Abstract
Plasmodium vivax is now recognized as a cause of severe and fatal infection in many parts of the world. This prospective observational study was undertaken in a tertiary health setting to understand the spectrum of the disease burden and associated complications due to P. vivax malaria in central India. A malaria clinic under Regional Medical Research Centre for Tribals is operational at Netaji Subhash Chandra Bose Medical College and Hospital, Jabalpur in central India, where all fever cases and cases with history of fever were referred for screening of malaria parasite by microscopy and rapid diagnostic test kits. Confirmation of all the cases was done by PCR targeting 18s ribosomal RNA gene of the parasite to exclude mixed infection with P. falciparum. Severe vivax malaria was found in 22 (11.1%) out of 198 vivax patients. Cerebral malaria, seizures, severe malaria anaemia, and respiratory distress each were observed in 32% subjects. Multi-organ dysfunction syndrome was common (36%). Mortality was recorded in two patients and neurological sequelae were also observed in two patients at the time of discharge. This is the first report from Central India where P. vivax has been shown to be associated with severe signs of malaria. Severe vivax malaria is a relatively new clinical entity and further studies from different parts of the world are needed to understand clinical spectrum and burden of P. vivax not only for successful treatment, but also for designing and developing effective malaria control measures.
Keywords: Plasmodium vivax, Severe malaria, Central India
Introduction
Global burden of Plasmodium vivax malaria is estimated to be 80 to 300 million clinical cases per year. Nearly 2.5 billion people worldwide are at risk of developing the disease.1 Being the largest contributor of malaria in the South East Asia region, India contributes a significant burden of P. vivax malaria in the world as about 50% of the total malaria in India is contributed by P. vivax.2 The infection due to P. vivax poses a greater challenge to roll back malaria programme as transmission of this parasite is hard to control, largely because of dormant hypnozoite stages.3 Hypnozoites in the liver can cause multiple disease relapses which occur in about 30% of cases without specific treatment.4
Recent studies have broken the myth that human vivax malaria is benign in nature.5,6 Although malaria researchers have made consistent progress towards understanding the biological basis of human severe malaria due to a more common cause i.e. P. falciparum, emerging potential of other non falciparum species as a cause of severe manifestations in humans is the new challenge. Plasmodium vivax in human is now recognized as a cause of severe and fatal malaria despite the facts of low parasite biomass (rarely exceed 2%), scanty sequestration evidences, and increased deformability of red blood cells (RBCs) compared to the P falciparum malaria parasite.7
Madhya Pradesh, located in the central part of India, stands among five highly malarious states in India,8 where both P. vivax and P. falciparum are equally prevalent. A series of 22 cases of vivax malaria is being presented in this report among whom chances of co-infection with P. falciparum were ruled out using microscopy, bivalent rapid diagnostic test kits (RDTs), and polymerase chain reaction (PCR). Precise description of P. vivax associated severe malaria cases helps to fill key gaps in our knowledge of its emerging potential as a cause of severe infection in different regions of the world where P. vivax malaria is endemic.
Material and Methods
Study site
A malaria clinic under Regional Medical Research Centre for Tribals (RMRCT) is operational at Netaji Subhash Chandra Bose (NSCB) Medical College and Hospital, where all fever cases and cases with history of fever (both indoor and outdoor patients) were referred for malaria diagnosis. This hospital serves as a tertiary health care facility for adjacent seven districts around Jabalpur. Catchment population of this hospital is around 12 million. Approval of the institutional ethics board (RMRCT and NSCB Hospital) and consent of the patient’s/guardian were obtained.
Study procedure
This prospective observational study was conducted on patients admitted with malaria. Diagnosis of malaria was made by peripheral blood smears and RDTs as described earlier.9,10 Reports of the blood smear were immediately provided to the hospital staff for initiation of the treatment. Parasite density was calculated in thick smear against 200 WBCs and parasite counts were multiplied by 40 (multiplication factor), assuming a total WBCs count of 8000/mm.10 PCR confirmation was done by targeting 18s ribosomal RNA gene of the parasite. Genomic DNA was isolated from blood using commercially available kits (HiPurA™ Blood Genomic DNA Miniprep Purification Spin Kit, HIMEDIA®, Mumbai, India) in accordance with the manufacturer's protocol. Species specific nested PCR was carried out to diagnose the malaria parasite using the 18s rRNA gene. Briefly, a two step PCR approach was utilized. The primary PCR was carried out for Plasmodium genus amplification and species specific nested PCR amplification for the detection of P. falciparum, P. vivax, P. malariae, and P. ovale by using primary PCR product as DNA template.11
Other laboratory investigations which were done in all patients with severe malaria included complete blood cell counts, blood glucose, blood urea, serum creatinine, and serum bilirubin. Depending upon the clinical manifestations, other specific tests included like arterial blood gas analysis for acute respiratory distress (RD), cerebrospinal fluid examination, ultrasonography of the abdomen, and specific test for hepatitis B and C in hepatic dysfunction and jaundice. Clinical information regarding patient’s illness history, biochemical and hematological investigations, and treatment details has been taken from hospital records. Patients with co-existent P. vivax and P. falciparum infections were excluded from the study.
