G., Berendt A. R., Pinches R., Nash G., Marsh K., Newbold C. I. Rapid switching to multiple antigenic and adhesive phenotypes in malaria. Nature. 1992 Jun 25;357(6380):689–692. doi: 10.1038/357689a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saag M. S., Hahn B. H., Gibbons J., Li Y., Parks E. S., Parks W. P., Shaw G. M. Extensive variation of human immunodeficiency virus type-1 in vivo. Nature. 1988 Aug 4;334(6181):440–444. doi: 10.1038/334440a0. [DOI] [PubMed] [Google Scholar]
- Shapiro S. Z., Murray M. African trypanosome antigens recognized during the course of infection in N'dama and Zebu cattle. Infect Immun. 1982 Feb;35(2):410–416. doi: 10.1128/iai.35.2.410-416.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sztein M. B., Kierszenbaum F. Mechanisms of development of immunosuppression during Trypanosoma infections. Parasitol Today. 1993 Nov;9(11):424–428. doi: 10.1016/0169-4758(93)90053-i. [DOI] [PubMed] [Google Scholar]
- Turner C. M., Barry J. D. High frequency of antigenic variation in Trypanosoma brucei rhodesiense infections. Parasitology. 1989 Aug;99(Pt 1):67–75. doi: 10.1017/s0031182000061035. [DOI] [PubMed] [Google Scholar]
- Vickerman K. On the surface coat and flagellar adhesion in trypanosomes. J Cell Sci. 1969 Jul;5(1):163–193. doi: 10.1242/jcs.5.1.163. [DOI] [PubMed] [Google Scholar]
- Vickerman K. Trypanosome sociology and antigenic variation. Parasitology. 1989;99 (Suppl):S37–S47. doi: 10.1017/s0031182000083402. [DOI] [PubMed] [Google Scholar]
- Wiley D. C., Skehel J. J. The structure and function of the hemagglutinin membrane glycoprotein of influenza virus. Annu Rev Biochem. 1987;56:365–394. doi: 10.1146/annurev.bi.56.070187.002053. [DOI] [PubMed] [Google Scholar]
- Wise K. S. Adaptive surface variation in mycoplasmas. Trends Microbiol. 1993 May;1(2):59–63. doi: 10.1016/0966-842X(93)90034-O. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer R. J., Boerlijst M. C. Diversity and virulence thresholds in AIDS. Proc Natl Acad Sci U S A. 1994 Jan 18;91(2):544–548. doi: 10.1073/pnas.91.2.544. [DOI] [PMC free article] [PubMed] [Google Scholar]



