Hypoxic ischemic encephalopathy (HIE) is one of the most serious birth complications affecting full term infants1 occurring in 1.5 to 2.5 per 1000 live births. 2, 3 HIE results in a brain injury from a hypoxic-ischemic event during the prenatal, intrapartum, or postnatal period preventing adequate blood flow to the infant's brain.4 Infants with HIE experience associated morbidities and a significant mortality rate with 40-83% not surviving past 2 years of age or having severe disabilities.5-7 The long-term neurological consequences of HIE include mental retardation, epilepsy, and cerebral palsy.7
The incidence of HIE has not declined with advances in obstetric care (i.e., fetal monitoring) aimed at preventing hypoxic-ischemic events;8 thus much of the current neonatal research focuses on preventing further brain injury after the hypoxic-ischemic event.9 In the past, treatment options were limited to standard medical treatment (e.g., antiepileptic medications or respiratory support) and maintaining normothermia.10, 11 Currently, moderate hypothermia5, 12-14 is utilized as a main treatment for infants with HIE, but the long-term success of the treatment on infant outcomes remain inconclusive.
The challenge with drawing conclusions about the effect of moderate hypothermia on infant outcomes relates, in part, to the multiple ways the treatment is administered. Large clinical trials have delivered the intervention using primarily two methods of delivery; selective head cooling (see Figure 1) or whole body cooling (see Figure 2). The complexity in this treatment modality includes the use of different target core temperatures and variable lengths of time for treatment. Tagin et al.15 conducted a systematic review identifying randomized controlled trials (n = 7) comparing moderate hypothermia to normothermia (standard care) in neonates with HIE. Of the seven studies, four of the studies employed whole body cooling5, 7, 14, 16 as the intervention and three of the studies used head cooling17-19 as the intervention comparing it to normothermia/control groups. The target core temperature for the intervention group ranged from 33 to 36.5°C. Table 1 illustrates the variations in hypothermia administration and the primary outcomes from the clinical trials identified by Tagin15 with updated primary outcomes for the NICHD12 and CoolCap13 studies.
Figure 1.
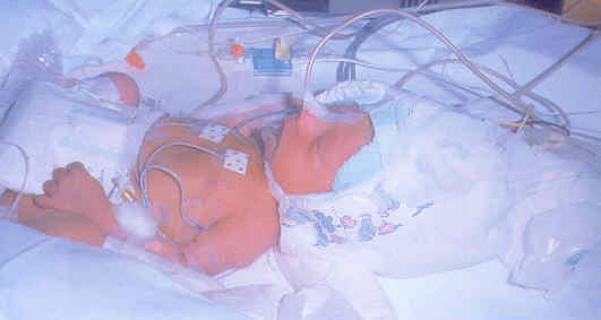
Selective Head Cooling
Figure 2.
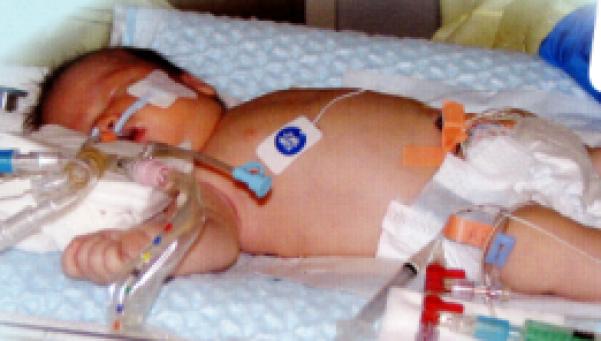
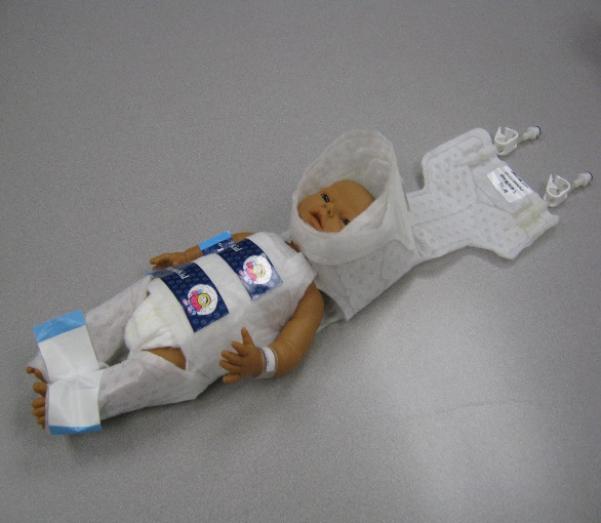
Whole body cooling
2a: whole body cooling with cooling blanket
2b: whole body cooling with body wrap
While the results of moderate hypothermia are inconclusive as to whether the intervention decreases death and/or severe neurological disability, moderate hypothermia is one of the few interventions for HIE that offers promise. Many neonatal intensive care units (NICUs) are implementing moderate hypothermia, but must decide whether whole body cooling or head cooling is better. Further exploration as to whether whole body cooling or head cooling produces better outcomes and fewer side effects is necessary. Therefore, the purpose of this review is to determine the level of evidence available comparing head cooling to whole body cooling for neonates with HIE.
