Abstract
Objective
Efficacy of artemisinin derivatives alone or in combination compared to praziquantel alone for the treatment of urinary schistosomiasis in schoolchildren.
Methods
Randomized clinical trials comparing praziquantel with artemisinin derivatives in the treatment of urinary schistosomiasis in schoolchildren were included. Medline, EMBASE, LILACS, CENTRAL, African Index Medicus, and Scielo were searched. We also analyzed the abstracts of the main conferences on infectious diseases and tropical medicine during the years 2009–2011. Google Scholar and OpenSIGLE were also searched. The last search was performed in July 2012. The primary endpoint was the cure rate. The main outcome data were retrieved using a standardized form; three independent researchers (WP, HC, and SS) performed the search, retrieved data, and evaluated the risk of bias. Disagreements were resolved by discussion. Risk ratios were used and heterogeneity was evaluated. A fixed or random-effects model was used according to the results of heterogeneity testing. An intention-to-treat analysis was done. Data were analyzed using Revman 5.0.24 (Copenhagen: The Nordic Cochrane Centre).
Results
Seven studies were selected for full text review and only five studies were finally included. The cure rate for praziquantel was superior to that of artesunate (RR: 1.66; 95% CI: 1.18–2.33). Artesunate was not clearly superior to placebo (artesunate versus placebo, RR: 3.21; 95% CI: 0.50–20.74). Combination of artesunate with praziquantel could prove more beneficial than praziquantel alone (RR: 1.15; 95% CI: 1.01–1.31). The frequency of adverse events was equivalent for both drugs (praziquantel versus artesunate, RR: 1.11; 95% CI: 0.80–1.55).
Conclusions
Our meta-analysis showed that praziquantel was significantly more effective than artesunate for the treatment of urinary schistosomiasis in schoolchildren. Artesunate at best had a marginal role in combination therapy.
Keywords: Artemisinin derivatives, Schistosomiasis, Praziquantel, Artesunate, Meta-analysis
Introduction
Schistosomiasis is one of the most prevalent helminthic infections in the world. In mid-2003, the population at risk was estimated to be 779 million individuals, and more than 200 million people were infected.1 This parasitosis affects people living in tropical and subtropical areas of Africa, South America, the Middle East, East Asia, and the Philippines. Schistosomiasis is acquired through the skin by contact with cercariae while bathing or wading in fresh water. Chronic infection in endemic areas is associated with liver and intestinal disease (Schistosoma mansoni, Schistosoma intercalatum, Schistosoma japonicum, and Schistosoma mekongi) or kidney and bladder dysfunction (Schistosoma haematobium).
Urinary schistosomiasis is an important cause of morbidity in Africa. It is normally acquired during childhood and up to 60% of schoolchildren have been found to be infected in some settings, even an 80% prevalence has been reported, indicating that schoolchildren is one of the most, if not the most affected group by chronic urinary schistosomiasis.2,3 Chronic infection can lead to chronic anemia, undernutrition, reduced exercise tolerance, and loss of performance.4 Schistosomiasis was responsible for the loss of at least 13–15 million disability-adjusted-life-years in 2004.5 Moreover, squamous cell carcinoma of the bladder is a well-known complication and has been reported as the most frequent cancer in endemic areas.6
The pyrazino-isoquinoline praziquantel is currently the drug of choice for the treatment of urinary schistosomiasis.7 It is administered as a single dose of 40 mg/kg. Praziquantel binds to the beta subunit of voltage-gated Ca2+ ion channels and disrupts Ca2+ homeostasis, although its mechanism of action is not completely understood.8,9 The drug causes tetanic contractions and tegumental vacuoles, which force the worms to detach from the veins and die.10 Praziquantel has been the main treatment for schistosomiasis for decades and is cheap and easy to administer, with an excellent efficacy and safety profile.7,11 It has also been shown that treatment with praziquantel reverses chronic injury caused by schistosomiasis, such as liver fibrosis and urinary tract abnormalities.12–16 Even during pregnancy and breastfeeding, praziquantel seems to be safe, and therapy should be encouraged.17,18 These attributes have made praziquantel the drug of choice in the treatment of schistosomiasis.18
Despite the advantages of praziquantel, massive preventive chemotherapy treatment programs (recommended and implemented by the World Health Organization) based on this agent maintain environmental pressure on the Schistosoma species and make the emergence of resistance a potential threat. In Senegal, the very low cure rates reported for S. mansoni infection are believed to be due to resistance.19 Failure of praziquantel treatment in travelers infected with S. haematobium has also been described.20 This resistance has been found in vivo and in vitro.21 A mechanism for resistance has also been suggested, as low cure rates with praziquantel have been described in one strain of S. mansoni from Egypt that overexpresses the protein SMDR2,22 an ATP-driven drug-efflux protein (homologous to P-glycoprotein) for which praziquantel is both a substrate and an inhibitor.23 Relying only on praziquantel for the treatment of schistosomiasis could prove problematic in the long term, and alternative drugs should be investigated. Similarly, it would be useful to have safe and effective alternative drugs for patients who experience intolerance or toxicity.
