Abstract
The blood-feeding behaviour of the Trinidad strain of Aedes aegypti was studied, under laboratory conditions, using one female per cage and monitoring blood feeding immediately, 12, and 24 hours after oviposition. To get large numbers of females that had newly completed their first gonotrophic cycle, the diel oviposition periodicity was conducted using single females per cage and monitoring at 2-hour intervals. The diel oviposition periodicity showed a small morning peak (8%) during the first 2 hours of the photophase after which oviposition declined: during the second half of the photophase, oviposition increased reaching a peak comprising 56% of eggs (G = 59.9, P>0.01) between 16:00 and 18:00 hours. At post-oviposition hour 0, only five (10%) of the females accepted a blood meal but at post-oviposition hour 12, significantly more (G = 46.98, P>0.02) females, 35 (70%) accepted a blood meal. This pattern was consistently observed when females were offered blood meals at 12 and 24 hours after completing their first gonotrophic cycle. Multiple feeding was observed among the blood-feeding females and the results of this study are discussed in the context of disease transmission patterns and physiological mechanisms which control their blood-feeding behaviours.
Keywords: Blood-feeding, Oviposition, Gonotrophic cycle, Aedes aegypti, Laboratory study
Introduction
Fundamental aspects of the gonotrophic cycle of nulliparous Aedes aegypti (L.) mosquitoes involve numerous physiological and behavioural components including: copulation and insemination, search for a suitable host, blood feeding, digestion of the blood meal, maturation of ovaries, and oviposition of mature eggs after foraging for oviposition sites.1–5 Indeed, much information exists which clearly demonstrates that the duration of the gonotrophic cycle is dependent on temperature,1 humidity5 size of the females,3 copulation status,6 and quality and quantity of blood meal taken.7,8 Studies show that females reared in less than optimal conditions are smaller in size and require at least two blood meals to mature a batch of eggs.9 Recent studies by Schneider et al.10 demonstrated that field-collected females were always smaller than laboratory-reared females and this small size may influence ‘double anautogeny’ or multiple feeding in the field and can influence vector competence because double feeding may improve the chance of acquiring an infected blood meal from a human host.9,10
Interestingly, Christophers,1 Judson,7 Jones and Madhukar,11 Klowden,12 and Nayar13 reported that blood seeking and subsequent feeding were independent of insemination but host seeking was enhanced if females were given a sugar meal.5,14 However, the quantity of blood imbibed by inseminated Ae. aegypti was reported to determine two important physiological mechanisms. First, the blood meal inhibits the host-seeking response of females;8,15 second, if the blood meal triggers vitellogenesis, a second inhibition accompanies oocyte development16,17 and ultimately influences the number of eggs which develop and are laid.
Very little is known about the blood-feeding behaviour of Ae. aegypti after oviposition. In fact, nothing is known about the duration of time needed for the resumption of blood feeding after oviposition, even though such information, which is closely related to disease transmission, is important for the control of the vectors, for standardization of laboratory methods, and for field use of transgenetically engineered mosquitoes,18 would clearly be helpful. The purpose of this study was to determine aspects of the physiology of blood feeding of Ae. aegypti mosquitoes. The central question concerns the number of blood meals that females require to develop a batch of eggs and to determine how soon after oviposition females take a blood meal under laboratory conditions. These questions have important implications for the dynamics of mosquito-borne viral infections and its subsequent transmission to man.
