Summary
The objective of the present study was to determine whether Fyn kinase participated in signaling events during sperm–egg interactions, sperm incorporation, and meiosis II. The functional requirement of Fyn kinase activity in these events was tested through the use of the protein kinase inhibitor SKI-606 (Bosutinib) and by analysis of Fyn-null oocytes. Suppression of Fyn kinase signaling prior to fertilization caused disruption of the functional polarity of the oocyte with the result that sperm were able to fuse with the oocyte in the immediate vicinity of the meiotic spindle, a region that normally does not allow sperm fusion. The loss of functional polarity was accompanied by disruption of the microvilli and cortical granule-free zone that normally overlie the meiotic spindle. Changes in the distribution of cortical granules and filamentous actin provided further evidence of disorganization of the oocyte cortex. Rho B, a molecular marker for oocyte polarity, was unaffected by suppression of Fyn activity; however, the polarized association of Par-3 with the cortex overlying the meiotic spindle was completely disrupted. The defects in oocyte polarity in Fyn-null oocytes correlated with a failure of the MII chromosomes to maintain a position close to the oocyte cortex which seemed to underlie the above defects in oocyte polarity. This was associated with a delay in completion of meiosis II, however, pronuclei eventually formed and subsequent mitotic cleavages and blastocyst formation occurred normally.
Introduction
Fertilization involves the preprogrammed activation of a series of protein kinase cascades that serve to establish the block to polyspermy (Runft et al., 2002; Wortzman-Show et al., 2007), initiate meiosis resumption (Ducibella and Fissore, 2008), pronuclear congression (Meng et al., 2006; McGinnis et al., 2007), activate egg metabolism, and trigger entry into the mitotic cell cycle (Liu and Maller, 2005). Src-family protein tyrosine kinases (PTKs) are cytosolic kinases, which contain Src homology 2 (SH2) and Src homology 3 (SH3) protein interaction domains as well as an N-terminal fatty acid acylation site that promotes membrane microdomain interactions (Bromann et al., 2004). These diverse features enable this family of PTKs to interact with signaling cascades located in discrete regions of the cell such as cell surface receptors, actin-based cytoskeletal elements (Angers-Loustau et al., 1999), and nuclear structures (Park et al., 1999; Haendeler et al., 2003; Zaidi et al., 2004; Coluccia et al., 2006). A large body of evidence obtained in species that fertilize externally, demonstrated that Src-family PTK signaling is activated at the site of sperm–egg interaction and progressed through the oocyte cortex eventually involving both the cortical and cytosolic compartments (reviewed in Sharma and Kinsey, 2008). In these species, Src-family PTKs play an important role during two phases of egg activation, sperm-induced calcium signaling (O'Neill et al., 2004) and progression from pronuclear to mitotic stages (Sharma and Kinsey, 2006). In mammalian fertilization, the global, high amplitude kinase activation typical of externally fertilized eggs has not been detected. However, evidence exists for localized, compartment-specific activation of Src-family PTKs in response to fertilization. For example, immunofluorescence detection of phosphotyrosine-containing proteins in the region of the actin cap and at sites of sperm incorporation provided indication that PTK activation had occurred, but these data did not identify the kinase(s) involved (McGinnis et al., 2007). The same study used a phosphorylation-site- specific antibody to detect active Src-family PTKs, which demonstrated that kinase activation occurs in proximity to the meiotic spindle and the pronuclear envelope, but no significant activation was detected at the cortex of the mouse oocyte. Functional studies of Fyn knockout mice have re- ported normal fertility (Stein et al., 1994); however, our detailed studies demonstrated that Fyn knockout mice exhibit impaired oocyte maturation (McGinnis et al., 2009), reduced developmental potential (unpublished), and smaller litter size (unpublished). Functional analyses using pharmacological inhibitors of Src-family kinases or dominant-nega- tive constructs support some roles in meiosis II (MII) resumption (Sette et al., 2002; Talmor-Cohen et al., 2004a), pronuclear congression (Moore and Kinsey, 1995; Wright and Schatten, 1995), and initiation of mitotic divisions (Besterman and Schultz, 1990; Jacquet et al., 1995; Meng et al., 2006). Whether or not this is due to effects on sperm-induced calcium signaling (Kurokawa et al., 2004); (Tomashov-Matar et al., 2008) remains an open question.