Defining complications
Severe vivax malaria in patients was defined as (1) no significant past medical history, (2) availability of clinical and biochemical evidences of complications, (3) presence of only P. vivax parasites in blood smear and HRP-2 (Pf histidine rich protein-2) and pLDH (Plasmodium lactate dehydrogenase) based bivalent RDT (FIRST RESPONSE) negative for P. falciparum and positive for P. vivax10,12 and, (4) confirmation of P. vivax mono infection by PCR. Cerebral malaria (CM) – unarousable coma (Glasgow coma score ≤10) or ≧3 seizures in 24 hours, in exclusion of hypoglycaemia and other encephalopathy’s by appropriate examinations, acute renal failure – serum creatinine >3 mg/dl, severe malaria anaemia – haemoglobin <5 g/dl, jaundice – serum bilirubin >3 mg/dl, hypotension – systolic blood pressure < 80 mm Hg for adults and <50 mm Hg for children (<5 years), thrombocytopenia – platelets<80 000/mm3, respiratory distress – age stratified increased respiratory rate (>32/minute for adults, >40 in children from 5 to 14 years, >50 in children less than 5 years or oxygen saturation <94% according to WHO definition.13,14 Multi-organ dysfunction syndrome (MODS) was defined by the presence of two or more complications, excluding thrombocytopenia. Neurological sequelae were defined as any kind of neurological/neuropsychiatric deficit at the time of discharge of the patient. Spleen infarct was confirmed by relevant clinical features (abdominal pain) and the ultrasonographic examination.
Treatment
Patients were treated promptly by a hospital physician according to hospital policy. All patients received intravenous artesunate daily till patient can swallow oral tablets of artemether-lumefantrine combination (3-day course) with supportive treatment. The supportive management included dextrose administration for hypoglycemia and paracetamol for hyperpyrexia. Additionally, cases of severe anaemia were treated with packed red cell transfusion and cases of severe thrombocytopenia required platelet transfusion. Subjects with seizures were treated with anticonvulsant drugs (phenytoin) and diazepam. Patients with renal failure were treated with fluid and diuretic therapy and one case required renal replacement therapy in the form of hemodialysis. Respiratory distress was managed with airway breathing in which nebulization/moist oxygenation was given. One patient was given ventilator support. Patients were followed up till discharge or death.
Statistical analysis
Data was entered into a Microsoft Excel (Redmond, WA) worksheet and data analysis was done using computer STATA-8.2 (Statacorp. College Station, TX, USA).
Results
As part of the hospital malaria surveillance at Medical College Hospital from 2008 to 2012, a total of 9191 patients with febrile illness were screened, out of which 581 were positive for malaria. Among positive cases, 383 had P. falciparum malaria (Adult; M = 97, F = 67 and Children; M = 135, F = 84) and 198 had P. vivax infection (Adults; M = 35, F = 33 and Children; M = 85, F = 45). Severe vivax malaria was found in 22 of 198 vivax patients (11.1%). The presence of P. vivax mono infection was confirmed by PCR (Fig. 1). Additionally, one pregnant woman with severe anaemia, an adult cerebral malaria patient with a history of tuberculosis, four sickle cell anaemia cases with severe signs of vivax infection and, four confirmed cases of mixed infection by PCR were also found, but these cases were not included in the analysis. Severe P. falciparum malaria was diagnosed in 160 cases out of 383 falciparum malaria patients (41.7%).
Figure 1.
