Abstract
Background:
Epidemics of febrile illnesses are often associated with rainy seasons in the tropics. During 2007–2008 an epidemic of dengue was identified in Jamaica based on serological testing of sera.
Methods:
A subset of 3165 of 5400 sera submitted for dengue analysis was tested for Leptospira IgM and malaria IgG using ELISA to determine their role in causing epidemic fever.
Findings:
Seropositivity for dengue, leptospirosis, and malaria were 38.4 and 6.0 and 6.5%, respectively, indicative of three concurrent epidemics. Mixed exposure to all three diseases was rare (0.1%), as were mixed dengue/malaria (2.4%); dengue/leptospirosis (1.6%), and leptospirosis/malaria (0.03%) exposure. Exposure to dengue and malaria appeared to occur most frequently among children while leptospirosis was more common among adults.
Conclusion:
While serological diagnosis definitively established that dengue was the main cause of the epidemic febrile illness, the data suggested that there may be other causes of fever, which may occur simultaneously. Consequently, leptospirosis and malaria should be considered as causes of fever during epidemics of dengue in endemic areas.
Keywords: Epidemic, Seropositive, Febrile illness, Diagnosis, Geographic distribution
Introduction
Dengue, leptospirosis, and malaria are tropical diseases that share similarities on presentation especially in the acute stages and have been increasingly reported as overlapping causes of fever in several countries.1,2 They are frequent causes of epidemics of fever in tropical settings although other etiologies include scrub typhus and viral hepatitis and HIV seroconversion.3,4 Determination of the causative agents of epidemics of fever is important for implementation of control strategies (such as vector and rodent control) and in the case of leptospirosis early treatment which is essential for best clinical outcome with this disease.5
Dengue is endemic in Jamaica and in the wider Caribbean and epidemics of infection continue to be recorded repeatedly.6,7 Exposure to the virus is widespread across Jamaica and one study reported a prevalence of 100% anti-dengue IgG and 3.6% anti-dengue IgM among 277 healthy Jamaicans.8 Furthermore, the prevalence of anti-dengue IgM was 15.4% in a sample of 1647 persons with suspected dengue between 2003 and 2006.9 The authors did not state the reported sensitivity and specificity of the test used or suggest possible causes of fever in dengue-IgM negative suspected cases during the inter-epidemic period.9 Epidemics of dengue are often associated with the more serious forms dengue hemorrhagic fever and dengue shock syndrome (DHF/DSS), which are known to have atypical presentations, thus posing further challenges to the diagnosis of acute febrile illnesses.7
Leptospirosis is the world’s most widespread diagnostic zoonotic infection and is caused by spirochetes of the genus Leptospira. Infection usually results when water or soil contaminated with the urine of an infected animal comes in contact with human skin or mucous membranes.10 Clinical manifestations of leptospirosis can range from a self-limited febrile syndrome to a fatal illness (Weil’s disease) characterized by hemorrhage, renal failure, and jaundice.10 In tropical settings, leptospirosis can be indistinguishable from other febrile illnesses such as malaria or dengue. The disease is increasingly seen as a co-infection with dengue and the need for improved diagnostics has been highlighted.3,4,11–18 Similarly while co-infections with dengue or malaria have not been well studied, their increasing importance has been highlighted.19,20 In particular, there is a need to distinguish between severe malaria (especially with potentially fatal Plasmodium falciparum) and severe dengue in emergency rooms.19 There is also a need to determine whether or not these two insults result in greater morbidity when compared to single infections.