Artemisia annua is the source of artemisinin, a sesquiterpene lactone whose derivatives are the first-line choice for the treatment of malaria.24 Artemisinin derivatives have been shown to have antischistosomal activity both in vitro and in animal models.25 Although the mechanism of action is not well understood, the glycogen content of the worms is reduced by a reduction in glucose uptake, an increase in glycogen phosphorylase activity, and inhibition of several other enzymes involved in glucose metabolism.25 The use of artemisinin derivatives could diminish the pharmacological pressure on praziquantel. Clinical trial results have also shown them to be effective in humans,26,27 although when compared to praziquantel, the results are not clear-cut. In 2008, Danso-Appiah et al. performed a thorough meta-analysis of currently available options for the treatment of urinary schistosomiasis. Only one clinical trial analyzed artesunate, and the authors concluded that further investigation was needed to elucidate the role of artemisinin derivatives in the treatment of urinary schistosomiasis.7 Recently, Liu et al.28 performed a meta-analysis regarding the efficacy of artemisinin derivatives versus praziquantel for prevention and treatment of schistosomiasis. Mainly this study speaks about prevention and species other than Schistosoma haematobium. However, in the small part of the analysis regarding artesunate for the treatment of S. haematobium infections, they found that it had a poor performance. A strong limitation is that only two studies were analyzed and no comparison to praziquantel was done. Another interesting finding was a possible role for artesunate in combination treatment with praziquantel. This could be explained by the theoretical synergistic effect of artemisinin derivatives acting on the immature forms of the fluke.29,30
The high burden of disease, the risk of resistance due to pharmacological pressure, the need for alternative drugs in cases of toxicity/intolerance, and the potential efficacy of artemisinin derivatives led us to review the role of these drugs in the treatment of urinary schistosomiasis. Our objective was to compare their efficacy alone or in combination with that of praziquantel for the treatment of chronic urinary schistosomiasis (defined as presence of S. haematobium ova in urine) in schoolchildren.
Methods
A protocol was specifically developed for this review (accessible by e-mail request to the corresponding author). Three independent researchers (WP, HC, and SS) performed the search, retrieved data, and evaluated the risk of bias. Disagreements were resolved by discussion.
Search methods
The electronic databases Medline, EMBASE, LILACS, the Cochrane Central Register of Controlled Trials, African Index Medicus, and Scielo were searched. In Medline, the terms entered were ‘schistosoma haematobium and: either artemisinin or arthemether or artesunate’. In LILACS, ‘schistosoma’ was entered in the fields ‘descriptor’ and ‘clinical trial registry’, in Scielo, the term entered was ‘schistosoma’, and in the Cochrane Central Register of Controlled Trials, the term entered was ‘schistosoma haematobium’.
The abstracts of the main conferences on infectious diseases and tropical medicine held in 2009, 2010 and 2011 (Interscience Conference on Antimicrobial Agents and Chemotherapy, European Congress on Clinical Microbiology and Infectious Diseases, Annual Meeting of the American Society for Tropical Medicine and Hygiene, and European Congress of Tropical Medicine and International Health) were analyzed, and the bibliographies of all recovered documents were searched for additional references. Google Scholar and OpenSIGLE were searched using the terms ‘schistosoma haematobium and: either artemisinin or artemether or artesunate’.
Study selection
No year of publication or language restrictions were applied; the last search was performed in July 2012. The results were screened by title. Potentially eligible studies were identified by reading the abstract and definitive decision about inclusion was done based on a reading of the full text. The only works included were comparative randomized or quasi-randomized clinical trials that studied schoolchildren with urinary schistosomiasis treated with an artemisinin derivative (with or without a secondary drug) or praziquantel (alone or in combination therapy).
Data extraction and management
The main outcome data were retrieved using a standardized form designed for this study. The primary endpoint was the cure rate, defined as the proportion of patients with no egg excretion in two urine samples on two consecutive days between 3 and 8 weeks after treatment. The secondary endpoints were egg reduction rate, resolution of hematuria, and comparison of the incidence of adverse events. The regimens compared for all endpoints were artesunate [with or without a secondary drug (not praziquantel)] versus praziquantel, artesunate plus praziquantel versus praziquantel alone, and artesunate versus placebo.
Risk of bias assessment
Risk of bias in each of the five studies was assessed using the Cochrane Collaboration tool.31 The following domains were evaluated: sequence generation, allocation concealment, blinding (investigators, outcome assessors, and participants), incomplete outcome data, selective outcome reporting, and other sources of bias. Each study was judged on each of the risk of bias domains as low risk of bias, high risk of bias, or ‘unclear risk of bias’.
Data synthesis
Data were analyzed using Revman 5.0.24 (Copenhagen: The Nordic Cochrane Centre). Risk ratios were used for dichotomous outcomes. Heterogeneity was evaluated visually using a forest plot and statistically using a Cochran Q test and Higgins I2 test32 to assess not only whether heterogeneity was present, but also its impact on outcome. We performed an intention-to-treat analysis to adjust for missing data due to dropouts, and dropouts were considered as treatment failure. A fixed or random-effects model was used according to the results of heterogeneity testing.