The objective of the present study was to test for a role of Src-family PTKs in the earliest stages of egg activation including sperm–egg interactions, sperm incorporation, and MII resumption. Since Fyn kinase was found to be the most highly expressed Src-family member in MII oocytes, the Fyn knockout model (Stein et al., 1992) was used to detect defects in egg activation. To rule out the possibility that some of the defects observed in Fyn-null oocytes may have resulted from indirect effects due to lack of Fyn in the maturing follicle cells, a second approach made use of the protein kinase inhibitor SKI-606 (Bosutinib; Wyeth, Pearl River, NY) to specifically inhibit Src-family PTK activity in wild-type oocytes prior to exposure to sperm. While the inhibitor was not specific for Fyn as opposed to Yes or other Src-family PTKs, it did provide an opportunity to confirm the results observed in the Fyn knockout oocyte.
The results presented below demonstrate that suppression of Fyn activity had no significant effect on sperm–egg fusion/incorporation. However, completion of anaphase II and pronuclear formation was reduced. The studies also revealed observations relevant to the functional polarity of the MII oocyte. Oocytes in which Fyn signaling was sup pressed exhibited a high frequency of sperm–egg fusion in the vicinity of the MII chromosomes. This was accompanied by disruption of the cortical granule and microvilli-free zone, as well as molecular markers of oocyte polarity such as f- actin and Par-3. The results suggest that Fyn kinase signaling played an important role in establishment and maintenance of the functional polarity of the MII oocyte.
Results
Functional Role of Src-Family PTKs in Sperm–Egg Interactions and Meiosis Resumption
Mammalian oocytes are known to express several members of the Src family of PTKs including Fyn, Yes, and Fgr which were detected by Western blot (Talmor et al., 1998; Mehlmann and Jaffe, 2005). Identification of other family members has been problematic largely because of limited material and antibody sensitivity. The recent availability of expression array data representing polysome-associated transcripts from mouse MII oocytes (Potireddy et al., 2006) presented an alternative method to characterize the expression of Src-family PTKs in oocytes. Analysis of the published data from four samples of oocytes revealed that the Fyn kinase was expressed at a level approximately ninefold higher than the average transcript and was the most highly expressed Src-family member in MII oocytes, followed by Yes kinase (Table 1). Other members of the Src family were detected at very low levels.
Table 1. Expression of Src-Family Protein Kinases in Mouse Oocytes.
| Kinase | Probe set | Rel. mRNA level | SD |
|---|---|---|---|
| Src | 1423518_at | 59.8 | 17.8 |
| Yes | 1449090_a_at | 542.4 | 64.4 |
| Fyn | 1448765_at | 1,365.3 | 320.1 |
| Fgr | 1419526_at | 98.1 | 12.9 |
| Lck | 1425396_a_at | 44.8 | 7.3 |
| Hck | 1449455_at | 61.5 | 4.7 |
| Lyn | 1425598_a_at | 62.9 | 9.9 |
| Blk | 1422775_at | 68.5 | 22.9 |
Relative expression values were obtained from published expression array data representing a set of four samples of polysomal mRNA prepared from MII mouse oocytes (GSM89969, GSM89970, GSM89971, GSM89972) (Potireddy et al., 2006). The mRNA was hybridized to Affymetrics MOE 430 2.0 chips. Expression values were normalized to a target value of 150. Values represent the average from the four data sets presented with the standard deviation (SD).
Fyn kinase activity is known to function during pronuclear congression and mitosis of mammalian zygotes (Meng et al., 2006; McGinnis et al., 2007), but limited information is available regarding the role of this kinase during the earliest stages of oocyte activation. The functional role of Fyn signaling events during sperm–egg interactions and anaphase resumption was tested by evaluating the effect of suppressed Fyn signaling on the ability of oocytes to fuse with and incorporate sperm, resume meiosis, form pronuclei, and divide following in vitro fertilization (IVF). Fyn signaling was suppressed by two approaches including a pharmacological inhibitor specific for Src-family PTKs, though not specific for Fyn, and through analysis of oocytes recovered from Fyn-null mice. In one experimental group, total Src-family PTK activity was suppressed in mature CF1 oocytes by incubating cumulus-free or zona-free oocytes in medium containing the inhibitor SKI-606 (25 mM) for 60 min. The oocytes were then washed free of inhibitor and fertilized in vitro as described in Materials and Methods Section. The second experimental group used oocytes obtained from Fyn-null females. These oocytes contained no detectable Fyn kinase (see below). Controls for the kinase inhibitor included cumulus-free CF1 oocytes treated with dimethylsulfoxide (DMSO) as a solvent control. Controls for the Fyn-null oocytes were the host strain B6/129SF2/J oocytes, representing a control for strain differences. Control and Fyn-null groups were fertilized in vitro by incubation with capacitated wild-type sperm for a minimum of 4 hr, then fixed and prepared for examination by confocal fluorescence microscopy to detect the number of incorporated sperm, their location relative to the MII spindle, the status of the metaphase to anaphase transition, formation of pronuclei, and the frequency of cell division at 25 hr post-insemination. Sperm incorporation was determined by confocal analysis of sperm heads stained with ethidium homodimer (EthD-2) and sperm were considered “incorporated” only if the sperm head showed clear evidence of decondensation. The results presented in Table 2 revealed that suppression of Fyn signaling in the oocyte had no significant effect on its ability to fuse with and incorporate sperm since the ratio of sperm incorporated per egg and the frequency of fertilization were similar among all groups. This level of analysis did allow us to identify the site of sperm incorporation and revealed the unusual result that oocytes in which Fyn signaling were suppressed had reduced capacity to prevent sperm–egg fusion in the region overlying the meiotic spindle. This was apparent in both SKI-606-treated and Fyn-null oocytes where the frequency of sperm incorporation within ±300 of the spindle was significantly higher than controls. Examples of this unusual event which is normally very rare in control oocytes are presented in Figure 1.