Cases of leptospirosis have been recognized in Jamaica since 1953 with the detection of anti-Leptospira antibodies varying between 50 000 and 100 000 persons by the mid-1960s.21,22 Batchelor et al., in 2012, reported this disease to be endemic with an estimated 153 cases reported annually.23 Jamaica is considered to have one of the highest incidence rates of leptospirosis in the Caribbean, which, in turn, is considered the region with the highest incidence globally.24,25 Despite this, risk factors for exposure in Jamaica have not been fully elucidated although infections are usually associated with Jamaica’s second rainy season between October and December. Low educational level has also been reported as a risk factor among butchers and other slaughterhouse workers.26 Risk factors for clinical leptospirosis in Western Jamaica included exposure to rodents and goats and outdoor labor.27 These factors were shown to be additive, with an increased risk associated with exposure to rodents and goats, outdoor labor and rodents, and outdoor labor and goats.27 On the other hand, knowledge of leptospirosis and its risk factors was protective.27
Malaria continues to be a global concern with 21 countries reporting more than 1 million cases per year, and 280 million people at risk.28 Previously a major cause of disease and death in the Caribbean, Jamaica, among several other Caribbean countries, successfully eradicated malaria during the global DDT campaign of the 1950s and early 1960s.29 Following eradication in the 1960s, Jamaica implemented strict surveillance protocols and has recorded only imported cases associated with travel by nationals or visitors.30 The peak of imported cases was associated with screening and treatment of a large number of Haitian refugees to Jamaica in 2005, which revealed 88 cases of predominantly P. falciparum but with some Plasmodium malariae.30,31 This increase in imported cases was followed by local transmission of malaria in Jamaica (after 45 years of malaria-free status), primarily from the western end of the capital, Kingston, between September 2006 and December 2009. The reintroduction of malaria to Jamaica resulted in the detection and confirmation of 406 cases of P. falciparum.28,30 These cases were tracked by a combination of several active and passive surveillance strategies and prompt treatment. This resulted in the interruption and control of transmission and the termination of the outbreak.
In Jamaica, health services are delivered through four semiautonomous regional health authorities (RHA) that have direct management responsibility within a geographically defined region. The northeast RHA (NERHA) comprises the parishes of St Ann, St Mary, and Portland; the southeast RHA includes Kingston, St Andrew, St Catherine, and St Thomas; the southern RHA comprises St Elizabeth, Manchester, and Clarendon; and the western RHA manages the parishes of Westmoreland, Hanover, St James, and Trelawny.
Since dengue, malaria, and early phase leptospirosis all present non-specific findings of fever, headache, and muscle pain, we hypothesized that all three illnesses may have been causes of fever during the dengue epidemic of 2007–2008. Further, some persons may be subjected to up to three simultaneous insults thereby increasing their morbidity during epidemics of febrile illnesses. The purpose of this study was to determine if leptospirosis and malaria were significant causes of febrile illnesses during the epidemic of dengue in Jamaica during 2007–2008. In addition, we sought to compare the epidemiological profile of seropositivity to the three diseases during the epidemic months and to determine their geographical distribution.
Materials and Methods
Samples
Between July 2007 and February 2008, 5400 blood samples were submitted to the Department of Microbiology at The University of the West Indies for testing for the presence of dengue IgM antibodies. This was part of a fever surveillance initiative by the local Ministry of Health to establish the cause of an apparent increase in cases of fever across the island. Samples were tested for dengue IgM using Dengue Virus Capture DxSelect Kit® (Focus Diagnostics, Cypress, CA, USA) ELISA. The manufacturers reported an overall sensitivity of 96% and specificity of 97% in trials.
For the purpose of this study, a sample of 3165 sera were randomly selected and tested for antibodies to Leptospira using the Leptospira IgM ELISA® (Panbio, Grenoble, France; sensitivity: 96.5%; specificity: 98.5%) and malaria IgG using the CELISA® (Cell Labs Pty, Brookvale, NSW, Australia; sensitivity: 94%; specificity: 100%) . Malaria IgG, rather than IgM, was assessed as it was only considered necessary to confirm exposure given that Jamaica has been malaria free since 1965, and any residual IgM would have waned over these years. All testing was conducted in duplicate according to the instructions of the manufacturers. Results of dengue and leptospirosis were given as positive, negative or equivocal while malaria results were given as positive or negative only. All equivocal results were excluded from analyses in the study.
Data recorded from laboratory records included age, sex, geographic health region of sample collection, and month of the year the sample were submitted. The geographic distribution of cases was based on Jamaica’s RHA of which there are four; the NERHA, the western regional health authority (WRHA), the southern regional health authority (SRHA), and the southeast regional health authority (SERHA) (Fig. 1).
Figure 1.
Map of Jamaica showing regional health authorities (RHA) (courtesy of Ralph Robinson, UWI, Jamaica).
Statistical analyses
Means of absorbance values for all three infections were converted to positive and negative based on the cut points noted in the manufacturers’ data sheets. Equivocal results were noted for those values that occurred between the negative and positive scores for dengue and leptospirosis. Seropositivity for all three infections were compiled and descriptive statistics, χ2, and student’s t-test were used to analyze the data using SPSS ver. 16 for Windows®. A P value of < 0.05 was taken as statistically significant.