Materials and Methods
The Ae. aegypti strain used during these studies originated in St. Joseph, Trinidad, collected as eggs from June to August (1986), and designated the Trinidad strain. This strain was accommodated in two light-proof rooms at 26±1°C and 70–75% relative humidity, and a regime of 12 hour light (06:00–18:00 hour) and either 12 hour dark (room 1) or 11 hour dark (room 2) with two 30-minute ‘twilight’ periods immediately before and after the scotophase. Illumination was as described by Corbet and Ali.19
The colony was maintained in room 1 in accordance with regimes that standardized density and nutrition of larvae as described by Corbet and Chadee.20 All Ae. aegypti used for experiments were transferred as eggs to room 2 and reared to adults. Female adults were selected so that the post-emergence age of each was the same, and known to within 1 hour. On the third day post-emergence, a sample of females was confirmed as inseminated by post-mortem dissection. Thereafter, females were allowed to engorge on blood from an experimenter’s arm within a 20-minute period centred on 17:20 hour, a time close to the main peak of landing and biting of Ae. aegypti in the field in Trinidad.21,22
Experiments
On the fourth day post-emergence, engorged females (assessed as such by eye) were placed individually, one per oviposition cage (30×30×30 cm) consisting of white cloth netting enclosing a wooden frame and a cube of white sugar in an uncovered Petri dish in the centre of the cage. In each cage, eight numbered small white polyethylene tubs (SWTs) (diameter of top 8.2 cm and bottom 7 cm, height 5.8 cm, and capacity 300 ml) painted black outside, containing 200 ml of temperature-equilibrated tap water with the inside of each tub lined with a white paper towel were placed as described in the oviposition assay method developed by Corbet and Chadee.20
In order to determine whether females took a blood meal soon after oviposition, it was necessary to monitor when eggs were laid, that is to determine the diel oviposition periodicity. The oviposition periodicity was monitored by manually placing eight pre-prepared SWTs into each cage labelled in accordance with the cage number. The eight SWTs were exposed for intervals of 2 hours and removed and replaced with another set bearing the time of exposure and cage number. These females were monitored every 2 hours for 48 hours. After each 2-hour exposure sequence, all oviposition paper towels were removed from the SWTs and placed onto white sheets of paper towel on the laboratory bench and eggs were counted under (×40 magnification) a stereomicroscope.
After oviposition had occurred, one group of females were individually offered a blood meal as described above between 18:00 hour+20 minutes on post-oviposition day 1 (POD) and again at 06:30 hour+20 minutes the following morning. After this, blood meals were offered continuously at the above mentioned times until the second gonotrophic cycle was completed.
The second group of mosquitoes were treated in a similar manner except that a blood meal was not offered until 12 hours had lapsed after the first gonotrophic cycle was completed. After this, these females were offered blood meals at morning and evenings times as in group 1.
The third group of mosquitoes were not offered a blood meal until 24 hours had elapsed after egg laying (first gonotrophic cycle) and thereafter were fed in accordance with the morning and evening feeding regimen outlined above.
The number of blood-feeding episodes recorded after oviposition was analysed to determine the time required for females to resume blood feeding and these data were analysed by transforming the data into contingency tables and a G-test as well as a Chi-square test23 was applied.
Results
Study 1
Fifty individuals of the Trinidad strain exhibited a distinct diel periodicity, with peak oviposition observed between 16:00 and 18:00 hour (Fig. 1). A small morning peak (8%) was observed during the first 2 hours of the photophase after which oviposition declined: during the second half of the photophase, oviposition increased reaching a peak, comprising 56% of eggs (G = 59.9, P>0.01) between 16:00 and 18:00 hour (Fig. 1). No eggs were laid during the scotophase. This is regarded as the definitive baseline oviposition periodicity of Ae. aegypti and is similar to that observed previously24-26 and these females were offered blood meals after different time intervals.
Figure 1.
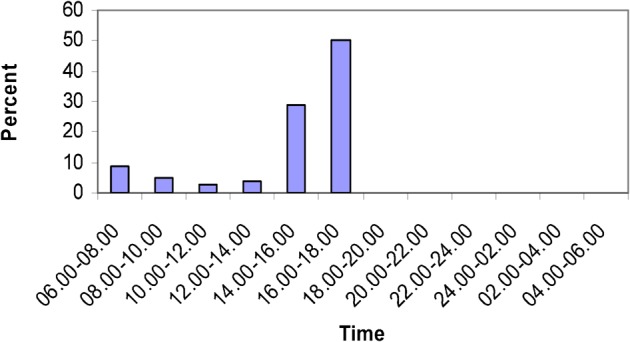
Aedes aegypti: diel oviposition periodicity in the laboratory using the 2-hour manual monitoring method.
Study 2
At post-oviposition hour 0, only five (10%) of the females accepted a blood meal but at post-oviposition hour 12, significantly more (G = 46.98, P>0.02) females, 35 (70%) accepted a blood meal (Table 1). At post-oviposition 36 hours, all females accepted a blood meal. Sixty-five percent of the females took two or more blood meals over the three successive days.