Table 2. Effect of Src-Family PTK Suppression on Oocyte Activation.
| Control (n ¼ 182) | SKI-606 (n ¼ 76) | Fyn−/− (n ¼ 90) | ||||
|---|---|---|---|---|---|---|
| Parameter | Mean | SEM | Mean | SEM | Mean | SEM |
| No. of sperm incorporated | 0.68 | 0.108 | 0.61 | 0.123 | 0.67 | 0.133 |
| % Fertilized | 62% | 9.5 | 54% | 11.7 | 65% | 12.9 |
| % Polyspermy | 5% | 3.3 | 23% | 15.6 | 3% | 2.0 |
| % Sperm at spindle | 4% | 2.1 | 40%* | 16.4 | 16%* | 5.1 |
| % Ana./tel. | 60 | 13.0 | 87% | 8.7 | 93% | 4.8 |
| % PN | 34% | 12.6 | 13% | 8.7 | 3%* | 2.5 |
| % Dspl. chrom. | 3 | 1.9 | 33%* | 10.5 | 17%* | 8.4 |
| % Two cells | 70% | 7.8 | 56% | 11.8 | 77 | 11.6 |
The effect of suppression of Src-family PTK signaling on oocyte activation was tested in two experimental groups of oocytes. Cumulus-free CF1 oocytes were incubated in KSOMaa containing 0.25% DMSO (control) or with 25 mM SKI-606 for 1 hr, then washed before in vitro fertilization. A second group of cumulus-free oocytes from Fyn-null females was used directly for in vitro fertilization. Controls for the Fyn-null oocytes were normal B6/129SF2/J oocytes. Results from both control groups were not statistically different and were combined in this table. Fertilization was performed as described in Materials and Methods Section, and oocytes were fixed at either 4.5 or 25 hr (two cells) post-insemination. Sperm were considered incorporated if the sperm head was surrounded by ooplasm and there was evidence of nuclear decondensation. Sperm were considered incorporated near the spindle (sperm at spindle) if the head was located within ±30o of the spindle. The frequency of cell division (two cells) was determined in zygotes fixed at 25 hr post-insemination.
indicates that the value is statistically different from control values (P < 0.05).
Figure 1.
