Abstract
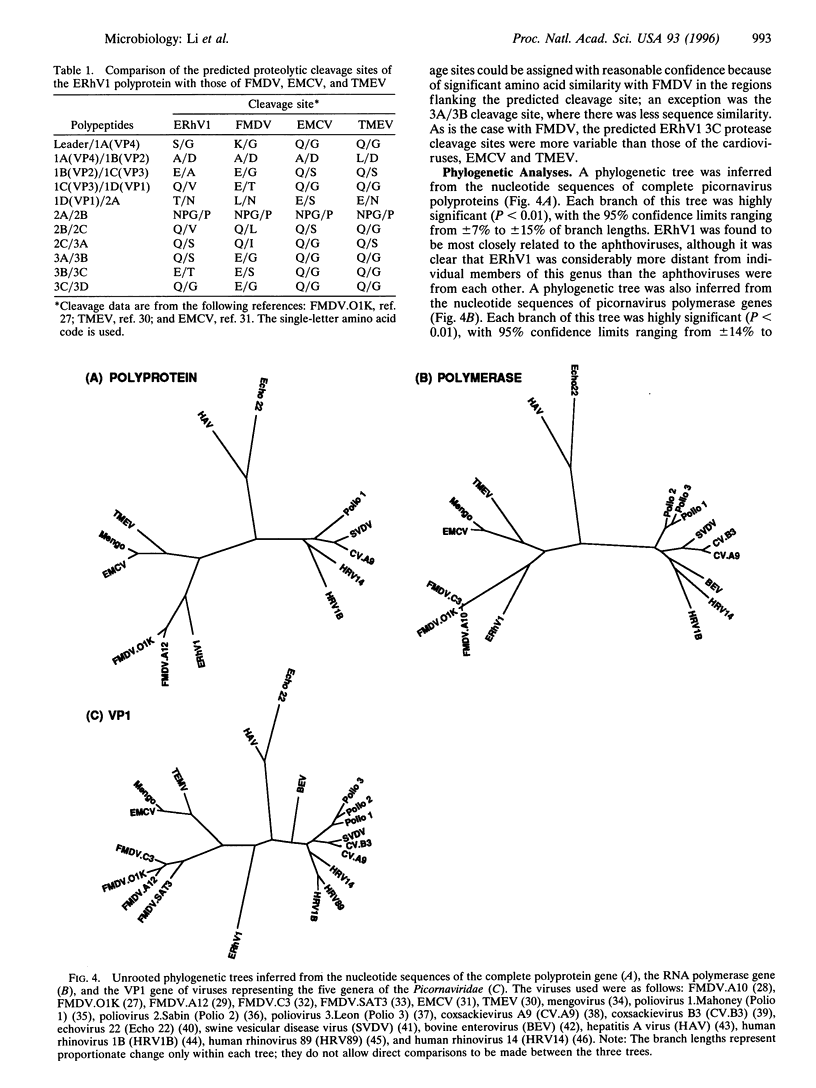
Equine rhinovirus 1 (ERhV1) is a respiratory pathogen of horses which has an uncertain taxonomic status. We have determined the nucleotide sequence of the ERhV1 genome except for a small region at the 5' end. The predicted polyprotein was encoded by 6741 nucleotides and possessed a typical picornavirus proteolytic cleavage pattern, including a leader polypeptide. The genomic structure and predicted amino acid sequence of ERhV1 were more similar to those of foot-and-mouth disease viruses (FMDVs), the only members of the aphthovirus genus, than to those of other picornaviruses. Features which were most similar to FMDV included a 16-amino acid 2A protein which was 87.5% identical in sequence of FMDV 2A, a leader (L) protein similar in size to FMDV Lab and the possibility of a truncated L protein similar in size to FMDV Lb, and a 3C protease which recognizes different cleavage sites. However, unlike FMDV, ERhV1 had only one copy of the 3B (VPg) polypeptide. The phylogenetic relationships of the ERhV1 sequence and nucleotide sequences of representative species of the five genera of the family Picornaviridae were examined. Nucleotide sequences coding for the complete polyprotein, the RNA polymerase, and VP1 were analyzed separately. The phylogenetic trees confirmed that ERhV1 was more closely related to FMDV than to other picornaviruses and suggested that ERhV1 may be a member, albeit very distant, of the aphthovirus genus.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham G., Colonno R. J. Many rhinovirus serotypes share the same cellular receptor. J Virol. 1984 Aug;51(2):340–345. doi: 10.1128/jvi.51.2.340-345.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsham G. J. Dual initiation sites of protein synthesis on foot-and-mouth disease virus RNA are selected following internal entry and scanning of ribosomes in vivo. EMBO J. 1992 Mar;11(3):1105–1110. doi: 10.1002/j.1460-2075.1992.tb05150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black D. N., Stephenson P., Rowlands D. J., Brown F. Sequence and location of the poly C tract in aphtho- and cardiovirus RNA. Nucleic Acids Res. 1979 Jun 11;6(7):2381–2390. doi: 10.1093/nar/6.7.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. L., Campbell R. O., Clarke B. E. The nucleotide sequence of the structural-protein-coding region of foot-and-mouth disease virus serotype SAT3. Gene. 1989 Feb 20;75(2):225–233. doi: 10.1016/0378-1119(89)90268-0. [DOI] [PubMed] [Google Scholar]
- Carroll A. R., Rowlands D. J., Clarke B. E. The complete nucleotide sequence of the RNA coding for the primary translation product of foot and mouth disease virus. Nucleic Acids Res. 1984 Mar 12;12(5):2461–2472. doi: 10.1093/nar/12.5.2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K. H., Auvinen P., Hyypiä T., Stanway G. The nucleotide sequence of coxsackievirus A9; implications for receptor binding and enterovirus classification. J Gen Virol. 1989 Dec;70(Pt 12):3269–3280. doi: 10.1099/0022-1317-70-12-3269. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- DITCHFIELD J., MACPHERSON L. W. THE PROPERTIES AND CLASSIFICATION OF TWO NEW RHINOVIRUSES RECOVERED FROM HORSES IN TORONTO, CANADA. Cornell Vet. 1965 Apr;55:181–189. [PubMed] [Google Scholar]
- Duechler M., Skern T., Sommergruber W., Neubauer C., Gruendler P., Fogy I., Blaas D., Kuechler E. Evolutionary relationships within the human rhinovirus genus: comparison of serotypes 89, 2, and 14. Proc Natl Acad Sci U S A. 1987 May;84(9):2605–2609. doi: 10.1073/pnas.84.9.2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earle J. A., Skuce R. A., Fleming C. S., Hoey E. M., Martin S. J. The complete nucleotide sequence of a bovine enterovirus. J Gen Virol. 1988 Feb;69(Pt 2):253–263. doi: 10.1099/0022-1317-69-2-253. [DOI] [PubMed] [Google Scholar]
