Abstract
The value of measuring IGF1 bioactivity in active acromegaly is unknown. Soluble Klotho (S-Klotho) level is elevated in active acromegaly and it has been suggested that S-Klotho can inhibit activation of the IGF1 receptor (IGF1R). A cross-sectional study was carried out in 15 patients with active acromegaly based on clinical presentation, unsuppressed GH during an oral glucose tolerance test, and elevated total IGF1 levels (>+2 s.d.). Total IGF1 was measured by immunoassay, IGF1 bioactivity by the IGF1R kinase receptor activation assay and S-Klotho by an ELISA. Quality of Life (QoL) was assessed by Acromegaly QoL (AcroQoL) Questionnaire and Short-Form-36 Health Survey Questionnaire (SF-36). Out of 15 patients, nine had IGF1 bioactivity values within the reference range. S-Klotho was higher in active acromegaly compared with controls. Age-adjusted S-Klotho was significantly related to IGF1 bioactivity (r=0.75, P=0.002) and to total IGF1 (r=0.62, P=0.02). IGF1 bioactivity and total IGF1 were inversely related to the physical component summary of the SF-36 (r=−0.78, P=0.002 vs r=−0.60, P=0.03). Moreover, IGF1 bioactivity, but not total IGF1, was significantly inversely related to the physical dimension of the AcroQoL Questionnaire (r=−0.60, P=0.02 vs r=−0.37, P=0.19). In contrast to total IGF1, IGF1 bioactivity was within the reference range in a considerable number of subjects with active acromegaly. Elevated S-Klotho levels may have reduced IGF1 bioactivity. Moreover, IGF1 bioactivity was more strongly related to physical measures of QoL than total IGF1, suggesting that IGF1 bioactivity may better reflect physical limitations perceived in active acromegaly.
Keywords: IGF1 bioactivity, soluble Klotho, total IGF1, acromegaly, quality of life
Introduction
Acromegaly is characterized by excess secretion of growth hormone (GH) causing multisystem-associated morbidities and increased mortality. GH is considered as the main regulator of circulating total insulin-like growth factor 1 (IGF1). Total IGF1 is therefore routinely used for diagnosis and monitoring treatment of GH deficiency and acromegaly. Nevertheless, discrepancies between clinical findings, GH, and total IGF1 levels are frequently encountered in clinical practice.
Many of the methods used for the measurement of circulating total IGF1 may be hampered by the interferences of insulin-like growth factor (IGF)-binding proteins (IGFBPs) remaining after extraction (1). The most important reason why immunoreactive total IGF1 levels are still used to assess IGF1 bioactivity has been the lack of reliable assays to measure IGF1 bioactivity. An IGF1 receptor (IGF1R)-specific kinase receptor activation (KIRA) assay has been developed as an alternative method to evaluate circulating bioavailable IGF1 (2). The principle of the IGF1R KIRA assay is based on quantification of serum-induced IGF1R phosphorylation in cells transfected with the human IGF1R (2). Compared with current IGF1 immunoassays, this assay theoretically has the advantage of measuring the net effects of serum on IGF1R activation, as it does not ignore the modifying effects of IGFBPs, IGFBP proteases, and other factors that interact between IGFs and the IGF1R (3, 4). One of these factors might be soluble Klotho (S-Klotho). S-Klotho is a protein that has been reported to inhibit IGF1R (and insulin receptor) signaling by inhibiting tyrosine phosphorylation of both receptors and their downstream signaling proteins (i.e. IRS) (5, 6, 7).
Acromegaly is characterized by excessively high GH and (immunoreactive) total IGF1 levels. Recent data suggest that S-Klotho level is also elevated in patients with active acromegaly and that S-Klotho level decreases toward normal following removal of the GH-producing pituitary adenoma (8, 9).
Previously, we found that determination of IGF1 bioactivity may offer advantages in the evaluation of adult GH deficiency compared with total IGF1 (10). In another study, we found that IGF1 bioactivity reflects different aspects of quality of life (QoL) than total IGF1 in GH-deficient patients during GH treatment (11).
The aim of the present study was to investigate the value of IGF1 bioactivity in the evaluation of active acromegaly and in the assessment of QoL in active acromegaly. In addition, we studied whether S-Klotho levels are elevated in active acromegaly and whether a relationship exists between S-Klotho levels and IGF1.
Subjects and methods
Study population
This was an investigator-initiated cross-sectional study. A total of 15 patients, with active acromegaly, were enrolled. Diagnosis of active acromegaly was based on clinical presentation, unsuppressed GH levels during an oral glucose tolerance test (OGTT), and elevated age-matched total IGF1 levels (and radiological detection of a pituitary tumor). Of them, ten patients had a macroadenoma of the pituitary gland and five had a microadenoma. Body weight, height, blood pressure, and ring size were measured. BMI was calculated. The following laboratory assessments were conducted in the fasting state; total IGF1, IGF1 bioactivity, IGFBP1, IGFBP3, glucose, insulin, and S-Klotho. Three questionnaires were used to assess symptoms and QoL (see below).