Table 1. Number of Aedes aegypti females blood feeding immediately, 12, and 24 hours after completing their first gonotrophic cycle in the laboratory.
| Post-oviposition hour | No. of blood feeding | % blood feeding |
| 0 | 5 | 10 |
| 12 | 35 | 70 |
| 24 | 10 | 100 |
At post-oviposition hour 12, significantly (G = 35.87, P>0.01) more females accepted a blood meal, i.e. over 85% or 43 females had blood fed. Therefore, by post-oviposition hour 36, all females had completed a blood meal to initiate the second gonotrophic cycle (Table 2). Fifty percent or 25 females took two or more blood meals over the three successive days
Table 2. Number of Aedes aegypti females blood feeding 12 and 24 hours after completing their first gonotrophic cycle in the laboratory.
| Post-oviposition hour | No. of blood feeding | % blood feeding |
| 12 | 43 | 85 |
| 24 | 5 | 10 |
| 36 | 2 | 5 |
At post-oviposition hour 24, 100% (50) of the females accepted a blood meal (Table 3) with 60% taking two or more blood meals over three successive days.
Table 3. Number of Aedes aegypti females blood feeding 24 hours after completing their first gonotrophic cycle in the laboratory.
| Post-oviposition hour | No. of blood feeding | % blood feeding |
| 24 | 50 | 100 |
| 36 | 50 | 0 |
| 48 | 50 | 0 |
Discussion
The results of this study showed laboratory-reared Ae. aegypti females blood-fed 12 hours after oviposition, with a significant peak (70%) restricted to the first 2 hours of the photophase (06:00–08:00 hour). This blood-feeding pattern is consistent with that observed in the field in Tanzania27 and in Trinidad21,22 when an early morning and later afternoon peak was observed. The present results showed that only 10% of the females took a blood meal immediately after oviposition with significantly (P>0.02) more Ae. aegypti females accepting a blood meal 12 hours post-oviposition after completing their first gonotrophic cycle. These results suggest that a period of ‘quiescence’ exists which may allow females to re-set their physiological processes before blood feeding, to kick-start their second gonotrophic cycle. Similar periods of inactivity or quiescence have been reported as ‘non-responsive females to human host’ within 24 hours of emergence,28 inactivity after insemination,29 inactivity after optimally laboratory-reared females blood feed to repletion,3 a 24-hour recovery period from the inhibition of host seeking,30 and now a rest of 12 hours post-oviposition (present study). It is quite possible that females (10%) that oviposited early using the 12-hour monitoring interval may have taken a blood meal immediately when offered at 18:00 hour but unfortunately this notion is not supported because females which oviposited at 06:00 hour did not take a blood meal until 18:00 hour, therefore individual variability and genetic differences may be responsible for this small atypical group of Ae. aegypti females.
It is well known that the quantity of blood imbibed by inseminated Ae. aegypti determines two physiological mechanisms. First, it inhibits the host-seeking response of females;8,15 second, if the blood meal triggers vitellogenesis, a second inhibition accompanies oocyte development16,17 and ultimately the number of eggs which develop and are laid. The abdominal distension following a blood meal usually terminates the host-seeking behaviour and if the blood meal triggers egg development, a second inhibition occurs and this haemolymph-borne factor is produced about 30 hours after blood feeding. In Ae. Aegypti, this inhibition normally lasts until oviposition and is called the oocyte-induced inhibition.17,31
Numerous workers have shown that the initiation of egg development in Ae. aegypti and other mosquitoes is controlled by the accessory gland.5 In spite of the endogenous mechanism which inhibits host seeking during oogenesis,6,15,17 host seeking may persist when the nutritional state is marginal, for example, when the quantity of blood and carbohydrates imbibed falls below 3 mg or 2.5 μl threshold for egg development.32,33 The present results showed that blood feeding lasted for 36 hours post-oviposition with many females multiple feeding thereafter triggering the second gonotrophic cycle. These results also support the concept of ‘distension-induced inhibition’ and ‘oocyte-induced inhibition’ after 36hours but females multiple fed despite being reared on standard diets. Therefore, it seems likely that multiple feeding is a strategy adopted by females whether they are standard size,9 disturbed while feeding in the experimental cages,24,25 or affected by temperature and humidity gradients.34,35
‘Double anautogeny’ or multiple blood feeding has been reported in adult females deprived of nutrients during the larval stage, and consequently cannot deposit yolk following the first blood meal. In such females, the ovarian follicles fail to develop to a pre-vitellogenic stage.36 Macdonald37 and Klowden38 reported that small females produced by an inadequate larval diet have a smaller blood meal capacity and may seek more than one blood meal. However, Klowden14,31 suggested that adult mosquitoes that produce eggs may seek another blood meal before oviposition. During the present study, females did not seek additional blood meals after 36 hours although they were offered, so it is quite likely that these differences may be due to geographical variation or because laboratory conditions did not fully replicate field conditions.