- Forss S., Strebel K., Beck E., Schaller H. Nucleotide sequence and genome organization of foot-and-mouth disease virus. Nucleic Acids Res. 1984 Aug 24;12(16):6587–6601. doi: 10.1093/nar/12.16.6587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes P. J., North C., Jellis C. H., Minor P. D., Stanway G. The nucleotide sequence of human rhinovirus 1B: molecular relationships within the rhinovirus genus. J Gen Virol. 1988 Jan;69(Pt 1):49–58. doi: 10.1099/0022-1317-69-1-49. [DOI] [PubMed] [Google Scholar]
- Hyypiä T., Horsnell C., Maaronen M., Khan M., Kalkkinen N., Auvinen P., Kinnunen L., Stanway G. A distinct picornavirus group identified by sequence analysis. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8847–8851. doi: 10.1073/pnas.89.18.8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T., Suzuki T., Sekiguchi K. The complete nucleotide sequence of swine vesicular disease virus. J Gen Virol. 1989 Apr;70(Pt 4):919–934. doi: 10.1099/0022-1317-70-4-919. [DOI] [PubMed] [Google Scholar]
- Kaminski A., Belsham G. J., Jackson R. J. Translation of encephalomyocarditis virus RNA: parameters influencing the selection of the internal initiation site. EMBO J. 1994 Apr 1;13(7):1673–1681. doi: 10.1002/j.1460-2075.1994.tb06431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump W. M., Bergmann I., Müller B. C., Ameis D., Kandolf R. Complete nucleotide sequence of infectious Coxsackievirus B3 cDNA: two initial 5' uridine residues are regained during plus-strand RNA synthesis. J Virol. 1990 Apr;64(4):1573–1583. doi: 10.1128/jvi.64.4.1573-1583.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koonin E. V., Dolja V. V. Evolution and taxonomy of positive-strand RNA viruses: implications of comparative analysis of amino acid sequences. Crit Rev Biochem Mol Biol. 1993;28(5):375–430. doi: 10.3109/10409239309078440. [DOI] [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989 Feb;108(2):229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M., Vriend G., Kamer G., Minor I., Arnold E., Rossmann M. G., Boege U., Scraba D. G., Duke G. M., Palmenberg A. C. The atomic structure of Mengo virus at 3.0 A resolution. Science. 1987 Jan 9;235(4785):182–191. doi: 10.1126/science.3026048. [DOI] [PubMed] [Google Scholar]
- Makoff A. J., Paynter C. A., Rowlands D. J., Boothroyd J. C. Comparison of the amino acid sequence of the major immunogen from three serotypes of foot and mouth disease virus. Nucleic Acids Res. 1982 Dec 20;10(24):8285–8295. doi: 10.1093/nar/10.24.8285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina M., Domingo E., Brangwyn J. K., Belsham G. J. The two species of the foot-and-mouth disease virus leader protein, expressed individually, exhibit the same activities. Virology. 1993 May;194(1):355–359. doi: 10.1006/viro.1993.1267. [DOI] [PubMed] [Google Scholar]
- Newman J. F., Rowlands D. J., Brown F. A physico-chemical sub-grouping of the mammalian picornaviruses. J Gen Virol. 1973 Feb;18(2):171–180. doi: 10.1099/0022-1317-18-2-171. [DOI] [PubMed] [Google Scholar]
- Newman J. F., Rowlands D. J., Brown F., Goodridge D., Burrows R., Steck F. Physicochemical characterization of two serologically unrelated equine rhinoviruses. Intervirology. 1977;8(3):145–154. doi: 10.1159/000148889. [DOI] [PubMed] [Google Scholar]
- PLUMMER G. An equine respiratory enterovirus. Some biological and physical properties. Arch Gesamte Virusforsch. 1963 Spring-Fall;12:694–700. doi: 10.1007/BF01246390. [DOI] [PubMed] [Google Scholar]
- PLUMMER G. An equine respiratory virus with enterovirus properties. Nature. 1962 Aug 4;195:519–520. doi: 10.1038/195519a0. [DOI] [PubMed] [Google Scholar]
- Palmenberg A. C., Kirby E. M., Janda M. R., Drake N. L., Duke G. M., Potratz K. F., Collett M. S. The nucleotide and deduced amino acid sequences of the encephalomyocarditis viral polyprotein coding region. Nucleic Acids Res. 1984 Mar 26;12(6):2969–2985. doi: 10.1093/nar/12.6.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmenberg A. C. Proteolytic processing of picornaviral polyprotein. Annu Rev Microbiol. 1990;44:603–623. doi: 10.1146/annurev.mi.44.100190.003131. [DOI] [PubMed] [Google Scholar]