All 15 patients had no previous history of surgery or radiotherapy for acromegaly. Informed consent was obtained from all subjects and the study was approved by the medical Ethics Committee of Erasmus MC.
Blood measurements
Serum total IGF1, IGFBP3, GH, and insulin were initially measured by solid-phase, enzyme-labeled chemiluminescent immunometric assays (intra-assay coefficients of variation (CV) values were 3.9, 4.4, 3.5, and 3.3–5.5% and inter-assay CV values were 7.7, 6.6, 6.5, and 4.1–7.3% respectively; Immulite 2000 supplied by Siemens Medical Solutions Diagnostics, Los Angeles, CA, USA). Serum IGFBP1 was measured by the ELISA (intra-assay CV value was 7.4% and inter-assay CV value was 6.8%) (Mediagnost GmBH, Reutlingen, Germany). Serum glucose was determined with a standard laboratory method. S-Klotho was determined using a sandwich ELISA described by Yamazaki et al. (12) (Kyowa Hakko Kirin Co. Ltd, Tokyo, Japan) according to the manufacturer's instructions. As control group we measured S-Klotho in 11 subjects with hypopituitarism, who were receiving complete hormone replacement therapies for all present pituitary deficiencies. These subjects appeared to have S-Klotho concentrations that were comparable with those found previously in a healthy population (12).
IGF1 bioactivity was determined by the IGF1R KIRA assay. The IGF1R KIRA assay has been described previously (2, 3). Briefly, the IGF1R KIRA assay uses a HEK cell line that is stably transfected with the human IGF1R gene and quantifies phosphorylation of tyrosine residues of the transfected IGF1R to assess IGF1R stimulating activity. All measurements were done in duplicate. The intra- and inter-assay CV values were <15%.
Total IGF1 concentrations, IGF1 bioactivity, and IGFBP3 were compared with the age-specific normative range values that have been published previously (3, 13). Total IGF1, IGFBP3, and IGF1 bioactivity individual Z-scores were calculated using the following formula: Z-score=(x−average x/s.d.), where x is the actual total IGF1 level or IGFBP3 or IGF1 bioactivity, average x is the mean total IGF1 level or IGFBP3 or IGF1 bioactivity at that age, and s.d. is for the mean at that age. The percentage IGF1 bioactivity of total IGF1 was calculated using the formula: IGF1 bioactivity (pmol/l)/total IGF1 (pmol/l)×100%.
QoL Questionnaires
Acromegaly Quality of Life Questionnaire
The Acromegaly Quality of Life (AcroQoL) Questionnaire comprises 22 questions. Each question has five possible answers scored 1–5, with a total maximum score of 110 and quoted as a percentage. The score of 110 reflects the best possible global AcroQoL score. The 22 questions are divided into two main categories: physical and psychological function. The psychological dimension is subdivided into appearance and personal relationships (14, 15). The AcroQoL Questionnaire has a good internal consistency (Cronbach's α>0.7) (16).
Short-Form-36 Health Survey Questionnaire
The Short-Form-36 Health Survey Questionnaire (SF-36) is a widely used generic measure of health status (17). The mental and physical component summaries (MCS and PCS respectively) of the SF-36 were calculated by standardizing the subscale scores using a linear Z-score transformation using national (Dutch) means and s.d. Then, Z-scores were multiplied by the US subscale factor score coefficients for PCS and MCS and summed over all eight subscales. Finally, T-scores were calculated by multiplying the obtained PCS and MCS sums by ten and adding 50 to the product, to yield a mean of 50 and a s.d. of ten for the norm population (18).
Patient-Assessed Acromegaly Symptom Questionnaire
The Patient-Assessed Acromegaly Symptom Questionnaire (PASQ) assesses symptoms and is a disease-specific questionnaire. It consists of six questions scoring 0–8 and the seventh question addressing the overall health status, based on the other six questions, scoring 0–10 (19). The first six questions evaluate symptoms such as headache, excessive sweating, joint pain, fatigue, soft tissue swelling, and numbness or tingling of the extremities. The maximum score of these six questions is 48 and indicates severe symptoms, with lower scores reflecting milder symptoms.
Statistical analysis
The clinical characteristics of the study population are presented as means with s.d. or ranges. The Kolmogorov–Smirnov test was used to test normality of the variables (data were considered to be normally distributed when P>0.05). For data that do not meet the criteria for normality, logarithmic transformations were applied. Age-adjusted Pearson's correlations were calculated to assess associations. Differences between groups were calculated by the Wilcoxon signed-rank test.
A P value of 0.05 or less was considered statistically significant. Data were analyzed using SPSS 20 for Windows (SPSS, Inc.).
Results
Clinical characteristics of study population
Table 1 lists the clinical characteristics of the study population. Three patients received metformin therapy for type 2 diabetes mellitus. Two of these three patients were treated with insulin and six patients were on antihypertensive treatment. One patient had abnormal renal function and one patient had a history of laryngeal cancer in situ. None of the subjects had abnormal liver functions.
Table 1.