Results
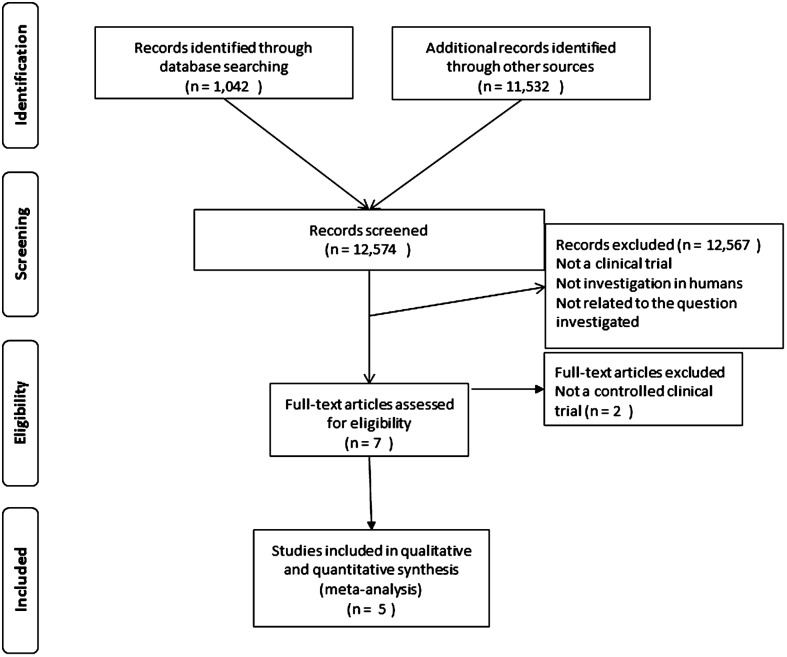
The search yielded 12 574 entries that were screened by title and abstract. Of these, only seven were selected for full text review and 5 studies were eventually included in the analysis (Fig. 1).33–37 Two studies were excluded because they were not controlled clinical trials. The characteristics of the studies included are shown in Table 1. The studies were performed during 2001–2010. All studies except one were randomized controlled clinical trials: three were open-label and two double-blind. All of the trials were conducted in Western Africa. The trial populations were 801 in one study, around 300 in three studies, and 83 in one study. Age ranged from 7 to 20 years. Diagnosis and cure assessment was performed with light microscopy of filtrated urine in all studies (current gold standard technique).38 For the purposes of this meta-analysis, the primary efficacy endpoint was measured at the earliest follow-up visit reported by the study even when the follow-up was longer (range: 26–56 days). The treatment arms compared were praziquantel (40 mg/kg in one dose), artesunate (in several dosing schedules) with or without a second drug (sulfamethoxypyrazine/pyrimethamine in one study and mefloquine in another), the combination of artesunate plus praziquantel, and placebo. One study had a mefloquine monotherapy arm. No studies evaluated any artemisinin derivatives other than artesunate for the treatment of S. haematobium infection in humans.
Figure 1.
Flow diagram of the search and study process.
Table 1. Characteristics of the studies included in the meta-analysis.
| Study | Methods | Participants | Sample size and follow-up | Interventions | Outcomes | ||
| CR (%) | ERR (%) | AE (%) | |||||
| Borrman et al. (2001)33 | Double-blind, Randomized, placebo controlled | Country: Gabon | n = 300 | Arm 1 (n = 90): PZQ (40 mg/kg) +PLB | 72.2 | NR | NR |
| Setting: rural | 56 days | Arm 2 (n = 90): ART (4 mg/kg/day 3 days)+PLB | 26.7 | NR | NR | ||
| Age (y): 10 (SD: 2.5) | Arm 3 (n = 90): ART (4 mg/kg/day 3 days)+PZQ (40 mg/kg) | 78.9 | NR | NR | |||
| Sex: 51% male | Arm 4 (n = 30): PLB | 20.0 | NR | NR | |||
| de Clerq et al. (2002)34 | Open-label | Country: Senegal | n = 288 | Arm 1 (n = 133): PZQ (40 mg/kg) | 37.6 | 84 | 14.3 |
| Setting: rural | 45 days | Arm 2 (n = 134): ART (150 mg 1st day, 100 mg 2nd day, 50 mg 3rd day, 50 mg 4th day, 50 mg 5th day) | 28.0 | 61 | 5.2 | ||
| Quasi-randomized | Age (y): 7–14 | ||||||
| Controlled | Sex: NR | ||||||
| Sissoko et al. (2009)35 | Double-blind, | Country: MaliSetting: peri-urban | 801 | Arm 1 (n = 400): ART (300 mg)+P (37.5 mg)+SM (750 mg) | 43.0 | 92.8 | 0.5 |