In addition, Macdonald37 and McClelland and Conway39 studying the frequency of blood feeding of Ae. aegypti under field conditions, found double feeding during the first and subsequent gonotrophic cycles to be fairly common among females collected. Laboratory and field studies have demonstrated that individuals reared under optimum laboratory conditions are significantly larger and show much less variability in size plasticity than their field-reared cohorts.10 Therefore, the present results suggest that the multiple feeding observed may have been due to disturbance during blood feeding or within the Ae. aegypti population atypical blooding feeding is found among a small sub-population.
Gillett40 reported no diel blood-feeding rhythm among Ae. aegypti, i.e. with no preference for diurnal, crepuscular or nocturnal periods under laboratory conditions. However, diel patterns have been demonstrated during field studies in many countries, including Tanzania27 and Trinidad.21,22 During the present study, mosquitoes peak blood feeding occurred between 06:00–08:00 and 16:00–1800 hour with infrequent feeding during other times during the day, which suggest the strength of the blood-feeding circadian rhythm.
In order to collect large numbers of Ae. aegypti females which had recently completed their first gonotrophic cycle, the manual oviposition monitoring methodology was adopted as described by Chadee.24 The results of the present laboratory study showed that the oviposition periodicity of Ae. aegypti was diurnal with significant peaks in oviposition restricted to the first 2 hours and the last 2 hours of the photophase. This pattern was consistently observed during field oviposition periodicity studies in Kenya when peak oviposition occurred at 14:00–18:00 hour41 and in Trinidad when peak oviposition occurred at 06:00–08:00 and 16:00–18:00 hour.4 Gillett et al.42 and Haddow et al.43 demonstrated the strength of the circadian rhythm with females having matured eggs at night and during different times during the day but waited until the afternoon period, 14:00–18:00 hour before laying her eggs. In the present study, similar results were observed for although females had matured eggs after 56–60 hours post-blood feeding, oviposition did not occur until 14:00–18:00 hour despite being offered oviposition sites from 06:00 hour (Fig. 1).
In conclusion, the present results demonstrate the strength of the circadian rhythms which regulates oviposition and blood feeding among Ae. aegypti mosquitoes. These results are relevant to vector control workers using genetically modified strains for these peak times of activity or periodicities can be incorporated in their monitoring and evaluation programmes or surveillance systems. In addition, conventional vector control programmes applying space spraying at dawn and dusk can certainly impact two important sectors of the Ae. aegypti population, i.e. the blood feeding and egg laying populations and once the spray applications are synchronized and applied in accordance with the manufacturers specifications, significant reductions in the vector population can be achieved, especially as Ae. aegypti multiple blood-fed in nature.
Acknowledgments
This study is dedicated to the memory of Professor Philip S. Corbet, University of Edinburgh, a mentor and friend who collaborated with me on the oviposition behaviour of mosquitoes for the last 25 years. I also thank Dr Joan M. Sutherland from the University of Dundee for assistance and for reviewing the manuscript. This study was supported by funds from the Government of Trinidad and Tobago Research Development Fund FSA-6.
References
- Christophers SR. Aedes aegypti (L.) The yellow fever mosquito. Its life history bionomics and structure. Cambridge: Cambridge University Press; 1960. [Google Scholar]
- Detinova TS. Age grouping methods in Diptera of medical importance. Geneva: WHO; 1962. WHO Monograph 47. [PubMed] [Google Scholar]