Ethical approval
The study was approved by the University of the West Indies/University Hospital of the West Indies/Faculty of Medical Sciences Ethics Committee.
Results
Age and sex distribution
The final sample size analyzed was 2419 after exclusion of equivocal results for dengue or leptospirosis. The mean age of the study population was 21.29 ± 0.30 and the mean age of females (22.20 ± 0.41 years; n = 1327) was significantly higher that the mean age of males (20.30 ± 0.46 years; n = 1092) [t = 3.077; P = 0.002]. The majority of the study population (46.6%) was 18 years old or younger and there was a decline in the population size with age, with the smaller age classes being 40–49 years (6.9%) and ⋛ 50 years (5.8%).
Serological evidence of infection and exposure
The prevalence of dengue infection in the study population was 34.8%, while the prevalence of leptospirosis was 6.5% and that of malaria 6.0%. Prevalence of infection was not different between males and females for leptospirosis (7.0 vs 5.4%, respectively; χ2 = 3.084; P = 0.214) or for malaria (6.4 vs 6.1%, respectively; χ2 = 0.193; P = 0.908). However, males (43.7%) were significantly less likely than females (51.6%) to be exposed to dengue (χ2 = 9.169; P = 0.010). A small proportion (0.2% or 4/2419) of samples were seropositive to all three infections studied. However, there were 47 (1.9%) cases of mixed dengue/leptospirosis, 70 (2.8%) cases of mixed dengue/malaria, and 10 (0.4%) cases of mixed malaria/leptospirosis. Details of the extent of morbidity and mortality were not available. Samples were submitted for surveillance of dengue and patients would have had fever indicative of dengue, and were submitted with minimal data (usually age sex and geographic location). We did not have permission or the resources to examine patients who were seropositive.
Age-prevalence profiles of seropositivity
During the epidemic, seropositivity in children decreased exponentially with increasing age except for leptospirosis, which showed a slight increase with age (Fig. 2A). In fact, seropositivity for leptospirosis continued to rise for all age groups (Fig. 2B); from 3.9% in the youngest age class (18–29 years) to 11.3% in the oldest individuals (⋛ 50 years) [χ2 = 29.901; P = 0.0001]. Children (< 18 years) had the highest prevalence (40.7%) of dengue IgM antibodies while the 18–29 years age class had 32.1% prevalence (Fig. 2B). However, among other age groups, seropositivity remained between 25.6 and 29.0% [χ2 = 36.459; P = 0.0001] (Fig. 2B). Exposure to malaria was also significantly different among age classes (χ2 = 12.29; P = 0.015) with highest seropositivity among the youngest (7.6%) and oldest (6.3%) age classes.
Figure 2.
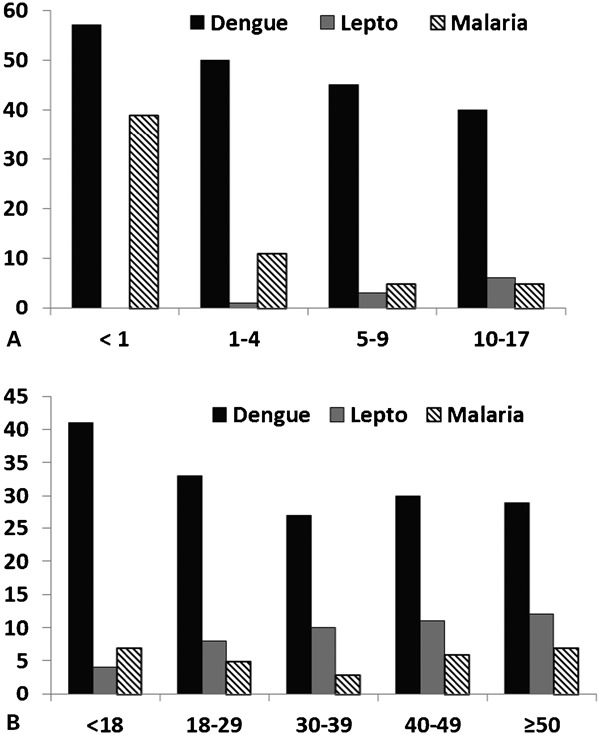
Age-prevalence profile of dengue, leptospirosis, and malaria among (A) Jamaican children and (B) Jamaicans during an epidemic of dengue (2007–2008).