PubMed was searched to obtain English language publications from 1998 to December 2013 for trials comparing safety and efficacy of whole body cooling or selective head cooling infants with HIE. The inclusion criteria of studies were clinical studies comparing whole body cooling to selective head cooling and included human infants with HIE. Studies were excluded if selective head cooling was compared to standard care only or whole body cooling was compared to standard care only because the purpose to was determine which method of cooling is better. Four studies20-23 were identified that met inclusion criteria.
The four studies all compared whole body cooling to selective head cooling in human neonates (see Table 2). One study23 compared magnetic resonance imaging (MRI) results between whole body cooling to selective head cooling in neonates within the first week of life. The remaining three studies20-22 compared the physiologic effects (temperature and organ dysfunction) of receiving whole body cooling to selective head cooling on neonates over the first 72 hours of life. These three studies were composed of two separate samples of neonates with HIE. Sarkar and colleagues21, 22 published study results comparing the physiologic effects including multisystem organ dysfunction22 and pulmonary dysfunction21 on a single sample of infants in two separate publications. Since the results are from the same sample of infants, the two publications will be referred to as a single study throughout the rest of this discussion. Each study is described and recommendations are offered as to the future directions of clinical and research needs in whole body cooling and selective head cooling for neonates with HIE.
A single clinical study23 was identified that compared radiographic outcomes in whole body cooling to head cooling. Generally, the distribution of lesions on MRI after treatment with hypothermia tends to predict long-term outcomes in neonates.24 The two main patterns of injury seen in hypoxic-ischemic events are the watershed (WS) predominant pattern and basal ganglia/thalamus distribution (BGT) pattern.25 The WS pattern involves the intervascular boundary-zone white matter with cortical gray matter when a severe insult was sustained. The BGT pattern involves the deep gray nuclei, hippocampi, and perirolandic cortex with additional cortical involvement if severe injury occurs.24 Sakar et al.23 sought to determine the difference in severity and distribution of brain lesions identified on MRI after treatment with hypothermia. This study was a retrospective medical chart review of MRIs obtained at 7 to 10 days of life in 83 infants cooled by either head cooling (n = 34) or whole body cooling (n = 49). HIE lesions were seen in 47 of the 83 cooled infants: BGT in 7, cortical not extending beyond the watershed regions in 16, cortical not extending beyond the watershed regions and basal nuclei in 5, and cortical extending beyond the watershed regions and basal nuclei in 19 infants. These findings indicate that neither method of cooling ‘cures’ nor ‘heals’ all of the lesions from the hypoxic-ischemic event. Significantly more abnormal brain MRIs were found in infants receiving selective head cooling compared to whole body cooling. The patterns visualized on MRI were also categorized based on the pattern and severity of injury using standardized scoring system (BG/W) with scores ranging from 0 being normal or no injury to 4 being abnormal signal in the cortex extending beyond the watershed areas and basal nuclei, indicating the most severe injury.26 MRIs of infants in the selective head cooling group had a median BG/W score of 2 and the whole body cooling group had a median BG/W score of 0, which is a significant difference indicating the head cooling group had a greater severity of lesions of MRI. These findings suggest that whole body cooling is the preferred method to selective head cooling for infants with HIE, at least within the first week of life. However, these are only the results of one small study and the findings were retrospective and thus, the level of evidence is not as high as with other types of research.