- Klowden MJ, Briegel H. Mosquito gonotrophic cycle and multiple feeding potential: contrast between Anopheles and Aedes (Diptera: Culicidae). J Med Entomol. 1994;30:618–22. doi: 10.1093/jmedent/31.4.618. [DOI] [PubMed] [Google Scholar]
- Chadee DD, Corbet PS. Seasonal incidence and diel patterns of oviposition in the field of the mosquito, Aedes aegypti (L.) (Diptera: Culicidae) in Trinidad, W.I. : a preliminary study. Ann Trop Med Parasitol. 1987;81:151–61. doi: 10.1080/00034983.1987.11812107. [DOI] [PubMed] [Google Scholar]
- Clements AN. The biology of mosquitoes. Wallingford: CABI Publishing; 1999; Vol. 2. [Google Scholar]
- Laviopierre MM. Biting behaviour of mated and unmated females of an African strain of Aedes aegypti. Nature. 1958;181:1781–2. doi: 10.1038/1811781a0. [DOI] [PubMed] [Google Scholar]
- Judson CL. Feeding and oviposition behaviour in the mosquito Aedes aegypti (L.). I. Preliminary studies of physiological control mechanisms. Biol Bull. 1967;133:369–78. doi: 10.2307/1539832. [DOI] [PubMed] [Google Scholar]
- Klowden MJ, Lea AO. Blood meal size as a factor affecting continued host-seeking by Aedes aegypti (L.). Am J Trop Med Hyg. 1978;77:827–31. doi: 10.4269/ajtmh.1978.27.827. [DOI] [PubMed] [Google Scholar]
- Chadee DD, Beier JC. Factors influencing blood-feeding duration for laboratory-reared and wild Aedes aegypti from Trinidad, West Indies. Ann Trop Med Parasitol. 1997;91:199–207. doi: 10.1080/00034983.1997.11813130. [DOI] [PubMed] [Google Scholar]
- Schneider JR, Chadee DD, Mori A, Romero-Severson R, Severson DW. Heritability and phenotype plasticity of adult body size in the mosquito Aedes aegypti and implications for dengue virus transmission. Infect Genet Evol. 2011;11:11–6. doi: 10.1016/j.meegid.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JC, Mudhukar BV. Effects of sucrose on blood avidity in mosquitoes. J Insect Physiol. 1976;3:357–60. doi: 10.1016/0022-1910(76)90001-9. [DOI] [PubMed] [Google Scholar]
- Klowden MJ. Blood intake by Aedes aegypti not regulated by insemination. J Insect Physiol. 1979;25:349–51. [Google Scholar]
- Nayar JK. Aedes aegypti (L.) (Diptera: Culicidae): observations on dispersal, survival, insemination, ovarian development and oviposition characteristics of a Florida population. J Florida Anti-Mosq Assoc. 1981;52:24–40. [Google Scholar]
- Klowden MJ. Effects of sugar deprivation on the host seeking behaviour of gravid Aedes aegypti mosquitoes. J Insect Physiol. 1986;32:479–83. [Google Scholar]
- Edman JD, Cody D, Lynn H. Blood-feeding activity of partially engorged Culex nigripalpus (Diptera: Culicidae). Entomol Exp Appl. 1975;18:261–8. [Google Scholar]
- Larsen JR, Bodenstein D. The humoral control of egg maturation in the mosquito Aedes aegypti. J Exp Zool. 1959;140:343–81. doi: 10.1002/jez.1401400208. [DOI] [PubMed] [Google Scholar]
- Klowden MJ, Lea AO. Abdominal distension terminates subsequent host seeking behaviour of Aedes aegypti following a blood meal. J Insect Physiol. 1979;25:583–5. doi: 10.1016/0022-1910(79)90073-8. [DOI] [PubMed] [Google Scholar]
- Chadee DD, Kittayapong P, Morrison AC, Tabachnick WJ. Genetics. A break through for global public health. Science. 2007;316:1703–4. doi: 10.1126/science.1138904. [DOI] [PubMed] [Google Scholar]
- Corbet PS, Ali AH. Diel patterns of pupation and emergence, and protogyny, in Toxorhynchites brevipalpis (Theobald) (Diptera: Culicidae): a laboratory study. Ann Trop Med Parasitol. 1987;81:437–43. doi: 10.1080/00034983.1987.11812141. [DOI] [PubMed] [Google Scholar]
- Corbet PS, Chadee DD. An improved method for detecting substrate preferences shown by mosquitoes than exhibit ‘skip’ oviposition. Physiol Entomol. 1993;18:114–8. [Google Scholar]
- Chadee DD. Landing periodicity of the mosquito Aedes aegypti (L.) in Trinidad in relation to the timing of insecticide space-spraying. Med Veter Entomol. 1988;2:189–92. doi: 10.1111/j.1365-2915.1988.tb00071.x. [DOI] [PubMed] [Google Scholar]