The mean age of persons who were seropositive for leptospirosis (26.71 ± 1.29 years) was significantly higher than that of persons positive for dengue (18.63 ± 0.54 years) and those exposed to malaria (19.15 ± 2.1 years) [F = 17.048; P < 0.0001]. However, there was no significant difference between the ages of persons exposed to malaria and those diagnosed with dengue (P = 0.960).
Temporal pattern of epidemic
The epidemic of febrile illness (notably dengue) began in July 2007 and peaked in October of that year after which there was a decline culminating in December 2007 and a small rise in January 2008 (Fig. 3). Seropositivity to leptospirosis and malaria began to increase in September 2007, three months after the start of the dengue epidemic (Fig. 3). All three causes of febrile illness peaked in October 2007 while both leptospirosis and malaria declined to a low point in December 2007. The start of the febrile illness epidemic coincided with the end of Jamaica’s first rainy season in May/June and peaked in the second rainy season in October. Thereafter, there was a decline with the average rainfall during the study period (Fig. 3).
Figure 3.
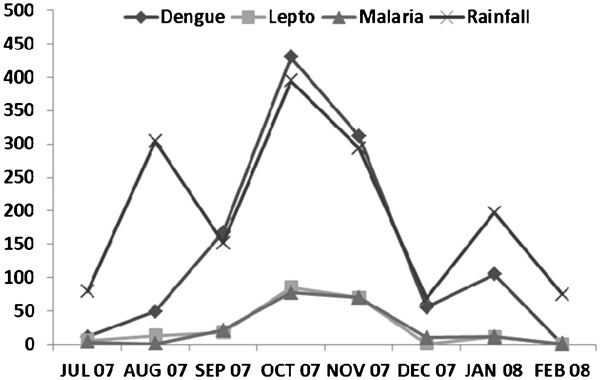
Epidemic curves for dengue, leptospirosis, and malaria among Jamaicans during an epidemic of dengue (2007–2008) and monthly rainfall pattern for Jamaica from July 2007 to February 2008.
Geographical distribution of infection
Overall, there were no significant differences in the seropositivity for dengue IgM antibodies across the four RHAs (χ2 = 3.231; P = 0.357) (Fig. 4). Positive serology for dengue was as follows: for SRHA (37.1%), SERHA (36.0%), WRHA (35.2%), and NERHA (31.0%). In contrast, seropositivity for both leptospirosis and malaria were not homogenous in their geographic distribution. In the case of leptospirosis, seropositivity was highest in SRHA (11.6%), followed by WRHA (6.9%), NRHA (5.7%), and SERHA (5.4%) [χ2 = 25.072; P = 0.0001]. Malaria had highest seropositivity in NERHA (6%), followed by WRHA (5.8%), SERHA (4.6%), and SRHA (3%) [χ2 = 9.00; P = 0.029]. When the data were analyzed by month for the different regions (not shown), only seropositivity to leptospirosis [χ2 = 10.524; P = 0.015] and dengue [χ2 = 7.807; P = 0.050] were significant for October, the peak of the second rainy period.
Figure 4.
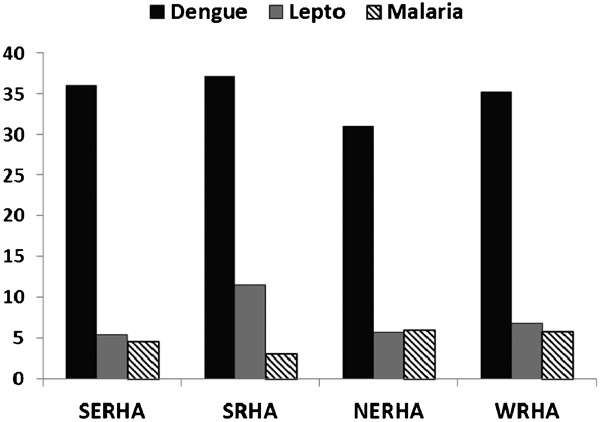
Geographic distribution of dengue, leptospirosis, and malaria in Jamaicans during an epidemic of dengue (2007–2008), Jamaica.