| Randomized. | Age (y): 10.4 (SD: 2.4) | 28 days | Arm 2 (n = 401): PZQ (40 mg/kg) | 51.4 | 95.6 | 2.3 | |
| Controlled | Sex: 64.2% male | ||||||
| Inyang-Etoh et al. (2009)36 | Open-label | Country: Nigeria | n = 327 | Arm 1 (n = 52): ART (4 mg/kg/day 3 days)+PZQ (40 mg/kg) | 75.0 | 93.6 | 30.1 |
| Randomized | Setting: rural | 56 days | Arm 2 (n = 52): PLB+ART (4 mg/kg/day 3 days) | 59.6 | 72.3 | 30.1 | |
| Controlled | Age (y): 7–20 | Arm 3 (n = 52): PLB+PZQ (40 mg/kg) | 61.5 | 79.3 | 36.5 | ||
| Sex: NR | Arm 4 (n = 52): PLB | 7.7 | 111.5 | 34.6 | |||
| Arm 5 (n = 52): ART (4 mg/kg/day 3 days) | 63.5 | 52.1 | 26.9 | ||||
| Arm 6 (n = 52): PZQ (40 mg/kg) | 59.6 | 76.7 | 26.9 | ||||
| Keiser et al. (2010)37 | Open-label | Country: Côte d’Ivoire | n = 83 | Arm 1 (n = 19): MFQ (25 mg/kg) | 21.0 | 74 | 100 |
| Randomized | Setting: rural | 26 days | Arm 2 (n = 20): ART (4 mg/kg/day 3 days) | 25.0 | 85 | 80 | |
| Controlled | Age (y): 9.4 | Arm 3 (n = 18): ART (100 mg 3 days)+MFQ (250 mg/3 days) | 61.0 | >95 | 94 | ||
| Sex: 45% male | Arm 4 (n = 26): PZQ (40 mg/kg) | 88.0 | >95 | 61 | |||
Note: AE, adverse events; ART, artesunate; CR, cure rate; ERR, egg reduction rate; MFQ, mefloquine; NR, not reported; SD, standard deviation; P, pyrimethamine; PLB, placebo; PZQ, praziquantel; SM, sulfamethoxypyrazine; y, years.
The risk of bias for each study is shown in Fig. 2. A potentially high risk was found in two of the studies included, one had an unclear risk, and only two trials were considered to have a low risk.
Figure 2.
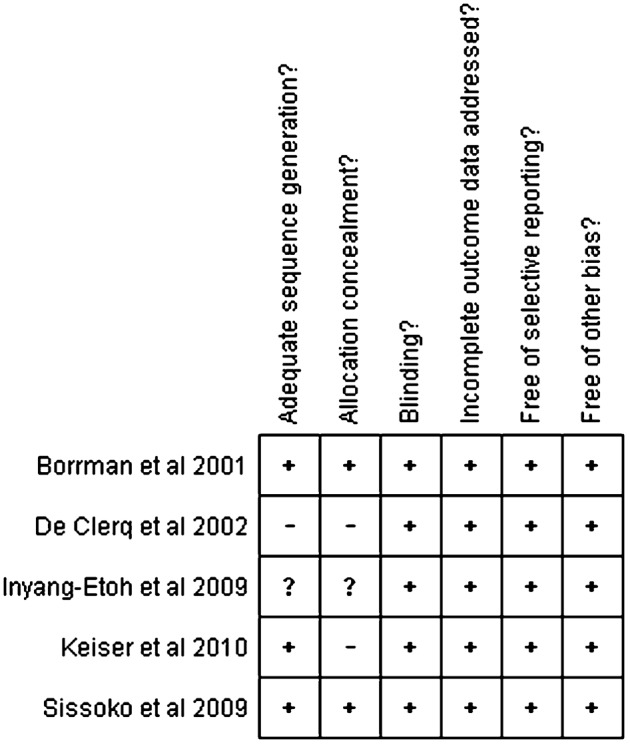
Summary of the risk of bias: +, low risk of bias; −, high risk; ?, unclear risk.
Figure 3 shows the results for the primary outcome expressed as the risk ratio for cure (praziquantel versus artesunate). Praziquantel showed a significantly better response rate than artesunate (RR: 1.66; 95% CI: 1.18–2.33). Analysis of the risk of bias showed that, in those studies with a low risk, praziquantel was superior to artesunate (RR: 2.16; 95% CI: 1.51–3.09), whereas in those with a potentially higher risk, the effect was not clearly better (RR: 1.35; 95% CI: 0.88–2.07).
Figure 3.
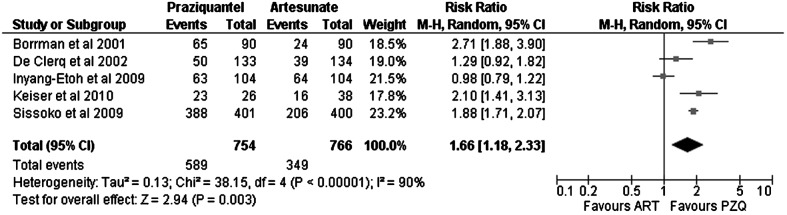
Evaluation of cure rates for praziquantel (PZQ) versus artesunate (ART) in urinary schistosomiasis. In the study by Sissoko et al., the artesunate+sulfamethoxypyrazine/pyrimethamine arm was counted as artesunate. In the study by Inyang-Etoh et al., the artesunate plus placebo and praziquantel plus placebo arms were counted as artesunate and as praziquantel, respectively. In the study by Keiser et al., artesunate plus mefloquine was counted as artesunate.