Clinical characteristics of the study population (n=15; 12 males and three females).
| Clinical characteristics | Mean (s.d.) | Range |
|---|---|---|
| Age (years) | 52.8 (13.9) | 24.1–69.7 |
| BMI (kg/m2) | 30.1 (6.2) | 21.4–41.1 |
| Systolic BP (mmHg) | 136 (20) | 109–179 |
| Diastolic BP (mmHg) | 84 (15) | 57–117 |
| Fasting insulin (pmol/l) | 142 (105) | 28–146 |
| Fasting glucose (mmol/l) | 5.5 (0.5) | 4.7–6.3 |
| GH (μg/l) | 7.2 (10.0) | 0.3–29.9 |
| Total IGF1 (nmol/l) | 79.7 (82.6) | 28.0–140.0 |
| IGFBP1 (μg/l) | 1.27 (1.28) | 0.00–4.20 |
| IGFBP3 (mg/l) | 7.0 (1.5) | 4.6–8.7 |
| IGF1 bioactivity (pmol/l) | 589 (237) | 218–1063 |
| S-Klotho (ng/l) | 2291 (2164) | 488–6823 |
| Ring size (mm) | 22.0 (2.5) | 17.5–24.5 |
| Comorbidity (n) | ||
| Secondary hypothyroidism | 3 | |
| Secondary hypogonadism | 3 | |
| Secondary adrenal failure | 1 | |
| Type 2 diabetes | 3 | |
| Hypertension | 6 |
BP, blood pressure.
Mean (median±s.d.) total IGF1 was 79.7 nmol/l (82.6±37.2) and the mean (median±s.d.) Z-score was 10.9 (11.8±6.4). Table 2 lists individual total IGF1 levels, IGF1 bioactivity, and Z-scores of all 15 untreated subjects with active acromegaly. All total IGF1 concentrations were above+2 s.d., which was a prerequisite criterion for the inclusion in this study (Table 2). Mean (median±s.d.) IGF1 bioactivity was 589 pmol/l (545±237); mean (median±s.d.) Z-score for IGF1 bioactivity was 1.77 (1.29±2.15); nine out of 15 patients (60%) had IGF1 bioactivity values within the reference range (Table 2). After excluding the diabetic subjects from the analysis, mean (median±s.d.) total IGF1 was 75.9 nmol/l (76.3±7.1) and the mean (median±s.d.) Z-score was 10.5 (10.7±7.1), while mean (median±s.d.) IGF1 bioactivity was 632 pmol/l (641±243) and the mean (median±s.d.) Z-score was 2.24 (2.19±2.13). The percentage IGF1 bioactivity (mean (median±s.d.) of total IGF1 was 0.81 (0.74±0.30). Mean (median±s.d.) IGFBP3 concentration was 7.0 mg/l (7.5±1.5) mg/l, mean (median±s.d.) Z-score for IGFBP3 was 2.58 (2.95±1.46) s.d. Out of 15 patients, eight had values for IGFBP3 above+2 s.d.
Table 2.
Individual age, total IGF1 levels, IGF1 bioactivity, and Z-scores of 15 subjects with active acromegaly.
| No. | Age (years) | Total IGF1 (nmol/l) | Z-score (s.d.) | IGF1 bioactivity (pmol/l) | Z-score (s.d.) |
|---|---|---|---|---|---|
| 1 | 65.2 | 32.0 | 3.70 | 450 | 1.92 |
| 2 | 54.0 | 40.6 | 4.10 | 545 | 1.10 |
| 3 | 46.5 | 113.1 | 16.40 | 822 | 3.36 |
| 4 | 24.1 | 127.9 | 12.50 | 819 | 2.45 |
| 5 | 59.6 | 140.0 | 23.60 | 1063 | 6.28 |
| 6 | 49.5 | 43.3 | 4.20 | 361 | 0.41 |
| 7a | 28.8 | 101.0 | 10.20 | 466 | −0.07 b |
| 8 | 52.6 | 82.6 | 11.80 | 430 | 0.42 |
| 9 | 68.0 | 95.4 | 17.70 | 713 | 4.55 |
| 10 a | 69.2 | 78.9 | 14.10 | 434 | 1.11 b |
| 11 | 50.8 | 70.0 | 9.50 | 568 | 1.29 |
| 12 | 57.1 | 31.0 | 2.70 | 218 | −1.55 |
| 13 | 38.2 | 104.4 | 13.20 | 770 | 2.56 |
| 14 a | 69.7 | 28.0 | 2.90 | 296 | −0.59 |
| 15 | 59.1 | 107.0 | 17.30 | 828 | 4.11 |
Type 2 diabetes; all three patients were using metformin.
Using insulin.