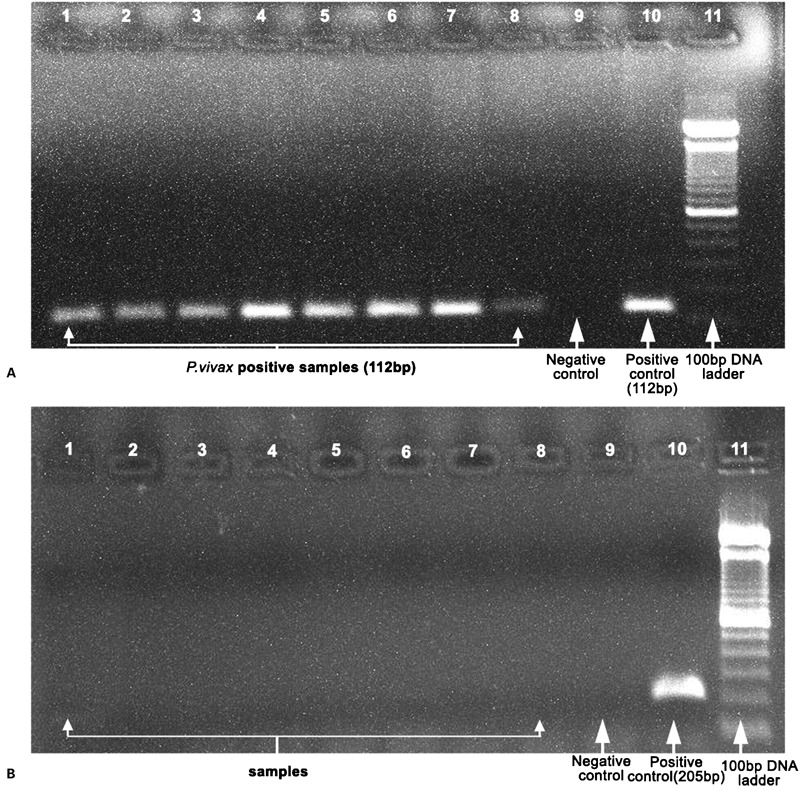
Gel picture showing the PCR amplification of Plasmodium vivax (1A, 112 bp) and P. falciparum (1B, 205 bp). Samples are in lane 1–8, lane 9 is negative control, lane 10 is positive control and lane 11 is 100 bp DNA ladder.
Analysis of severe vivax malaria (N = 22, M/F = 12/10) revealed that 50% patients were under 5 years of age, 27% in the age group of >5–10 years and only 23% were above 10 years of age (Table 1). Out of 22 severe vivax patients, seven patients have satisfied the WHO criteria for CM (32%). Seizures, severe malaria anaemia and RD each were observed in 32% subjects. Thrombocytopenia was observed in 50% subjects (7/14) (Table 1). Other complications such as jaundice and renal failure were observed in 13.6% subjects each and the frequency of gastrointestinal bleeding (GI bleed) was 4.5%. Mortality was recorded in two patients and both of them had CM and RD as common complications. At the time of discharge neurological sequelae were also recorded in two subjects. High parasite density (120000 parasites/μl) in peripheral blood smears was found only in one case. This patient was also having severe anaemia, thrombocytopenia, and splenic infarct.
Table 1. Clinical characteristics of hospitalized P. vivax malaria cases in a tertiary health facility of central India.
| Cases | Age in years | Sex | Fever history/pretreatment | Seizures/GCS | Hb (g/dl) | TLC | Platelets | Bilirubin (mg%) | Creatinine (mg%) | Parasite (per μl) | Complications | Outcome |
| 1 | 3 | F | 4 days/N | Yes/5 | 8 | 9300 | 120000 | na | 1.07 | 1600 | CM, RD | Died |
| 2 | 4 | M | 4 days/N | Yes/7 | 10 | 5400 | 54000 | na | na | 4520 | CM, Thp | Discharge |
| 3 | 32 | F | 3 days/Y | Yes/12 | 11 | na | na | na | na | 5960 | – | Discharge |
| 4 | 10 | M | 3 days/N | Yes/9 | 7.1 | 6100 | 78000 | na | na | 5280 | CM, Thp | Discharge |
| 5 | 6 | M | 2 days/N | Yes/12 | 10 | 4500 | 170000 | na | 0.67 | 4600 | – | Altered behaviour at discharge |
| 6 | 2 | M | 5 days/N | Yes/8 | 8.5 | 4900 | 81000 | na | 0.97 | 6840 | CM, RD | Discharge |
| 7 | 2 | M | 5 days/N | No/14 | 6.3 | 18000 | 54000 | na | na | 1800 | RD, Thp | Discharge |
| 8* | 4 | F | 8 days/Y | No/10 | 2.3 | na | 88000 | na | 6.37 | 200 | CM, RD, RF, SMA | Left against advise |
| 9 | 8 | M | 7 days/Y | No/14 | 7.8 | 8400 | 100000 | na | 4.99 | 240 | RD, RF, GI bleed | Discharge |
| 10 | 18 | M | 4 days/N | No/14 | 10.0 | na | na | 3.8 | na | 9400 | J | Discharge |
| 11 | 16 | M | 4 days/N | No/14 | 10.6 | na | 60000 | 3.4 | 0.9 | 720 | J, Thp | Discharge |
| 12 | 40 | F | 8 days/N | No/13 | 3.8 | 17600 | 59000 | na | na | 120000 | Spleen infarct, SMA,Thp | Discharge |
| 13 | 10 | F | 3 days/N | No/14 | 8.0 | na | na | na | na | 33000 | RD | Discharge |
| 14 | 3 | M | 7 days/Y | No/14 | 6.4 | 11300 | 180000 | na | na | 6000 | RF, Severe dehydration | Discharge |
| 15 | 9 | M | 7 days/N | No/14 | 8.2 | 4900 | 33000 | na | na | 8480 | RD, Thp | Discharge |
| 16 | 5 | F | 5 days/Y | No/9 | 9.2 | 11400 | 48000 | na | na | 45000 | CM, S, Thp | Prostration(unable to sit or walk without support) at discharge |
| 17 | 2 | M | 4 days/N | Yes/14 | 7.2 | na | na | na | na | 60000 | Many seizures (CM) | Discharge |