Discussion
The study showed that malaria and leptospirosis were likely contributors to the epidemic of febrile illnesses during the dengue epidemic of 2007–2008 in Jamaica. Both infections were suggested in about 6.0% of a large sample of sera, which were submitted for dengue diagnosis. This confirms the findings from other endemic areas that leptospirosis should be considered as a cause of fever during epidemics of dengue.2,3,12,15,31 The study also highlighted the importance of conducting serological tests for leptospirosis during dengue epidemics since early diagnosis and treatment are crucial for the best clinical outcome.32 The reintroduction and limited spread of malaria in Jamaica provided a unique opportunity to study another possible underlying cause of fever during an epidemic.30 We were able to use serology (IgG) for exposure to malaria in a relatively naïve population to show that this vector-borne protozoan infection was a possible important cause of morbidity during the epidemic. While IgG does not indicate acute infection, it was sufficient to show that persons had been previously exposed to the parasite.
There were no attempts to determine the prevalence of infection with malaria parasites since smears were not available to us and we did not quantify morbidity caused by leptospirosis. While epidemics of jaundice and acute renal failure have been associated with outbreaks of leptospirosis,33 we were unaware of such an outbreak during the 2007–2008 dengue epidemic in Jamaica. It should be noted that leptospirosis diagnosis in Jamaica is primarily carried out by the veterinary services of the Ministry of Agriculture and that Jamaica’s healthcare system could be improved by integrating testing for both leptospirosis and dengue in the same laboratory.34 It was reported that the malaria epidemic was limited to western Kingston and was controlled and it was not unclear if positive IgG status represented antibodies from persons who were already free of malaria.30 As Jamaica moves to re-establish its malaria-free status, serology may play an important role in highlighting geographic regions and at risk groups for malaria. While we were not able to describe the differences in clinical picture of dengue, malaria, and leptospirosis positive patients as we did not collect this data, primarily because of the limits of the study objectives, ethical approval, and resources, the Ministry of Health will use the data in planning future surveillance activities.
The age distribution of the seropositive individuals did not parallel each other. Persons who were exposed to leptospirosis were significantly older than those positive for both malaria IgG and dengue IgM. Evidence of both mosquito-borne diseases was highest in children while leptospirosis seropositivity was highest in adults. This may represent the lack of immunity to dengue in the young who had experienced low rates of exposure in the inter-epidemic period between 1995 and 20076,11 and the fact that leptospirosis is primarily an occupational and recreational disease.10,26 The age-prevalence profile of dengue in Jamaica between 2003 and 2006 did not show a correlation between age or sex of study participants and positive dengue IgM titers. A similar age-prevalence pattern was seen in the 2005 epidemic in Singapore that occurred after successful control of dengue over many years.34,35,36 In the case of malaria, the Jamaican population is likely to be uniformly immunologically naive and high prevalence at the extremes of age may be associated with mosquito biting rates.
The prevalence of Leptospira IgM in suspected dengue cases in the inter-epidemic period of 2002–2007 was reported at 2.5%,11 whereas the current estimate of 6.0% shows a clear epidemic. A study of dengue and leptospirosis among children during an epidemic of the former during in Thailand showed that dengue accounted for 61% of the cases while leptospirosis accounted for 6.0%.37 While leptospirosis showed two epidemic peaks at ages 6 and 11 years, dengue had a single peak at age 9 years. There was a male predominance in cases of leptospirosis. The study also showed that there were no differences in mean age, sex distribution, onset time, and frequency of various symptoms between the two diseases.37
Like age, there were no similarities in the frequency of seropositivity based on the RHA. In the case of dengue positive IgM samples, the distribution was not different across health regions, while leptospirosis seropositive samples were highest in the SRHA, which has predominant farming and large rural communities, and lowest in the health authority with the largest urban population (SERHA). This confirms earlier findings that leptospirosis is primarily a rural disease in Jamaica; however, it must be noted that leptospirosis can shift to become a serious urban disease.13
Conclusion
This study confirms that febrile illnesses in Jamaica can have multiple etiologies, and that co-infections (although of low frequencies) can occur. As these febrile illnesses coincide with the rainy seasons in Jamaica, health education campaigns to reduce mosquito breeding sites and increase rodent control may be effective in reducing further local outbreaks of these diseases.
Acknowledgments
The study was funded by the Culture, Health, Arts, Sports and Education Fund (CHASE Fund), Jamaica. We thank Kadri-Anne Jackson and Carol Khan for technical support and Jascinth Lindo for statistical analysis.