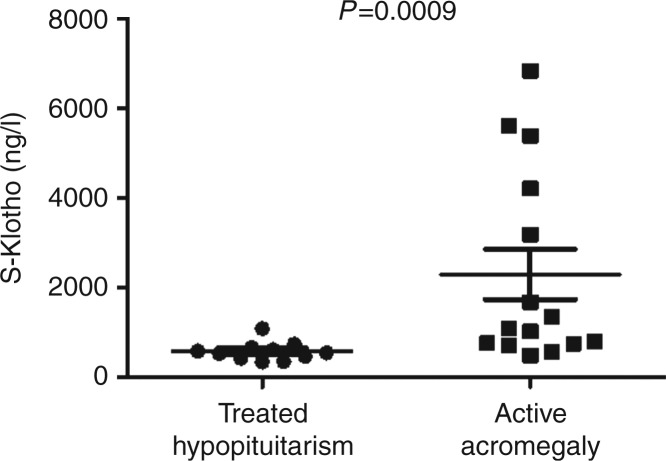
Serum S-Klotho levels in subjects with active acromegaly were significantly higher than that in subjects with hypopituitarism, who were receiving complete hormone replacement therapies for all pituitary deficits (n=11): 2291 ng/l (488–6823) (mean (range)) vs 574 ng/l (341–1084); P=0.0009 (Fig. 1). Thus, in our study, subjects with hypopituitarism had comparable S-Klotho levels as was reported previously in a healthy population (12).
Figure 1.
Mean (±s.e.m.) S-Klotho levels in subjects with active acromegaly (right) and in subjects with hypopituitarism, who were receiving complete hormone replacement therapies for all present pituitary deficiencies (left) (see text for details).
Age-adjusted relationships between IGF1, IGFBPs, GH, and S-Klotho
Total IGF1 was positively related to IGF1 bioactivity (r=0.86, P<0.001; R 2=0.70) and IGFBP3 (r=0.64, P=0.02), while the relationships between total IGF1 and random GH (r=0.52, P=0.07) and between total IGF1 and IGFBP1 (r=−0.55, P=0.05) just missed statistical significance. IGF1 bioactivity was positively related to IGFBP3 (r=0.59, P=0.03) and random GH (r=0.63, P=0.02) and negatively to IGFBP1 (r=−0.58, P=0.04).
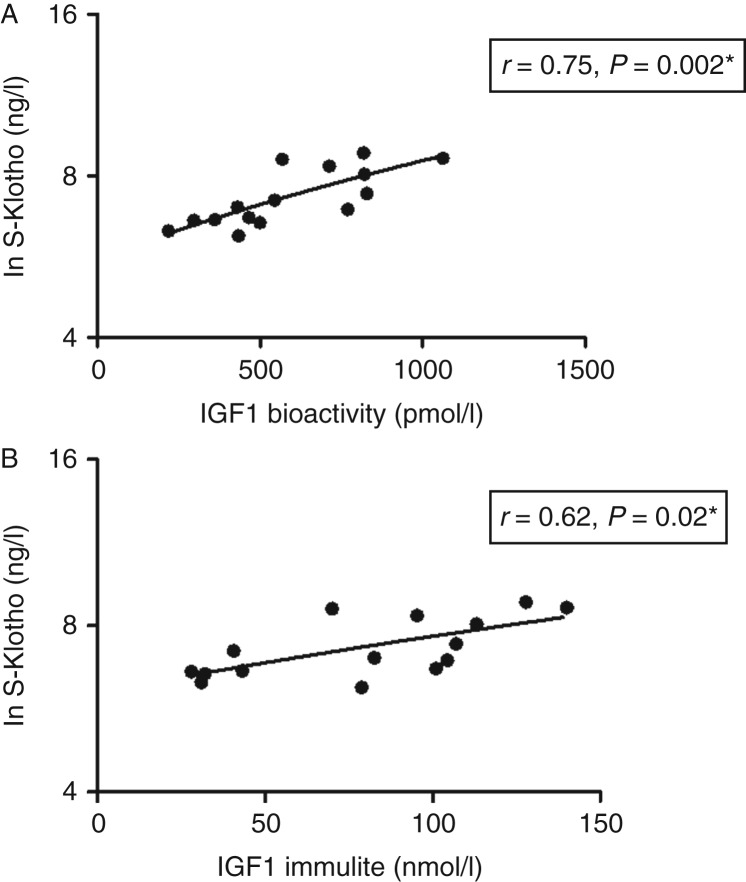
S-Klotho was positively related to IGF1 bioactivity (r=0.75, P=0.002; Fig. 2A) and total IGF1 (r=0.62, P=0.02; Fig. 2B). There were no relationships between S-Klotho, IGFBP3 (r=0.40, P=0.18), IGFBP1 (r=−0.43, P=0.14), and random GH (r=0.37, P=0.21). In addition, the age-adjusted relationships between S-Klotho and insulin (r=0.48, P=0.08) and between S-Klotho and fasting glucose levels (r=0.55, P=0.08) just missed statistical significance.
Figure 2.
Relationships between the natural logarithm of serum S-Klotho (Ln S-Klotho) levels and IGF1 bioactivity (A) and total IGF1 levels (B) in active acromegaly. *Age-adjusted.
QoL measurements and PASQ
The mean (s.d.) and ranges of the PCS of the SF-36, the MCS of the SF-36, the AcroQoL Questionnaire, and the PASQ score in 15 patients with active acromegaly are given in Table 3. The age-adjusted relationships between IGF1 parameters, measures of QoL, and the PASQ are given in Table 4. Both total IGF1 and IGF1 bioactivity were inversely related to the PCS of the SF-36 and were positively related to PASQ. Moreover, IGF1 bioactivity, but not total IGF1, was significantly inversely related to the physical dimension of the AcroQoL Questionnaire. S-Klotho was not related to any of the QoL measurements (data not shown).