Sarkar et al.21, 22 conducted an observational study examining organ function in whole body cooling and selective head cooling. A total of 59 infants were enrolled with 28 infants receiving whole body cooling and 31 infants receiving selective head cooling. No differences were found between groups in regard to needing mechanical ventilation during cooling or being able to be extubated during cooling or the incidence of persistent pulmonary hypertension (PPHN).21 Ventilatory parameters during cooling including maximum peak inspiratory pressure (PIP), maximum positive end-expiratory pressure (PEEP), maximum FiO2 (fraction of inspired oxygen), and highest PaCO2 (partial pressure of carbon dioxide) were also not different between whole body and selective head cooling.21 Indicators of organ dysfunction were also similar between group including coagulopathy treated with fresh frozen plasma (FFP), thrombocytopenia treated with platelet transfusion, hypotension treated with vasopressors for > 24 hours, urine output < 0.5 ml/kg/hr for > 24 hours after birth, and electrolyte abnormalities (hyponatremia, hypokalemia, hypocalcemia).22 This study suggests that neither cooling method offers less risk of organ dysfunction.
Hoque et al.20 performed an observational study with four group receiving moderate hypothermia to determine the differences in temperature and hemodynamic stability across groups. The four group had a total of 73 infants with HIE: selective head cooling (n = 20), whole body cooling with mattress manually controlled (n = 23), whole body cooling with body wrap servo-controlled (n = 28), and whole body with water-filled gloves (n = 2). During the initiation of hypothermia, the target core temperature was ‘overshoot’ when cooling with the manually controlled mattress. There was significantly greater overshoot in range of hypothermia in the manually controlled group (head cooling and mattress) compared with the servo-controlled group. Additionally during the maintenance period of the hypothermia, greater variability in the core temperature was observed in the manually controlled group compared to the servo-controlled group. In rewarming a similar pattern was observed with greater variation in core temperature in the head cooling group compared to the servo-controlled group. Hemodynamic variation in mean arterial blood pressure (MABP) was also greater in the whole body manually controlled group compared to the whole body servo-controlled group. These results suggest that manually controlled hypothermia may be more difficult to achieve and maintain temperature control with, which could affect long-term outcomes.
Based on the evidence from the literature reviewed, whole body appears to offer the most promise. However, this must be interpreted with significant caution. Three clinical studies is not enough evidence to determine which method of moderate hypothermia is safer and more effective. Future research with larger samples is needed to identify which method is best for which infants with HIE to ensure these infants have reduced morbidity. Additionally, long-term follow-up studies with previous large randomized clinical trials are also necessary to evaluate whether moderate hypothermia is a worth-while treatment for infants with HIE.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Nursing Research (NINR) Training Grant T32NR007106.