| 18 | 2 | F | 4 days/N | No/14 | 4.2 | na | na | 3.2 | na | 9000 | SMA, J | Discharge |
| 19 | 9 | F | 3 days/N | No/14 | 2.1 | na | na | na | na | 1800 | SMA | Discharge |
| 20 | 20 | M | 3 days/N | No/14 | 3.9 | na | na | na | na | 1920 | SMA | Discharge |
| 21 | 3 | F | 3 days/Y | No/14 | 4.8 | na | na | na | na | 920 | SMA | Discharge |
| 22 | 1 | F | 5 days/N | No/14 | 4.2 | 11000 | 166000 | na | na | 36000 | SMA | Discharge |
Patient died after 2 weeks at home; na = not available, CM = Cerebral malaria, J = Jaundice, RF = Renal failure, RD = Respiratory distress, S = Shock, SMA = Severe malaria anaemia, Thp = Thrombocytopenia, na No complication present other than seizure, GCS = Glasgow coma score.
In adults, cerebral signs were found in only single female patient who gave history of one episode of seizure followed by impaired consciousness for 6 hours, but the patient regained consciousness shortly after intravenous dextrose infusion (50%) in the hospital. Because of the doubtful coma score, this patient was not classified as CM. Multi-organ dysfunction syndrome was common in severe vivax subjects (36.4%), four in CM cases and four in non-cerebral severe malaria cases. These patients took 2–3 weeks to recover completely. Cerebral malaria and RD together had the worst prognosis.
Discussion
Classically P. vivax causes an acute febrile illness with no complications or death. However, in recent years complications due to P. vivax are being increasingly reported from different parts of the world.15,16 The reason for appearance of severe vivax malaria in many parts of the world may be linked with declining efficacy of chloroquine, global warming, and lack of primaquine alternative due to its long course of treatment (14 days) for liver stage clearance of infection.13 Further it is only in 1990s that clinical laboratory parameters of severe malaria (severe anaemia and RD) other than coma is defined.5,17 Severe anaemia and RD are commonly found in P. vivax malaria.18 A molecular tool like PCR is now widely used in malaria research. Moreover, large-scale disease surveillance system in different epidemiological settings has also improved the ability to capture unexpected trends.5
The complications that are seen with P. vivax, in this study are similar to those seen often with P. falciparum. These include thrombocytopenia, severe anaemia, jaundice, RD, renal impairment, and cerebral involvement. Fever is one feature that is almost invariably present during a malaria paroxysm in all these cases followed by anaemia/severe anaemia. Pathogenesis of severe anaemia is multifactorial. It may be due to red blood cell destruction, phagocytosis of non-parasitized red cells, increased splenic clearance, and dyserythropoiesis in bone marrow.19 Interestingly, thrompocytopenia was not found associated with abnormal bleeding in this study. Median 3 days were required for normalization of platelet count after starting therapy. Jaundice is also multi-factorial and it returns to normal after treatment. The mean duration of recovery from jaundice in this study was about 1 week. Exact pathogenesis and organ specific morbidity caused by P. vivax infection remains unrecognized and poorly studied because of a paucity of research in this area.