Table 3.
PCS of the SF-36, the MCS of the SF-36, the AcroQoL score, and PASQ in 15 patients with active acromegaly.
| Baseline mean (s.d.) | Range | |
|---|---|---|
| SF-36 | ||
| PCS | 45.6 (9.6) | 28.6–58.7 |
| MCS | 43.1 (12.1) | 24.6–55.6 |
| AcroQol Questionnaire | 67 (19) | 32–97 |
| Physical dimension | 28 (9) | 13–40 |
| Psychological dimension | 53 (10) | 32–67 |
| Appearance | 22 (6) | 13–32 |
| Personal relations | 30 (5) | 19–35 |
| PASQ | 21.5 (13.3) | 0.00–46.0 |
SF-36, Short-Form Health Survey-36 Questionnaire; PCS, physical component summaries; MCS, mental component summaries; AcroQol Questionnaire, Acromegaly Quality of Life Questionnaire; PASQ, Patient-Assessed Acromegaly Symptom Questionnaire.
Table 4.
Age-adjusted relations in 15 subjects with active acromegaly between total IGF1 and IGF1 bioactivity, and the PCS of the SF-36, the MCS of the SF-36, the global AcroQol score, and the physical and psychological dimensions of the AcroQol Questionnaire and the PASQ.
| Baseline | Total IGF1 | IGF1 bioactivity | ||
|---|---|---|---|---|
| r | P value | r | P value | |
| SF-36 | ||||
| PCS | −0.60 | 0.03* | −0.78 | 0.002* |
| MCS | −0.02 | 0.95 | −0.18 | 0.55 |
| AcroQol Questionnaire | −0.24 | 0.43 | −0.39 | 0.19 |
| Physical dimension | −0.37 | 0.19 | −0.60 | 0.02* |
| Psychological dimension | −0.13 | 0.68 | −0.19 | 0.53 |
| Appearance | −0.23 | 0.44 | −0.20 | 0.51 |
| Personal relations | 0.01 | 0.98 | −0.16 | 0.61 |
| PASQ | 0.65 | 0.02* | 0.57 | 0.05* |
Statistically significant (*P<0.05). SF-36, Short-Form Health Survey-36 Questionnaire; PCS, physical component summaries; MCS, mental component summaries; AcroQol Questionnaire, Acromegaly Quality of Life Questionnaire; PASQ, Patient-Assessed Acromegaly Symptom Questionnaire.
Discussion
In this cross-sectional study of newly diagnosed patients with active acromegaly based on clinical presentation, unsuppressed GH levels during an OGTT, and elevated age-matched immunoreactive total IGF1 levels, IGF1 bioactivity was within the reference range in a considerable number of patients.
This result suggests that, in subjects with active acromegaly, total IGF1 level is more often elevated than IGF1 bioactivity as all subjects had total IGF1 outside the reference range. However, clinical presentation with elevated age-matched total IGF1 levels was a prerequisite criterion for the inclusion in this study and this may have introduced a selection bias.
Most recent studies have consistently found that IGF1 levels are lower in diabetic patients than in healthy volunteers (20). In our study, three subjects received metformin for type 2 diabetes mellitus and two were additionally treated with insulin. Excluding diabetic subjects from the analysis did not change the mean results for total IGF1. However, the mean IGF1 bioactivity became >+2 s.d. suggesting that diabetes can reduce IGF1 bioactivity in acromegalic subjects.
Previously, we have shown in a healthy population that 70% of the variation in IGF1 bioactivity could not be explained by levels of total IGF1 (3). In this study, the R 2 value was 0.70 suggesting that 30% of the variation in IGF1 bioactivity could not be explained by levels of total IGF1, again demonstrating that IGF1 bioactivity is only partly dependent on total IGF1. In addition, the mean percentage of IGF1 bioactivity over total IGF1 was 0.81% in the 15 subjects with active acromegaly indicating that the IGF1 KIRA assay provides information about the circulating IGF1 system that fundamentally differs from that obtained by IGF immunoassays.
IGF1 bioactivity was within the reference range in a considerable number of subjects. However, this finding does not mean that IGF1 bioactivity was normal for these subjects. The overall variation in the healthy population determines the width of the population-based reference range for IGF1, which is defined by a distribution that includes 95% of the test results in a healthy population (21). As a consequence, any pathological change in IGF1 in a (acromegalic) subject must be very large (id est outside the boundaries of the population-based reference range) to be clinically considered as abnormal. However, the dispersion of ‘normal’ IGF1 in a healthy individual will usually span only a small part of the conventional population-based reference range (22). Therefore, an IGF1 bioactivity value within the reference range may be still abnormal for an individual (and provides arguments for developing specific individual reference range values for IGF1 (bioactivity) in this era of personalized medicine).
Interestingly, our finding that IGF1 bioactivity was within the reference range in a considerable number of patients with active acromegaly is in agreement with the results published in the 1970s and 1980s, which were obtained using classical IGF1 bioassays based on cartilage stimulation (23, 24). In those days, it was already postulated that discrepancies between IGF1 bioassays and total IGF1 immunoassays were reflecting effects of somatomedin inhibitors and other biologically relevant factors that were apparently detected by bioassays but recognized poorly by immunoassays (23).