References
- 1.Schiariti V, Klassen AF, Hoube JS, Synnes A, Lisonkova S, Lee SK. Perinatal characteristics and parents’ perspective of health status of NICU graduates born at term. J Perinatol. 2008;28:368–76. doi: 10.1038/jp.2008.9. doi: 10.1038/jp.2008.9. [DOI] [PubMed] [Google Scholar]
- 2.Graham EM, Ruis KA, Hartman AL, Northington FJ, Fox HE. A systematic review of the role of intrapartum hypoxia-ischemia in the causation of neonatal encephalopathy. Am J Obstet Gynecol. 2008;199:587–95. doi: 10.1016/j.ajog.2008.06.094. doi: 10.1016/j.ajog.2008.06.094. [DOI] [PubMed] [Google Scholar]
- 3.Kurinczuk JJ, White-Koning M, Badawi N. Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum Dev. 2010;86:329–38. doi: 10.1016/j.earlhumdev.2010.05.010. doi: 10.1016/j.earlhumdev.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 4.Volpe J. Neurology of the Newborn. 5th ed. Saunders; Philadelphia: 2008. [Google Scholar]
- 5.Jacobs SE, Morley CJ, Inder TE, et al. Whole-body hypothermia for term and near-term newborns with hypoxic-ischemic encephalopathy: a randomized controlled trial. Arch Pediatr Adolesc Med. 2011;165:692–700. doi: 10.1001/archpediatrics.2011.43. doi: 10.1001/archpediatrics.2011.43. [DOI] [PubMed] [Google Scholar]
- 6.Kwon JM, Guillet R, Shankaran S, et al. Clinical seizures in neonatal hypoxic-ischemic encephalopathy have no independent impact on neurodevelopmental outcome: secondary analyses of data from the neonatal research network hypothermia trial. J Child Neurol. 2011;26:322–8. doi: 10.1177/0883073810380915. doi: 10.1177/0883073810380915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simbruner G, Mittal RA, Rohlmann F, Muche R. Systemic hypothermia after neonatal encephalopathy: outcomes of neo.nEURO.network RCT. Pediatrics. 2010;126:e771–8. doi: 10.1542/peds.2009-2441. doi: 10.1542/peds.2009-2441. [DOI] [PubMed] [Google Scholar]
- 8.Kumar S, Paterson-Brown S. Obstetric aspects of hypoxic ischemic encephalopathy. Early Hum Dev. 2010;86:339–44. doi: 10.1016/j.earlhumdev.2010.05.009. doi: 10.1016/j.earlhumdev.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Shankaran S. Neonatal encephalopathy: treatment with hypothermia. J Neurotrauma. 2009;26:437–43. doi: 10.1089/neu.2008.0678. doi: 10.1089/neu.2008.0678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vannucci RC, Perlman JM. Interventions for perinatal hypoxicischemic encephalopathy. Pediatrics. 1997;100:1004–14. doi: 10.1542/peds.100.6.1004. doi. [DOI] [PubMed] [Google Scholar]
- 11.Hoehn T, Hansmann G, Buhrer C, et al. Therapeutic hypothermia in neonates. Review of current clinical data, ILCOR recommendations and suggestions for implementation in neonatal intensive care units. Resuscitation. 2008;78:7–12. doi: 10.1016/j.resuscitation.2008.04.027. doi: 10.1016/j.resuscitation.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 12.Shankaran S, Pappas A, McDonald SA, et al. Childhood outcomes after hypothermia for neonatal encephalopathy. N Engl J Med. 2012;366:2085–92. doi: 10.1056/NEJMoa1112066. doi: 10.1056/NEJMoa1112066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guillet R, Edwards AD, Thoresen M, et al. Seven- to eight-year follow-up of the CoolCap trial of head cooling for neonatal encephalopathy. Pediatr Res. 2012;71:205–9. doi: 10.1038/pr.2011.30. doi: 10.1038/pr.2011.30. [DOI] [PubMed] [Google Scholar]
- 14.Azzopardi D, Strohm B, Edwards AD, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361:1349–58. doi: 10.1056/NEJMoa0900854. doi: 10.1056/NEJMoa0900854. [DOI] [PubMed] [Google Scholar]
- 15.Tagin MA, Woolcott CG, Vincer MJ, Whyte RK, Stinson DA. Hypothermia for neonatal hypoxic ischemic encephalopathy: an updated systematic review and meta-analysis. Arch Pediatr Adolesc Med. 2012;166:558–66. doi: 10.1001/archpediatrics.2011.1772. doi: 10.1001/archpediatrics.2011.1772. [DOI] [PubMed] [Google Scholar]