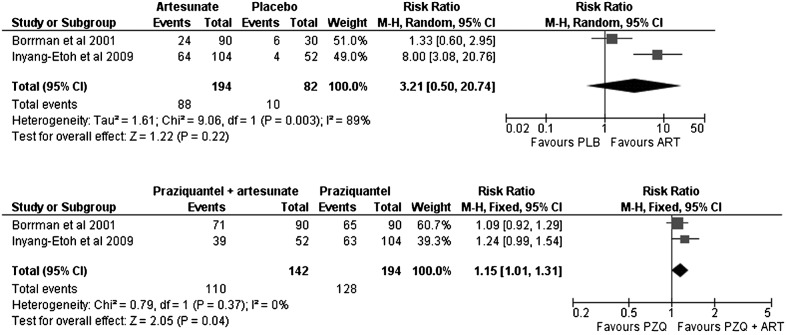
Artesunate was not clearly superior to placebo in cure rates (Fig. 4) (artesunate versus placebo, RR: 3.21; 95% CI: 0.50–20.74). Nevertheless, the combination of artesunate with praziquantel (Fig. 4) was slightly superior to praziquantel monotherapy (RR: 1.15; 95% CI: 1.01–1.31).
Figure 4.
Evaluation of cure rates for artesunate (ART) versus placebo (PLB) in urinary schistosomiasis. In the study by Inyang-Etoh et al., the arm of artesunate plus placebo was counted as artesunate. Evaluation of cure rates for praziquantel+artesunate (PZQ+ART) versus praziquantel (PZQ) alone in urinary schistosomiasis.
The egg reduction rate could not be evaluated in a pooled analysis, given that not all studies reported enough information to perform a pooled analysis. We provide the crude data in Table 1. Although not formally meta-analyzed, the egg reduction rate was consistently higher in the praziquantel groups than in the artesunate groups in all studies except in the study by Keiser et al., where it was reported to be the same in both the praziquantel and the artesunate groups (>95%). Resolution of hematuria could not be evaluated because of the diverse nature of the definitions used in the different trials.
No differences were found in the comparison of the proportion of adverse events reported between the praziquantel and artesunate regimens (RR: 1.49; 95% CI: 0.82–2.71) (Fig. 5).
Figure 5.
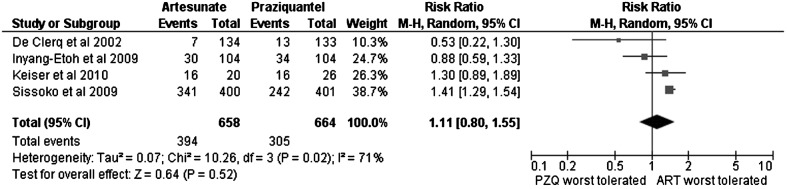
Evaluation of the proportion of patients who developed any adverse event during therapy with praziquantel (PZQ) versus artesunate (ART). In the study by Sissoko et al., the artesunate+sulfamethoxypyrazine/pyrimethamine arm was counted as artesunate. In the study by Inyang-Etoh et al., the artesunate plus placebo and praziquantel plus placebo arms were counted as artesunate and as praziquantel, respectively. In the study by Keiser et al., artesunate plus mefloquine was counted as artesunate.
Discussion
In our meta-analysis, praziquantel showed significantly better efficacy than artesunate for the treatment of urinary schistosomiasis in schoolchildren. At best, artesunate had a marginal role in combination therapy.
Artesunate cannot be considered an alternative to praziquantel. In our study, it was not clearly superior to placebo, although it could be used in combination treatment if the need for combination therapy ever arose. Administration of artesunate to treat schistosomiasis is further restricted because of high concern over the emergence of resistant strains of Plasmodium spp. in malaria-endemic areas. Plasmodium spp. and Schistosoma spp. coexist in many regions, and co-infection affects 4.6–25% of patients,26,27 depending on the species of Schistosoma and the geographical location. The use of artesunate in monotherapy and in suboptimal doses for treatment of malaria is risky, as this approach is a known risk factor for the emergence of resistant strains of Plasmodium spp.24,39
Treatment compliance within the regimens could potentially have affected the outcomes as praziquantel is taken as a single dose and artesunate as multiple doses. Nevertheless, we do not believe this to be the case, as except in the studies of Sisoko et al. and de Clerq et al., treatment was directly observed and if the patient spat out or vomited the drug it was readministrated. Even if this difference in complexity of the regimens contributes to a difference in the outcomes, this would just be another argument that favors praziquantel.
Our meta-analysis is limited by the small number of studies included, especially in the analysis of secondary endpoints, as conclusions drawn from a meta-analysis of only two studies are questionable, and we believe that they could be regarded, at best, as a hypothesis for further investigation. Nonetheless, we believe that the conclusion drawn for our primary endpoint is solid, as all the studies reveal similar findings and the studies with the highest methodological quality and least risk of bias reach the same conclusion.