Recent data suggest that S-Klotho level is dramatically elevated in patients with active acromegaly and decreases toward normal following removal of the GH-producing pituitary adenoma (8, 9). As levels of S-Klotho are markedly elevated in relation to GH excess in acromegaly, it has been suggested that S-Klotho levels depend on GH to a comparable extent as IGF1 (7). In our study, we found elevated S-Klotho levels in active acromegaly. In addition, we observed a significant positive relationship between S-Klotho and IGF1. There are at least two potential explanations for these positive relationships between S-Klotho and IGF1 in our study. First, S-Klotho and IGF1 may both be independent markers for the severity of acromegaly. Another possibility could be that the elevation in S-Klotho levels represents an adaptation mechanism by which the body attenuates increased IGF1 action. In favor of this latter possibility, it has been found that S-Klotho can inhibit the activation of the IGF1R in a dose-dependent manner (5, 25). It suppresses not only ligand-stimulated autophosphorylation of the IGF1R but also activation of signaling events downstream of the IGF1R (5, 26), which in turn may reduce the activation of the IGF1R in patients with active acromegaly. To the best of our knowledge, we are the first to present data suggesting that increased S-Klotho levels are part of a physiological mechanism dampening IGF1 actions in active acromegaly patients.
S-Klotho-induced IGF1R resistance may help to explain why diabetogenic effects of elevated GH levels in active acromegalic subjects are often not completely counteracted and neutralized by IGF1 (27, 28).
As discussed above, the IGF1 KIRA assay, in contrast to total IGF1 immunoassays, quantifies autophosphorylation of the IGF1R. This opens the possibility that the KIRA assay is sensitive to the modifying actions of circulating S-Klotho and this may have contributed to the observed discrepancy between IGF1 bioactivity and total IGF1 in our study.
It has been suggested that patients' perception of QoL cannot be inferred directly from circulating hormone levels (29). However, there was an inverse relationship between the PCS of the SF-36 and IGF1 bioactivity and total IGF1. This latter relationship was stronger for IGF1 bioactivity than for total IGF1. In addition, IGF1 bioactivity, but not total IGF1, was significantly related to the physical dimension of the disease-specific AcroQoL Questionnaire.
Our results suggest that IGF1 bioactivity may better reflect physical limitations perceived by untreated acromegalic patients than total IGF1. In this respect, it would be interesting to measure IGF1 bioactivity, total IGF1, and S-Klotho levels in a group of patients with acromegaly, in which total IGF1 levels remain high during treatment with somatostatin analogs despite improvement in clinical symptoms. Such a study may help to give more insight into the known lack of correlation between total IGF1 and clinical scores during medical therapy of some acromegalic patients.
Both IGF1 bioactivity and total IGF1 were not related to MCS. In this respect, it is important to emphasize that it was previously found that physical function dimensions of the SF-36 are perceived lower in subjects with acromegaly than in the general population, while no differences have been found in the mental dimensions of the SF-36 (30).
Finally, though the disease-specific PASQ still requires validation before it can be used in everyday clinical practice, both IGF1 bioactivity and total IGF1 were significantly related to the PASQ. However, this latter relationship was stronger for total IGF1 than for IGF1 bioactivity.
Although the IGF1 KIRA assay provides a reproducible method for quantitatively assessing IGF1 bioactivity, currently it does not have a clear place in clinical routine due to several limitations such as assay run time, its labor intensiveness, and expenses higher than IGF1 immunoassays. Taking this into consideration, an important step toward implementation of the IGF1 KIRA assay into clinical routine is to run the assays on an automated system so that the assay can be performed with minimal personnel involvement at each step from beginning to end.
It should be stressed that our study has a considerable number of limitations. First of all, only a small number of acromegalic patients were studied and it had a cross-sectional study design. Patients were initially selected on clinical presentation and elevated total IGF1 levels. Moreover, only fasting hormone levels were measured, and there was no information on 24 h GH secretion nor on IGFBP protease activity.
In conclusion, IGF1 bioactivity was within the reference range in a considerable number of patients with active acromegaly in contrast to total IGF1. As the IGF1 KIRA assay, unlike the IGF1 immunoassay, does not ignore the modifying effects of factors that interact between IGFs and the IGF1R, S-Klotho may have reduced IGF1 bioactivity. In addition, IGF1 bioactivity was also more strongly related to physical measures of QoL than total IGF1, suggesting that IGF1 bioactivity may better reflect physical limitations perceived in patients with active acromegaly.
Our study suggests that measuring IGF1 bioactivity in subjects with active acromegaly provides information about the IGF1 system, which fundamentally differs from that obtained by common available IGF immunoassays. Larger studies are needed to confirm our findings.
Author contribution statement
A J Varewijck researched data and wrote the manuscript, A J van der Lely, S W J Lamberts, and L J Hofland reviewed/edited the manuscript and contributed to the discussion, S J C M M Neggers researched data, reviewed/edited the manuscript, and contributed to the discussion, and J A M J L Janssen researched data, wrote the manuscript, and reviewed/edited the manuscript.