Splenomegaly is considered a typical finding in the physical examination of a patient with malaria.20 However, splenic infarct/spleen rupture is an uncommon complication in P. vivax which may occur due to severe infection and during primary attack.21 The probable cause for splenic infarction in one patient in this study may be sudden enlargement of the spleen due to severe infection as parasitaemia was unusually high with subsequent hypoxic injury and the patient had to undergo surgical intervention. This is uncommon in chronically enlarged spleen.20 Thus, patient with vivax malaria referring abdominal pain should be investigated for this complication.22
Case fatality rate due to P. vivax was between 1.6 and 3.8% among children.6,18 However in adults case fatality rate was 9.5% in a tertiary care centre in Mumbai.23 Multi-organ dysfunction syndrome in this study was present in 36% subjects while Kochar et al. recorded 61% MODS among children in Rajasthan.6 Forms of neurological sequelae are very rarely defined in the literature in vivax malaria although one case of psychosis and another with extreme weakness (unable to walk or sit without assistance) was recorded at the time of discharge in this study. These cases raise the possibility that severe, complicated, and life-threatening vivax malaria may be confused for falciparum malaria and highlights the importance of applying PCR diagnostics in severely ill patients. It is likely that in this hospital based study, the incidence of various complications may be higher than the incidence in the community and this is a limitation of the study.
Further, P. vivax is much less researched than P. falciparum.24 Therefore, systematic documentation of severe vivax malaria is required to better understand the features of severity caused due to P. vivax malaria.25 As efforts diverted to limit transmission of more dangerous P. falciparum malaria parasite in different parts of the world may further increase P. vivax relative to P. falciparum which may expose the population at greater risk of developing severe vivax infection.3,13 Thus every effort to reduce or eliminate the malaria burden must also target P. vivax along with P. falciparum in regions where both species coexist. Severe vivax malaria is a relatively new clinical entity and further studies from different parts of the world are needed to understand clinical spectrum and burden of P. vivax not only for successful treatment, but also for designing and developing effective malaria control measures.
Funding
This study has been carried out as part of the intramural service component.
Authors Contribution
All the authors have contributed significantly in all the fields. NS was involved in the conception and design of the study and drafting of the article, AA did a clinical evaluation of the patients, analysis, and drafting of the manuscript and VJ contributed in acquisition of data, analysis, and interpretation of data and prepared first draft of the manuscript. All the authors have approved the final version of the manuscript.
Acknowledgments
Grateful thanks are due to Dr Praveen Bharti, Scientist C, RMRCT for conducting PCR on severe P. vivax subjects. We thank all the patients and their relatives for their kind consent to take part in the study. VJ is supported by the senior research fellowship from Indian Council of Medical Research, New Delhi during this work.
References
- 1.Mueller I, Galinski MR, Baird JK, Carlton JM, Kochar DK, Alonso PL, et al. Key gaps in the knowledge of Plasmodium vivax, a neglected human malaria parasite. Lancet Infect Dis. 2009;9:555–66 [DOI] [PubMed] [Google Scholar]
- 2.Singh N, Dash AP, Thimasarn K. Fighting malaria in Madhya Pradesh (Central India): are we losing the battle? Malar J. 2009;8:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mendis K, Sina BJ, Marchesini P, Carter R. The neglected burden of Plasmodium vivax malaria. Am J Trop Med Hyg. 2001;64:97–106 [DOI] [PubMed] [Google Scholar]
- 4.Dastur FD. The changing scenario of malaria in India. J Assoc Physicians India. 2012;60:9. [PubMed] [Google Scholar]