- 16.Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–84. doi: 10.1056/NEJMcps050929. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 17.Gunn AJ, Gluckman PD, Gunn TR. Selective head cooling in newborn infants after perinatal asphyxia: a safety study. Pediatrics. 1998;102:885–92. doi: 10.1542/peds.102.4.885. doi. [DOI] [PubMed] [Google Scholar]
- 18.Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365:663–70. doi: 10.1016/S0140-6736(05)17946-X. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- 19.Zhou WH, Cheng GQ, Shao XM, et al. Selective head cooling with mild systemic hypothermia after neonatal hypoxic-ischemic encephalopathy: a multicenter randomized controlled trial in China. J Pediatr. 2010;157:367–72. 72, e1–3. doi: 10.1016/j.jpeds.2010.03.030. doi: 10.1016/j.jpeds.2010.03.030. [DOI] [PubMed] [Google Scholar]
- 20.Hoque N, Chakkarapani E, Liu X, Thoresen M. A comparison of cooling methods used in therapeutic hypothermia for perinatal asphyxia. Pediatrics. 2010;126:e124–30. doi: 10.1542/peds.2009-2995. doi: 10.1542/peds.2009-2995. [DOI] [PubMed] [Google Scholar]
- 21.Sarkar S, Barks JD, Bhagat I, Dechert R, Donn SM. Pulmonary dysfunction and therapeutic hypothermia in asphyxiated newborns: whole body versus selective head cooling. Am J Perinatol. 2009;26:265–70. doi: 10.1055/s-0028-1103154. doi: 10.1055/s-0028-1103154. [DOI] [PubMed] [Google Scholar]
- 22.Sarkar S, Barks JD, Bhagat I, Donn SM. Effects of therapeutic hypothermia on multiorgan dysfunction in asphyxiated newborns: whole-body cooling versus selective head cooling. J Perinatol. 2009;29:558–63. doi: 10.1038/jp.2009.37. doi: 10.1038/jp.2009.37. [DOI] [PubMed] [Google Scholar]
- 23.Sarkar S, Donn SM, Bapuraj JR, Bhagat I, Barks JD. Distribution and severity of hypoxic-ischaemic lesions on brain MRI following therapeutic cooling: selective head versus whole body cooling. Arch Dis Child Fetal Neonatal Ed. 2012;97:F335–9. doi: 10.1136/fetalneonatal-2011-300964. doi: 10.1136/fetalneonatal-2011-300964. [DOI] [PubMed] [Google Scholar]
- 24.Steinman KJ, Gorno-Tempini ML, Glidden DV, et al. Neonatal watershed brain injury on magnetic resonance imaging correlates with verbal IQ at 4 years. Pediatrics. 2009;123:1025–30. doi: 10.1542/peds.2008-1203. doi: 10.1542/peds.2008-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller SP, Ramaswamy V, Michelson D, et al. Patterns of brain injury in term neonatal encephalopathy. J Pediatr. 2005;146:453–60. doi: 10.1016/j.jpeds.2004.12.026. doi: 10.1016/j.jpeds.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 26.Barkovich AJ, Hajnal BL, Vigneron D, et al. Prediction of neuromotor outcome in perinatal asphyxia: evaluation of MR scoring systems. AJNR Am J Neuroradiol. 1998;19:143–9. doi. [PMC free article] [PubMed] [Google Scholar]
- 27.Pimentel-Coelho PM, Rosado-de-Castro PH, da Fonseca LM, Mendez-Otero R. Umbilical cord blood mononuclear cell transplantation for neonatal hypoxic-ischemic encephalopathy. Pediatr Res. 2012;71:464–73. doi: 10.1038/pr.2011.59. doi: 10.1038/pr.2011.59. [DOI] [PubMed] [Google Scholar]
- 28.Zhu C, Kang W, Xu F, et al. Erythropoietin improved neurologic outcomes in newborns with hypoxic-ischemic encephalopathy. Pediatrics. 2009;124:e218–26. doi: 10.1542/peds.2008-3553. doi: 10.1542/peds.2008-3553. [DOI] [PubMed] [Google Scholar]
- 29.Elmahdy H, El-Mashad AR, El-Bahrawy H, El-Gohary T, El-Barbary A, Aly H. Human recombinant erythropoietin in asphyxia neonatorum: pilot trial. Pediatrics. 2010;125:e1135–42. doi: 10.1542/peds.2009-2268. doi: peds.2009-2268 [pii] 10.1542/peds.2009-2268. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