Acknowledgements
The authors thank the patients for their participation and the nursing staff of the Clinical Research Unit from the Erasmus MC, Rotterdam, the Netherlands, for their excellent patient care. In addition, they would like to thank P Uitterlinden (Erasmus MC, Rotterdam, The Netherlands) for performing the immunoassays.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This work was supported by an unrestricted grant from Novo Nordisk A/S (Alphen aan de Rijn, The Netherlands). Novo Nordisk A/S had no involvement in the study design and in the collection, analysis, and interpretation of data.
References
- 1.Clemmons DR. IGF-I assays: current assay methodologies and their limitations. Pituitary. 2007;10:121–128. doi: 10.1007/s11102-007-0032-z. [DOI] [PubMed] [Google Scholar]
- 2.Chen JW, Ledet T, Orskov H, Jessen N, Lund S, Whittaker J, De Meyts P, Larsen MB, Christiansen JS, Frystyk J, et al. A highly sensitive and specific assay for determination of IGF-I bioactivity in human serum. American Journal of Physiology. Endocrinology and Metabolism. 2003;284:E1149–E1155. doi: 10.1152/ajpendo.00410.200200410.2002. [DOI] [PubMed] [Google Scholar]
- 3.Brugts MP, Ranke MB, Hofland LJ, van der Wansem K, Weber K, Frystyk J, Lamberts SW, Janssen JA. Normal values of circulating insulin-like growth factor-I bioactivity in the healthy population: comparison with five widely used IGF-I immunoassays. Journal of Clinical Endocrinology and Metabolism. 2008;93:2539–2545. doi: 10.1210/jc.2007-2454. [DOI] [PubMed] [Google Scholar]
- 4.Janssen JA. Insulin-like growth factor I: pros and cons of a bioassay. Hormone Research in Paediatrics. 2011;76(Suppl 1):106–110. doi: 10.1159/000329191. [DOI] [PubMed] [Google Scholar]
- 5.Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, et al. Suppression of aging in mice by the hormone Klotho. Science. 2005;309:1829–1833. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolf I, Levanon-Cohen S, Bose S, Ligumsky H, Sredni B, Kanety H, Kuro-o M, Karlan B, Kaufman B, Koeffler HP, et al. Klotho: a tumor suppressor and a modulator of the IGF-1 and FGF pathways in human breast cancer. Oncogene. 2008;27:7094–7105. doi: 10.1038/onc.2008.292. [DOI] [PubMed] [Google Scholar]
- 7.Chateau MT, Araiz C, Descamps S, Galas S. Klotho interferes with a novel FGF-signalling pathway and insulin/Igf-like signalling to improve longevity and stress resistance in Caenorhabditis elegans . Aging. 2010;2:567–581. doi: 10.18632/aging.100195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sze L, Bernays RL, Zwimpfer C, Wiesli P, Brandle M, Schmid C. Excessively high soluble Klotho in patients with acromegaly. Journal of Internal Medicine. 2012;272:93–97. doi: 10.1111/j.1365-2796.2012.02542.x. [DOI] [PubMed] [Google Scholar]
- 9.Neidert MC, Sze L, Zwimpfer C, Sarnthein J, Seifert B, Frei K, Leske H, Rushing EJ, Schmid C, Bernays RL, et al. Soluble α-klotho: a novel serum biomarker for the activity of GH-producing pituitary adenomas. European Journal of Endocrinology. 2013;168:575–583. doi: 10.1530/EJE-12-1045. [DOI] [PubMed] [Google Scholar]
- 10.Varewijck AJ, Lamberts SW, Uitterlinden P, Hofland LJ, Janssen JA. IGF-I bioactivity better reflects growth hormone deficiency than total IGF-I. Journal of Clinical Endocrinology and Metabolism. 2011;96:2248–2254. doi: 10.1210/jc.2011-0051. [DOI] [PubMed] [Google Scholar]
- 11.Varewijck AJ, Lamberts SW, Neggers SJ, Hofland LJ, Janssen JA. IGF-I bioactivity might reflect different aspects of quality of life than total IGF-I in GH-deficient patients during GH treatment. Journal of Clinical Endocrinology and Metabolism. 2013;98:761–768. doi: 10.1210/jc.2012-2901. [DOI] [PubMed] [Google Scholar]
- 12.Yamazaki Y, Imura A, Urakawa I, Shimada T, Murakami J, Aono Y, Hasegawa H, Yamashita T, Nakatani K, Saito Y, et al. Establishment of sandwich ELISA for soluble α-Klotho measurement: age-dependent change of soluble α-Klotho levels in healthy subjects. Biochemical and Biophysical Research Communications. 2010;398:513–518. doi: 10.1016/j.bbrc.2010.06.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elmlinger MW, Kuhnel W, Weber MM, Ranke MB. Reference ranges for two automated chemiluminescent assays for serum insulin-like growth factor I (IGF-I) and IGF-binding protein 3 (IGFBP-3) Clinical Chemistry and Laboratory Medicine. 2004;42:654–664. doi: 10.1515/CCLM.2004.112. [DOI] [PubMed] [Google Scholar]