- Paul A. V., Tada H., von der Helm K., Wissel T., Kiehn R., Wimmer E., Deinhardt F. The entire nucleotide sequence of the genome of human hepatitis A virus (isolate MBB). Virus Res. 1987 Aug;8(2):153–171. doi: 10.1016/0168-1702(87)90026-8. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pevear D. C., Calenoff M., Rozhon E., Lipton H. L. Analysis of the complete nucleotide sequence of the picornavirus Theiler's murine encephalomyelitis virus indicates that it is closely related to cardioviruses. J Virol. 1987 May;61(5):1507–1516. doi: 10.1128/jvi.61.5.1507-1516.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccone M. E., Sira S., Zellner M., Grubman M. J. Expression in Escherichia coli and purification of biologically active L proteinase of foot-and-mouth disease virus. Virus Res. 1995 Mar;35(3):263–275. doi: 10.1016/0168-1702(94)00084-P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter A. G. Picornavirus nonstructural proteins: emerging roles in virus replication and inhibition of host cell functions. J Virol. 1993 Dec;67(12):6917–6921. doi: 10.1128/jvi.67.12.6917-6921.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racaniello V. R., Baltimore D. Molecular cloning of poliovirus cDNA and determination of the complete nucleotide sequence of the viral genome. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4887–4891. doi: 10.1073/pnas.78.8.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson B. H., Grubman M. J., Weddell G. N., Moore D. M., Welsh J. D., Fischer T., Dowbenko D. J., Yansura D. G., Small B., Kleid D. G. Nucleotide and amino acid sequence coding for polypeptides of foot-and-mouth disease virus type A12. J Virol. 1985 Jun;54(3):651–660. doi: 10.1128/jvi.54.3.651-660.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanway G., Cann A. J., Hauptmann R., Hughes P., Clarke L. D., Mountford R. C., Minor P. D., Schild G. C., Almond J. W. The nucleotide sequence of poliovirus type 3 leon 12 a1b: comparison with poliovirus type 1. Nucleic Acids Res. 1983 Aug 25;11(16):5629–5643. doi: 10.1093/nar/11.16.5629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanway G., Hughes P. J., Mountford R. C., Minor P. D., Almond J. W. The complete nucleotide sequence of a common cold virus: human rhinovirus 14. Nucleic Acids Res. 1984 Oct 25;12(20):7859–7875. doi: 10.1093/nar/12.20.7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanway G. Structure, function and evolution of picornaviruses. J Gen Virol. 1990 Nov;71(Pt 11):2483–2501. doi: 10.1099/0022-1317-71-11-2483. [DOI] [PubMed] [Google Scholar]
- Studdert M. J., Gleeson L. J. Isolation and characterisation of an equine rhinovirus. Zentralbl Veterinarmed B. 1978;25(3):225–237. doi: 10.1111/j.1439-0450.1978.tb01180.x. [DOI] [PubMed] [Google Scholar]
- Studdert M. J., Gleeson L. J. Isolation of equine rhinovirus type 1. Aust Vet J. 1977 Sep;53(9):452–452. doi: 10.1111/j.1751-0813.1977.tb05504.x. [DOI] [PubMed] [Google Scholar]
- Thompson J. D., Higgins D. G., Gibson T. J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994 Nov 11;22(22):4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda H., Kohara M., Kataoka Y., Suganuma T., Omata T., Imura N., Nomoto A. Complete nucleotide sequences of all three poliovirus serotype genomes. Implication for genetic relationship, gene function and antigenic determinants. J Mol Biol. 1984 Apr 25;174(4):561–585. doi: 10.1016/0022-2836(84)90084-6. [DOI] [PubMed] [Google Scholar]
- Ward C. W. Progress towards a higher taxonomy of viruses. Res Virol. 1993 Nov-Dec;144(6):419–453. doi: 10.1016/S0923-2516(06)80059-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