When interpreting the results, it is important to remember that in two studies, artesunate was combined with a second drug, namely, mefloquine in one and sulfamethoxypyrazine/pyrimethamine in the other, and that these drugs could affect the outcome of the artesunate arm. Intuitively, one might expect both drugs to improve the outcome of the artesunate arm, implying that the difference observed in our study is smaller than the real difference between artesunate and praziquantel. In fact, no differences were found between artesunate and placebo. However, the number of patients in this comparison was small.
Another potential limitation of this study is the presence of heterogeneity, which could be due to the different treatment regimens administered in studies where artesunate is combined with a second drug, the different doses of artesunate used, differences in study design and risk of bias, and even the different geographic locations where the studies were performed (reflecting differences in the susceptibility of the parasite to treatment, different reinfection rates, and different severities of infection). Differences in the time at which treatment outcomes were measured could also be a source of heterogeneity, as the longer time to measurement the higher the possibility that the patient becomes re-infected. Although, heterogeneity is a common feature of meta-analyses,40 we must establish whether it affects the results to the extent that it invalidates them. We do not believe that heterogeneity significantly affects the results of our meta-analysis: the forest plot shows the same trend for all trials, a random-effects model was used to minimize the effect of heterogeneity on pooled estimates, and the studies with least risk of bias showed that praziquantel was more efficacious than artesunate.
Artesunate and other derivates of artemisinin (e.g. artemether, dihydroartemisinin, and arteether) have shown antischistosomal properties in experimental animal models and in humans.25,41,42 However, since we only examined trials involving artesunate in S. haematobium infections, we are unable to extrapolate the results for artesunate to other derivatives of artemisinin or to infections by Schistosoma species other than S. haematobium. Liu et al. found a very good performance of artesunate in preventing S. japonicum infections,28 reflecting the possibility that artesunate could be effective against only some species of Schistosoma.
In any case, the high burden of disease induced by schistosomiasis is unlikely to be resolved by relying only on pharmacological approaches and treatment of individual cases. Public health strategies including snail control with molluscicides and population-based chemotherapy have proven effective and drastically reduce the prevalence of infection.43 These programs lead to a reduction in morbidity due to schistosomiasis that lasts up to 2 years.44 However, their sustainability has been questioned, and the eradication of schistosomiasis will also depend on health education, behavioral changes, sanitation, and safe water supply.45
In summary, based on the results of this meta-analysis, artesunate is clearly inferior to praziquantel for the treatment of urinary schistosomiasis. The question of whether combination therapy could be of interest or whether other artemisinin derivatives could be useful remains unanswered.
Funding
This study was supported in part by the Red de Investigación de Centros de Enfermedades Tropicales RED: RD06/0021/0020.
References
- 1.Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis. 2006;6(7):411–25. doi: 10.1016/S1473-3099(06)70521-7. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed AA, Afifi AA, Adam I. High prevalence of Schistosoma haematobium infection in Gereida Camp, in southern Darfur, Sudan. Ann Trop Med Parasitol. 2009;103(8):741–3. doi: 10.1179/000349809X12502035776234. [DOI] [PubMed] [Google Scholar]
- 3.Ekpo UF, Laja-Deile A, Oluwole AS, Sam-Wobo SO, Mafiana CF. Urinary schistosomiasis among preschool children in a rural community near Abeokuta, Nigeria. Parasit Vectors. 2010;3:58. doi: 10.1186/1756-3305-3-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.King CH, Dickman K, Tisch DJ. Reassessment of the cost of chronic helmintic infection: a meta-analysis of disability-related outcomes in endemic schistosomiasis. Lancet. 2005;365(9470):1561–9. doi: 10.1016/S0140-6736(05)66457-4. [DOI] [PubMed] [Google Scholar]
- 5.King CH. Parasites and poverty: the case of schistosomiasis. Acta Trop. 2010;113(2):95–104. doi: 10.1016/j.actatropica.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mostafa MH, Sheweita SA, O’Connor PJ. Relationship between schistosomiasis and bladder cancer. Clin Microbiol Rev. 1999;12(1):97–111. doi: 10.1128/cmr.12.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Danso-Appiah A, Utzinger J, Liu J, Olliaro P. Drugs for treating urinary schistosomiasis. Cochrane Database Syst Rev. 2008;(3):CD000053. doi: 10.1002/14651858.CD000053.pub2. [DOI] [PubMed] [Google Scholar]