- 14.Webb SM, Prieto L, Badia X, Albareda M, Catalá M, Gaztambide S, Lucas T, Páramo C, Picó A, Lucas A, et al. Acromegaly Quality of Life Questionnaire (ACROQOL) a new health-related quality of life questionnaire for patients with acromegaly: development and psychometric properties. Clinical Endocrinology. 2002;57:251–258. doi: 10.1046/j.1365-2265.2002.01597.x. [DOI] [PubMed] [Google Scholar]
- 15.Badia X, Webb SM, Prieto L, Lara N. Acromegaly Quality of Life Questionnaire (AcroQoL) Health and Quality of Life Outcomes. 2004;2:13. doi: 10.1186/1477-7525-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Webb SM. Quality of life in acromegaly. Neuroendocrinology. 2006;83:224–229. doi: 10.1159/000095532. [DOI] [PubMed] [Google Scholar]
- 17.Aaronson NK, Muller M, Cohen PD, Essink-Bot ML, Fekkes M, Sanderman R, Sprangers MA, te Velde A, Verrips E. Translation, validation, and norming of the Dutch language version of the SF-36 Health Survey in community and chronic disease populations. Journal of Clinical Epidemiology. 1998;51:1055–1068. doi: 10.1016/S0895-4356(98)00097-3. [DOI] [PubMed] [Google Scholar]
- 18.Taft C, Karlsson J, Sullivan M. Do SF-36 summary component scores accurately summarize subscale scores? Quality of Life Research. 2001;10:395–404. doi: 10.1023/A:1012552211996. [DOI] [PubMed] [Google Scholar]
- 19.Trainer PJ, Drake WM, Katznelson L, Freda PU, Herman-Bonert V, van der Lely AJ, Dimaraki EV, Stewart PM, Friend KE, Vance ML, et al. Treatment of acromegaly with the growth hormone-receptor antagonist pegvisomant. New England Journal of Medicine. 2000;342:1171–1177. doi: 10.1056/NEJM200004203421604. [DOI] [PubMed] [Google Scholar]
- 20.Chestnut RE, Quarmby V. Evaluation of total IGF-I assay methods using samples from type I and type II diabetic patients. Journal of Immunological Methods. 2002;259:11–24. doi: 10.1016/S0022-1759(01)00478-1. [DOI] [PubMed] [Google Scholar]
- 21.Andersen S, Bruun NH, Pedersen KM, Laurberg P. Biologic variation is important for interpretation of thyroid function tests. Thyroid. 2003;13:1069–1078. doi: 10.1089/105072503770867237. [DOI] [PubMed] [Google Scholar]
- 22.Borofsky ND, Vogelman JH, Krajcik RA, Orentreich N. Utility of insulin-like growth factor-1 as a biomarker in epidemiologic studies. Clinical Chemistry. 2002;48:2248–2251. [PubMed] [Google Scholar]
- 23.Phillips LS, Vassilopoulou-Sellin R. Somatomedins (first of two parts) New England Journal of Medicine. 1980;302:371–380. doi: 10.1056/NEJM198002143020704. [DOI] [PubMed] [Google Scholar]
- 24.Wiedemann E, Uthne E, Tang RG, Spencer M, Saito T, Linfoot JA. Serum somatomedin activity by rat cartilage bioassay and human placental membrane radioreceptor assay in acromegaly and Cushings disease. In: Giordano G, Van Wyk JJ, Minuto F, editors. Somatomedins and Growth. Academic Press; New York: 1979. pp. 289–294. [Google Scholar]
- 25.Wang Y, Chen L, Huang G, He D, He J, Xu W, Zou C, Zong F, Li Y, Chen B, et al. Klotho sensitizes human lung cancer cell line to cisplatin via PI3k/Akt pathway. PLoS ONE. 2013;8:e57391. doi: 10.1371/journal.pone.0057391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bartke A. Long-lived Klotho mice: new insights into the roles of IGF-1 and insulin in aging. Trends in Endocrinology and Metabolism. 2006;17:33–35. doi: 10.1016/j.tem.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 27.Kaplan SA, Cohen P. The somatomedin hypothesis 2007: 50 years later. Journal of Clinical Endocrinology and Metabolism. 2007;92:4529–4535. doi: 10.1210/jc.2007-0526. [DOI] [PubMed] [Google Scholar]
- 28.Janssen JA. Advantages and disadvantages of GH/IGF-I combination treatment. Reviews in Endocrine & Metabolic Disorders. 2009;10:157–162. doi: 10.1007/s11154-008-9081-5. [DOI] [PubMed] [Google Scholar]
- 29.Waters MJ, Brooks AJ. Growth hormone and cell growth. Endocrine Development. 2012;23:86–95. doi: 10.1159/000341761. [DOI] [PubMed] [Google Scholar]
- 30.Johnson MD, Woodburn CJ, Vance ML. Quality of life in patients with a pituitary adenoma. Pituitary. 2003;6:81–87. doi: 10.1023/B:PITU.0000004798.27230.ed. [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a