References
- 1.Shah I, Katira B. Clinical and laboratory profile of dengue, leptospirosis and malaria in children: a study from Mumbai. Arch Dis Child. 2007:92:561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zaki SA, Shanbag P. Clinical manifestations of dengue and leptospirosis in children in Mumbai: an observational study. Infection. 2010:38:285–91 [DOI] [PubMed] [Google Scholar]
- 3.Behera B, Chaudhry R, Pandey A, Mohan A, Dar L, Premlatha MM, et al. Co-infections due to Leptospira, dengue and hepatitis E: a diagnostic challenge. J Infect Dev Ctries. 2009:4:48–50 [DOI] [PubMed] [Google Scholar]
- 4.LaRocque RC, Breiman RF, Ari MD, Morey RE, Janan FA, Hayes JM, et al. Leptospirosis during dengue outbreak, Bangladesh. Emerg Infect Dis. 2005:11:766–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watt G, Padre LP, Tuazon ML, Calubaquib C, Santiago E, Ranoa CP, et al. Placebo-controlled trial of intravenous penicillin for severe and late leptospirosis. Lancet. 1988:1:433–5 [DOI] [PubMed] [Google Scholar]
- 6.Castle T, Amador M, Rawlins S, Figueroa JP, Reiter P. Absence of impact of aerial malathion treatment on Aedes aegypti during a dengue outbreak in Kingston, Jamaica. Rev Panam Salud Publica. 1999:5:100–5 [DOI] [PubMed] [Google Scholar]
- 7.Gubler D. The emergence of epidemic dengue fever and dengue hemorrhagic fever in the Americas: a case of failed public health policy. Rev Panam Salud Publica. 2005:17:221–4 [DOI] [PubMed] [Google Scholar]
- 8.Brown MG, Vickers IE, Salas RA, Smikle MF. Seroprevalence of dengue virus antibodies in healthy Jamaicans. Hum Antibodies. 2009:18:123–6 [DOI] [PubMed] [Google Scholar]
- 9.Brown MG, Vickers IE, Salas RA, Smikle MF. Patterns of dengue virus IgM and IgG antibodies in suspected cases of dengue in Jamaica, 2003–2006. Hum Antibodies. 2009:18:29–34 [DOI] [PubMed] [Google Scholar]
- 10.Levett PN. Leptospirosis. Clin Microbiol Rev. 2001:14:296–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown MG, Vickers IE, Salas RA, Smikle MF. Leptospirosis in suspected cases of dengue in Jamaica, 2002–2007. Trop Doct. 2010:40:92–4 [DOI] [PubMed] [Google Scholar]
- 12.Bruce MG, Sanders EJ, Leake JA, Zaidel O, Bragg SL, Aye T, et al. Leptospirosis among patients presenting with dengue-like illness in Puerto Rico. Acta Trop. 2005:96:36–46 [DOI] [PubMed] [Google Scholar]
- 13.Flannery B, Pereira MM, Velloso LdeF, Carvalho CdeC, De Codes LG, Orrico G de S, et al. Referral pattern of leptospirosis cases during a large urban epidemic of dengue. Am J Trop Med Hyg. 2001:65:657–63 [DOI] [PubMed] [Google Scholar]
- 14.Manock SR, Jacobsen KH, de Bravo NB, Russell KL, Negrete M, Olson JG, et al. Etiology of acute undifferentiated febrile illness in the Amazon basin of Ecuador. Am J Trop Med Hyg. 2009:81:146–51 [PubMed] [Google Scholar]
- 15.Ellis T, Imrie A, Katz AR, Effler PV. Underrecognition of leptospirosis during a dengue fever outbreak in Hawaii, 2001–2002. Vector Borne Zoonotic Dis. 2008:8:541–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karande S, Gandhi D, Kulkarni M, Bharadwaj R, Pol S, Thakare J, et al. Concurrent outbreak of leptospirosis and dengue in Mumbai, India, 2002. J Trop Pediatr. 2005:51:174–81 [DOI] [PubMed] [Google Scholar]
- 17.Kaur H, John M. Mixed infection due to Leptospira and dengue. Indian J Gastroenterol. 2002:1:206. [PubMed] [Google Scholar]
- 18.Levett PN, Branch SL, Edwards CN. Detection of dengue infection in patients investigated for leptospirosis in Barbados. Am J Trop Med Hyg. 2000:62:112–4 [DOI] [PubMed] [Google Scholar]