- 8.Greenberg RM. Are Ca2+ channels targets of praziquantel action? Int J Parasitol. 2005;35(1):1–9. doi: 10.1016/j.ijpara.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Doenhoff MJ, Cioli D, Utzinger J. Praziquantel: mechanisms of action, resistance and new derivatives for schistosomiasis. Curr Opin Infect Dis. 2008;21(6):659–67. doi: 10.1097/QCO.0b013e328318978f. [DOI] [PubMed] [Google Scholar]
- 10.Gray DJ, Ross AG, Li YS, McManus DP. Diagnosis and management of schistosomiasis. BMJ. 2011;342:d2651. doi: 10.1136/bmj.d2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olliaro PL, Vaillant MT, Belizario VJ, Lwambo NJ, Ouldabdallahi M, Pieri OS, et al. A multicentre randomized controlled trial of the efficacy and safety of single-dose praziquantel at 40 mg/kg vs. 60 mg/kg for treating intestinal schistosomiasis in the Philippines, Mauritania, Tanzania and Brazil. PLoS Negl Trop Dis. 2011;5(6):e1165. doi: 10.1371/journal.pntd.0001165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dietze R, Prata A. Rate of reversion of hepatosplenic schistosomiasis after specific therapy. Rev Soc Bras Med Trop. 1986;19(2):69–73. doi: 10.1590/s0037-86821986000200003. [DOI] [PubMed] [Google Scholar]
- 13.Doehring-Schwerdtfeger E, Abdel-Rahim IM, Kardorff R, Kaiser C, Franke D, Schlake J, et al. Ultrasonographical investigation of periportal fibrosis in children with Schistosoma mansoni infection: reversibility of morbidity twenty-three months after treatment with praziquantel. Am J Trop Med Hyg. 1992;46(4):409–15. doi: 10.4269/ajtmh.1992.46.409. [DOI] [PubMed] [Google Scholar]
- 14.Gryseels B. The relevance of schistosomiasis for public health. Trop Med Parasitol. 1989;40(2):134–42. [PubMed] [Google Scholar]
- 15.Richter J. Evolution of schistosomiasis-induced pathology after therapy and interruption of exposure to schistosomes: a review of ultrasonographic studies. Acta Trop. 2000;77(1):111–31. doi: 10.1016/s0001-706x(00)00125-x. [DOI] [PubMed] [Google Scholar]
- 16.Hatz CF, Vennervald BJ, Nkulila T, Vounatsou P, Kombe Y, Mayombana C, et al. Evolution of Schistosoma haematobium-related pathology over 24 months after treatment with praziquantel among school children in southeastern Tanzania. Am J Trop Med Hyg. 1998;59(5):775–81. doi: 10.4269/ajtmh.1998.59.775. [DOI] [PubMed] [Google Scholar]
- 17.Olds GR. Administration of praziquantel to pregnant and lactating women. Acta Trop. 2003;86(2–3):185–95. doi: 10.1016/s0001-706x(03)00033-0. [DOI] [PubMed] [Google Scholar]
- 18.WHO. Prevention and control of schistosomiasis and soil-transmitted helminthiasis. World Health Organ Tech Rep Ser. 2002;912:i–vi. 1–57. back cover. [PubMed] [Google Scholar]
- 19.Danso-Appiah A, de Vlas SJ. Interpreting low praziquantel cure rates of Schistosoma mansoni infections in Senegal. Trends Parasitol. 2002;18(3):125–9. doi: 10.1016/s1471-4922(01)02209-7. [DOI] [PubMed] [Google Scholar]
- 20.Alonso D, Munoz J, Gascon J, Valls ME, Corachan M. Failure of standard treatment with praziquantel in two returned travelers with Schistosoma haematobium infection. Am J Trop Med Hyg. 2006;74(2):342–4. [PubMed] [Google Scholar]
- 21.Ismail M, Botros S, Metwally A, William S, Farghally A, Tao LF, et al. Resistance to praziquantel: direct evidence from Schistosoma mansoni isolated from Egyptian villagers. Am J Trop Med Hyg. 1999;60(6):932–5. doi: 10.4269/ajtmh.1999.60.932. [DOI] [PubMed] [Google Scholar]
- 22.Messerli SM, Kasinathan RS, Morgan W, Spranger S, Greenberg RM. Schistosoma mansoni P-glycoprotein levels increase in response to praziquantel exposure and correlate with reduced praziquantel susceptibility. Mol Biochem Parasitol. 2009;167(1):54–9. doi: 10.1016/j.molbiopara.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kasinathan RS, Goronga T, Messerli SM, Webb TR, Greenberg RM. Modulation of a Schistosoma mansoni multidrug transporter by the antischistosomal drug praziquantel. FASEB J. 2010;24(1):128–35. doi: 10.1096/fj.09-137091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.WHO. Geneva: WHO; 2010. Guidelines for the treatment of malaria. 2nd ed. [Google Scholar]
- 25.Utzinger J, Xiao S, Keiser J, Chen M, Zheng J, Tanner M. Current progress in the development and use of artemether for chemoprophylaxis of major human schistosome parasites. Curr Med Chem. 2001;8(15):1841–60. doi: 10.2174/0929867013371581. [DOI] [PubMed] [Google Scholar]