- Chadee DD, Martinez R. Landing periodicity of Aedes aegypti with Implications for dengue transmission in Trinidad, West Indies. J Vector Ecol. 2000;25:158–63. [PubMed] [Google Scholar]
- Sokal RR, Rohlf FR. Biometry. 2nd ed. New York: Freeman; 1981. [Google Scholar]
- Chadee DD. The diel oviposition periodicity of Aedes aegypti (L.) (Diptera: Culicidae) in the laboratory: density disturbance. Ann Trop Med Parasitol. 2007;101:353–8. doi: 10.1179/136485907X176409. [DOI] [PubMed] [Google Scholar]
- Chadee DD. The diel oviposition periodicity of Aedes aegypti (L.) (Diptera: Culicidae) in the laboratory: substrate-movement effect. Ann Trop Med Parasitol. 2008;102:259–65. doi: 10.1179/136485908X278739. [DOI] [PubMed] [Google Scholar]
- Chadee DD. Diel oviposition periodicity of Aedes aegypti (L.) (Diptera: Culicidae): effects of forced egg retention. Bull Entomol Res. 2010;100:599–603. doi: 10.1017/S0007485309990666. [DOI] [PubMed] [Google Scholar]
- Corbet PS, Smith SM. Diel periodicities of landing of nulliparous and parous Aedes aegypti L. at Dar es Salaam, Tanzania (Diptera: Culicidae). Bull Entomol Res. 197464111–21. [Google Scholar]
- Bowen MF. The sensory physiology of host-seeking behavior in mosquitoes. Annu Rev Entomol. 1991;36:139–58. doi: 10.1146/annurev.en.36.010191.001035. [DOI] [PubMed] [Google Scholar]
- Fuchs MS, Kang SH. Evidence for a naturally occurring inhabitor of oviposition in Aedes aegypti. Ann Entomol Soc Am. 1978;71:473–5. [Google Scholar]
- Klowden MJ. Endogenous regulation of the attraction of Aedes aegypti mosquitoes. J Am Mosq Control Assoc. 1994;10:326–32. [PubMed] [Google Scholar]
- Klowden MJ. Initiation and termination of host-seeking inhibition in Aedes aegypti during oocte maturation. J Insect Physiol. 1981;27:799–803. [Google Scholar]
- Edman JD, Lynn H. Relationship between blood-meal volume and ovarian development in Culex nigripalpus (Diptera: Culicidae). Entomol Exp Appl. 1975;18:492–6. [Google Scholar]
- Nayar JK, Sauerman DM. The effect of nutrition on survival and fecundity in Florida mosquitoes. Part 2. Utilization of a blood meal for survival. J Med Entomol. 1975;12:99–103. doi: 10.1093/jmedent/12.1.99. [DOI] [PubMed] [Google Scholar]
- Chadee DD, Beier JC. Natural variation in blood feeding kinetics of four mosquito vectors. J Vector Ecol. 1996;21:151–5. [Google Scholar]
- Mohammed A, Chadee DD. Effects of different temperature regimens on the development of Aedes aegypti (L.) (Diptera: Culicidae) mosquitoes. Acta Trop. 2011;119:38–43. doi: 10.1016/j.actatropica.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Feinsod FM, Spielman A. Nutrients mediated juvenile hormone secretion in mosquitoes. J Insect Physiol. 1980;26:113–7. [Google Scholar]
- Macdonald WW. Aedes aegypti in Malaysia. II. Larval and adult biology. Ann Trop Med Parasitol. 1956;50:399–414. [PubMed] [Google Scholar]
- Klowden MJ.The physiological control of mosquito host-seeking behaviour In: Harris KF, editor. Current topics in vector research.New York: Harris-Praeger Publishers; 1983. p.93–116. [Google Scholar]
- McClelland GAH, Conway GR. Frequency of blood-feeding in the mosquito Aedes aegypti. Nature. 1971. 232:485–6. doi: 10.1038/232485a0. [DOI] [PubMed] [Google Scholar]
- Gillett JD. Contribution to the oviposition cycle by the individual mosquitoes in a population. J Insect Physiol. 1962;8:665–81. [Google Scholar]
- McClelland GAH. Field observations on periodicity and site preferences in oviposition by Aedes aegypti (L.) and related mosquitoes (Diptera: Culicidae) in Kenya. Proc R Entomol Soc Lond. 1968;43:147–54. [Google Scholar]
- Gillett JD, Corbet PS, Haddow AJ. Observations on the oviposition-cycle of Aedes (Stegomyia) aegypti (Linnaeus) III. Ann Trop Med Parasitol. 1959;53:132–6. doi: 10.1080/00034983.1959.11685910. [DOI] [PubMed] [Google Scholar]
- Haddow AJ, Gillett JD, Corbet PS. Observations on the oviposition-cycle of Aedes (Stegomyia) aegypti (Linnaeus) V. Ann Trop Med Parasitol. 1961;55:343–56. doi: 10.1080/00034983.1961.11686057. [DOI] [PubMed] [Google Scholar]