- 19.Carme B, Matheus S, Donutil G, Raulin O, Nacher M, Morvan J. Concurrent dengue and malaria in Cayenne Hospital, French Guiana. Emerg Infect Dis. 2009:15:668–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santana Vdos S, Lavezzo LC, Mondini A, Terzian AC, Bronzoni RV, Rossit AR, et al. Concurrent Dengue and malaria in the Amazon region. Rev Soc Bras Med Trop. 2010:43:508–11 [DOI] [PubMed] [Google Scholar]
- 21.Segree W, Fitz-Henly M, Rawlins J, Bowen-Wright C. Leptospirosis: a review of the Jamaican experience compared with other Caribbean territories. West Indian Med J. 1982:31:54–60 [PubMed] [Google Scholar]
- 22.Bras G. Leptospirosis in Jamaica; case report. West Indian Med J. 1955:4:126–8 [PubMed] [Google Scholar]
- 23.Batchelor T, Stephenson T, Brown PD, Amarakoon D, Taylor MA. Impact of climate variability on the incidence of leptospirosis in Jamaica. Clim Res. 2012:55:79–90 [Google Scholar]
- 24.Everard JD, Everard CO. Leptospirosis in the Caribbean. Rev Med Microbiol. 1993:4:114–22 [Google Scholar]
- 25.Pappas G, Papadimitriou P, Siozopoulou V, Christou L, Akritidis N. The globalization of leptospirosis: worldwide incidence trends. Int J Infect Dis. 2008:12:351–7 [DOI] [PubMed] [Google Scholar]
- 26.Brown PD, McKenzie M, Pinnock M, McGrowder D. Environmental risk factors associated with leptospirosis among butchers and their associates in Jamaica. Int J Occup Environ Med. 2011:2:47–57 [PubMed] [Google Scholar]
- 27.Keenan J, Ervin G, Aung M, McGwin G, Jr, Jolly P. Risk factors for clinical leptospirosis from Western Jamaica. Am J Trop Med Hyg. 2010:83:633–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.WHO. WHO World Malaria Report 2008. Geneva: WHO; 2008. Available from http://malaria.who.int/wmr2008/malaria2008.pdf. [Google Scholar]
- 29.Pan American Health Organization. Status of malaria eradication in the Americas, 18th report. PAHO CSP 18/7 September–October, 1970. Available from http://hist.library.paho.org/English/GOV/CSP/18_7.pdf [Google Scholar]
- 30.Webster-Kerr K, Peter Figueroa J, Weir PL, Lewis-Bell K, Baker E, Horner-Bryce J, et al. Success in controlling a major outbreak of malaria because of Plasmodium falciparum in Jamaica. Trop Med Int Health. 2011:16:298–306 [DOI] [PubMed] [Google Scholar]
- 31.Lindo JF, Bryce JH, Ducasse MB, Howitt C, Barrett DM, Lorenzo Morales J, et al. Plasmodium malariae in Haitian refugees, Jamaica. Emerg Infect Dis. 2007:13:931–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watt G, Jongsakul K, Ruangvirayuth R, Kantipong P, Silpapojakul K. Short report: prospective evaluation of a multi-test strip for the diagnoses of scrub and murine typhus, leptospirosis, dengue fever, and Salmonella typhi infection. Am J Trop Med Hyg. 2005:72:10–2 [PubMed] [Google Scholar]
- 33.Koh BK, Ng LC, Kita Y, Tang CS, Ang LW, Wong KY, et al. The 2005 dengue epidemic in Singapore: epidemiology, prevention and control. Ann Acad Med Singapore. 2008:37:538–45 [PubMed] [Google Scholar]
- 34.Nsubuga P, Nwanyanwu O, Nkengasong JN, Mukanga D, Trostle M. Strengthening public health surveillance and response using the health systems strengthening agenda in developing countries. BMC Public Health. 2010:10:S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goh KT. Dengue – a re-emerging infectious disease in Singapore. Ann Acad Med Singapore. 1997:26:664–70 [PubMed] [Google Scholar]
- 36.Ooi EE, Goh KT, Gubler DJ. Dengue prevention and 35 years of vector control in Singapore. Emerg Infect Dis. 2006:12:887–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Libraty DH, Myint KS, Murray CK, Gibbons RV, Mammen MP, Endy TP, et al. A comparative study of leptospirosis and dengue in Thai children. PLoS Negl Trop Dis. 2007:26:e111. [DOI] [PMC free article] [PubMed] [Google Scholar]