- 26.Adam I, Elhardello OA, Elhadi MO, Abdalla E, Elmardi KA, Jansen FH. The antischistosomal efficacies of artesunate–sulfamethoxypyrazine–pyrimethamine and artemether–lumefantrine administered as treatment for uncomplicated, Plasmodium falciparum malaria. Ann Trop Med Parasitol. 2008;102(1):39–44. doi: 10.1179/136485908X252214. [DOI] [PubMed] [Google Scholar]
- 27.Boulanger D, Dieng Y, Cisse B, Remoue F, Capuano F, Dieme JL, et al. Antischistosomal efficacy of artesunate combination therapies administered as curative treatments for malaria attacks. Trans R Soc Trop Med Hyg. 2007;101(2):113–6. doi: 10.1016/j.trstmh.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 28.Liu R, Dong HF, Guo Y, Zhao QP, Jiang MS. Efficacy of praziquantel and artemisinin derivatives for the treatment and prevention of human schistosomiasis: a systematic review and meta-analysis. Parasit Vectors. 2011;4:201. doi: 10.1186/1756-3305-4-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shuhua X, Jiqing Y, Jinying M, Huifang G, Peiying J, Tanner M. Effect of praziquantel together with artemether on Schistosoma japonicum parasites of different ages in rabbits. Parasitol Int. 2000;49(1):25–30. doi: 10.1016/s1383-5769(00)00029-5. [DOI] [PubMed] [Google Scholar]
- 30.Xiao S, Utzinger J, Chollet J, Endriss Y, N’Goran EK, Tanner M. Effect of artemether against Schistosoma haematobium in experimentally infected hamsters. Int J Parasitol. 2000;30(9):1001–6. doi: 10.1016/s0020-7519(00)00091-6. [DOI] [PubMed] [Google Scholar]
- 31.Higgins JP, Altman D. Chichester: John Wiley & Sons; 2008. Assessing risk of bias in included studies. In: Higgins J, Green S, editors. Cochrane handbook of systematic reviews of interventions. pp. 187–241. [Google Scholar]
- 32.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 33.Borrmann S, Szlezak N, Faucher JF, Matsiegui PB, Neubauer R, Binder RK, et al. Artesunate and praziquantel for the treatment of Schistosoma haematobium infections: a double-blind, randomized, placebo-controlled study. J Infect Dis. 2001;184(10):1363–6. doi: 10.1086/324004. [DOI] [PubMed] [Google Scholar]
- 34.de Clercq D, Vercruysse J, Kongs A, Verle P, Dompnier JP, Faye PC. Efficacy of artesunate and praziquantel in Schistosoma haematobium infected schoolchildren. Acta Trop. 2002;82(1):61–6. doi: 10.1016/s0001-706x(02)00003-7. [DOI] [PubMed] [Google Scholar]
- 35.Sissoko MS, Dabo A, Traore H, Diallo M, Traore B, Konate D, et al. Efficacy of artesunate + sulfamethoxypyrazine/pyrimethamine versus praziquantel in the treatment of Schistosoma haematobium in children. PLoS ONE. 2009;4(10):e6732. doi: 10.1371/journal.pone.0006732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Inyang-Etoh PC, Ejezie GC, Useh MF, Inyang-Etoh EC. Efficacy of a combination of praziquantel and artesunate in the treatment of urinary schistosomiasis in Nigeria. Trans R Soc Trop Med Hyg. 2009;103(1):38–44. doi: 10.1016/j.trstmh.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 37.Keiser J, N’Guessan NA, Adoubryn KD, Silue KD, Vounatsou P, Hatz C, et al. Efficacy and safety of mefloquine, artesunate, mefloquine-artesunate, and praziquantel against Schistosoma haematobium: randomized, exploratory open-label trial. Clin Infect Dis. 2010;50(9):1205–13. doi: 10.1086/651682. [DOI] [PubMed] [Google Scholar]
- 38.Gryseels B, Polman K, Clerinx J, Kestens L. Human schistosomiasis. Lancet. 2006;368(9541):1106–18. doi: 10.1016/S0140-6736(06)69440-3. [DOI] [PubMed] [Google Scholar]
- 39.White N. Antimalarial drug resistance and combination chemotherapy. Philos Trans R Soc Lond B Biol Sci. 1999;354(1384):739–49. doi: 10.1098/rstb.1999.0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Utzinger J, N’Goran EK, N’Dri A, Lengeler C, Xiao S, Tanner M. Oral artemether for prevention of Schistosoma mansoni infection: randomised controlled trial. Lancet. 2000;355(9212):1320–5. doi: 10.1016/s0140-6736(00)02114-0. [DOI] [PubMed] [Google Scholar]
- 42.Li YS, Chen HG, He HB, Hou XY, Ellis M, McManus DP. A double-blind field trial on the effects of artemether on Schistosoma japonicum infection in a highly endemic focus in southern China. Acta Trop. 2005;96(2–3):184–90. doi: 10.1016/j.actatropica.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 43.Zhang W, Wong CM. Evaluation of the 1992–1999 World Bank Schistosomiasis Control Project in China. Acta Trop. 2003;85(3):303–13. doi: 10.1016/s0001-706x(02)00263-2. [DOI] [PubMed] [Google Scholar]
- 44.Magnussen P. Treatment and re-treatment strategies for schistosomiasis control in different epidemiological settings: a review of 10 years’ experiences. Acta Trop. 2003;86(2–3):243–54. doi: 10.1016/s0001-706x(03)00045-7. [DOI] [PubMed] [Google Scholar]
- 45.Minai M, Hosaka Y, Ohta N. Historical view of schistosomiasis japonica in Japan: implementation and evaluation of disease-control strategies in Yamanashi Prefecture. Parasitol Int. 2003;52(4):321–6. doi: 10.1016/s1383-5769(03)00047-3. [DOI] [PubMed] [Google Scholar]