- 5.Genton B, D’Acremont V, Rare L, Baea K, Reeder JC, Alpers MP, et al. Plasmodium vivax and mixed infections are associated with severe malaria in children: a prospective cohort study from Papua New Guinea. PLoS Med. 2008;5:e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kochar DK, Tanwar GS, Khatri PC, Kochar SK, Sengar GS, Gupta A, et al. Clinical features of children hospitalized with malaria – a study from Bikaner, northwest India. Am J Trop Med Hyg. 2010;83:981–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anstey NM, Russell B, Yeo TW, Price RN. The pathophysiology of vivax malaria. Trends Parasitol. 2009;25:220–7 [DOI] [PubMed] [Google Scholar]
- 8.WHO. World malaria report. Geneva: World Health Organization; 2010, p. 95 [Google Scholar]
- 9.Jain V, Nagpal AC, Joel PK, Shukla M, Singh MP, Gupta RB, et al. Burden of cerebral malaria in central India (2004–2007). Am J Trop Med Hyg. 2008;79:636–42 [PMC free article] [PubMed] [Google Scholar]
- 10.Singh N, Bharti PK, Singh MP, Mishra S, Shukla MM, Sharma RK, et al. Comparative evaluation of bivalent malaria rapid diagnostic tests versus traditional methods in field with special reference to heat stability testing in Central India. PLoS ONE. 2013;8:e58080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Snounou G, Viriyakosol S, Zhu XP, Jarra W, Pinheiro L, do Rosario VE, et al. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol Biochem Parasitol. 1993;61:315–20 [DOI] [PubMed] [Google Scholar]
- 12.Bharti PK, Silawat N, Singh PP, Singh MP, Shukla M, Chand G, et al. The usefulness of a new rapid diagnostic test, the First Response Malaria Combo (pLDH/HRP2) card test, for malaria diagnosis in the forested belt of central India. Malar J. 2008;7:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kochar DK, Das A, Kochar SK, Saxena V, Sirohi P, Garg S, et al. Severe Plasmodium vivax malaria: a report on serial cases from Bikaner in northwestern India. Am J Trop Med Hyg. 2009;80:194–8 [PubMed] [Google Scholar]
- 14.World Health Organization. Severe falciparum malaria. Trans R Soc Trop Med Hyg. 2000;94:1–75 [PubMed] [Google Scholar]
- 15.Lacerda MV, Mourão MP, Alexandre MA, Siqueira AM, Magalhães BM, Martinez-Espinosa FE, et al. Understanding the clinical spectrum of complicated Plasmodium vivax malaria: a systematic review on the contributions of the Brazilian literature. Malar J. 2012;11:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaikh S, Memon H, Iohano B, Shaikh A, Ahmed I, Baird JK. Severe disease in children hospitalized with a diagnosis of Plasmodium vivax in south-eastern Pakistan. Malar J. 2012;11:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marsh K, Forster D, Waruiru C, Mwangi I, Winstanley M, Marsh V, et al. Indicators of life-threatening malaria in African children. N Engl J Med. 1995;332:1399–404 [DOI] [PubMed] [Google Scholar]
- 18.Tjitra E, Anstey NM, Sugiarto P, Warikar N, Kenangalem E, Karyana M, et al. Multidrug-resistant Plasmodium vivax associated with severe and fatal malaria: a prospective study in Papua, Indonesia. PLoS Med. 2008;6:e128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gehlawat VK, Arya V, Kaushik JS, Gathwala G. Clinical spectrum and treatment outcome of severe malaria caused by Plasmodium vivax in 18 children from northern India. Pathog Glob Health. 2013;107:210–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shukla M, Singh N, Singh MP. Spleen rates and infant parasite rates as surveillance tool for malaria control in remote hard to reach areas of central India. Malar J. 2011;10:381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imbert P, Rapp C, Buffet PA. Pathological rupture of the spleen in malaria: analysis of 55 cases (1958–2008). Travel Med Infect Dis. 2009;7:147–59 [DOI] [PubMed] [Google Scholar]
- 22.Thabah MM, Kumar M, Ramesh A, Suri Subrahmanyam DK, Elangovan S. A case of vivax malaria with splenic infarction. J Vector Borne Dis. 2013;50:74–6 [PubMed] [Google Scholar]
- 23.Nadkar MY, Huchche AM, Singh R, Pazare AR. Clinical profile of severe Plasmodium vivax malaria in a tertiary care centre in Mumbai from June 2010-January 2011. J Assoc Physicians India. 2012;60:11–3 [PubMed] [Google Scholar]
- 24.Kute VB, Trivedi HL, Vanikar AV, Shah PR, Gumber MR, Patel HV, et al. Plasmodium vivax malaria-associated acute kidney injury, India, 2010–2011. Emerg Infect Dis. 2012;18:842–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Limaye CS, Londhey VA, Nabar ST. The study of complications of vivax malaria in comparison with falciparum malaria in Mumbai. J Assoc Physicians India. 2012;60:15–8 [PubMed] [Google Scholar]