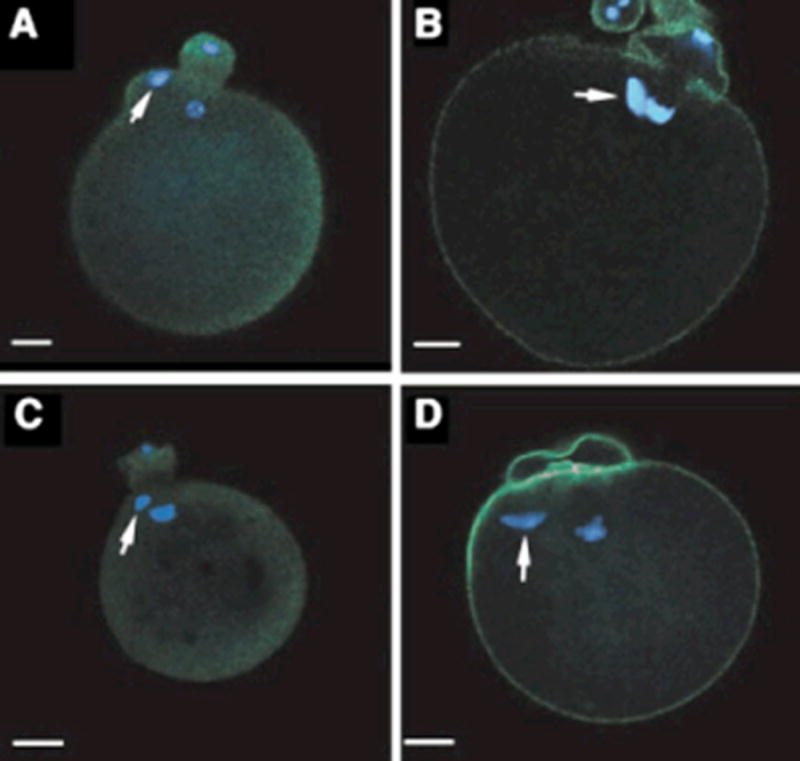
Sperm incorporation near the meiotic spindle in oocytes deficient in Src-family PTK signaling. Oocytes from CF1 females were denuded of cumulus cells, treated with 25 mM SKI-606 in mKSOMaa, then washed by passage through a drop of mKSOMaa (panels A,B). Oocytes from Fyn-null females were denuded of cumulus cells and washed as above (panels C,D). Both groups of oocytes were fertilized in vitro by addition of capacitated sperm as described in Materials and Methods Section. Fertilization was allowed to proceed for 4.5 hr after which time the oocytes were fixed and processed for confocal fluores- cence microscopy and stained with ethidium homodimer to label DNA (blue) and either anti-P-Tyr antibody (green, panels A,C) or Oregon green-phalloidin (panels B,D) (green). The presence of sperm heads within the egg cytoplasm is indicated by the white arrows, while the unlabeled chromatin is the oocyte chromosomal complement arranged on the telophase spindle. Magnification is indicated by the bar which represents 10 mm.
Meiosis resumption was not affected in Fyn-null oocytes or in oocytes pretreated with 25 mM SKI-606 as shown by the fact that over 96% of oocytes containing sperm entered anaphase before fixation at 4.5 hr post-insemination. How- ever, both SKI-606 treatment and Fyn knockout delayed completion of meiosis as detected by pronuclear formation with the result that the frequency of pronuclear stage zygotes was significantly less than in controls (Table 2). The above experiments with SKI-606 required treatment with high (25 mM) concentrations of SKI-606 because the inhibitor was washed out prior to insemination to prevent the effects on sperm. Separate experiments in which zona-free oocytes were pretreated with SKI-606, then washed and fertilized via a short 35 min insemination, followed by re- application of the inhibitor, confirmed the above delay of pronuclear formation and increased chromosome displacement at SKI-606 concentrations as low as 1 mM (not shown). Unfortunately, zona-free eggs were found to be undesireable for our morphological studies (below) because they exhibited an abnormal pattern of P-Tyr immunofluorescence in the egg cortex (not shown). The following studies were therefore performed with zona-intact oocytes.
One mechanism for the delay in pronuclear formation may be that suppression of Fyn activity caused an increased frequency of displaced chromosomes (Table 1; Fig. 2) which may have caused activation of the spindle assembly check- point at MII (Brunet and Maro, 2005). In any case, the chromosome displacement was not permanent, and oocytes were able to form normal pronuclei after a delay.
Figure 2.
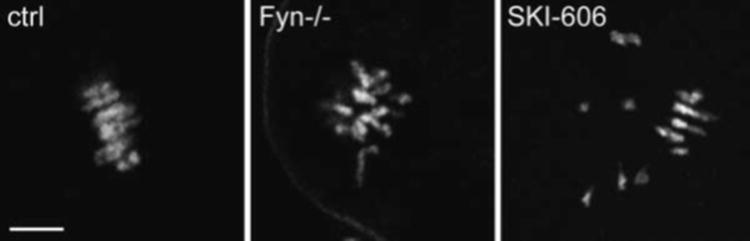
Suppression of Src-family PTK activity leads to disorganization of MII chromosomes. Oocytes from CF1 females were denuded of cumulus cells, treated with DMSO (ctrl) or with 25 mM SKI-606 in mKSOMaa for 1 hr, then fixed for immunofluorescence microscopy. Oocytes from Fyn-null females were denuded of cumulus cells and fixed similarly. Samples were labeled with Oregon green-phalloidin to stain actin filaments and with ethidium homodimer to label chromatin. The oocytes from Fyn-null or SKI-606-treated groups had a high frequency of disorganized chromosomes (middle and right panels). Magnification is indicated by the bar which represents 10 mm.
Fyn Activity Is Required for the Normal Morphology and Distribution of Microvilli on Oocytes
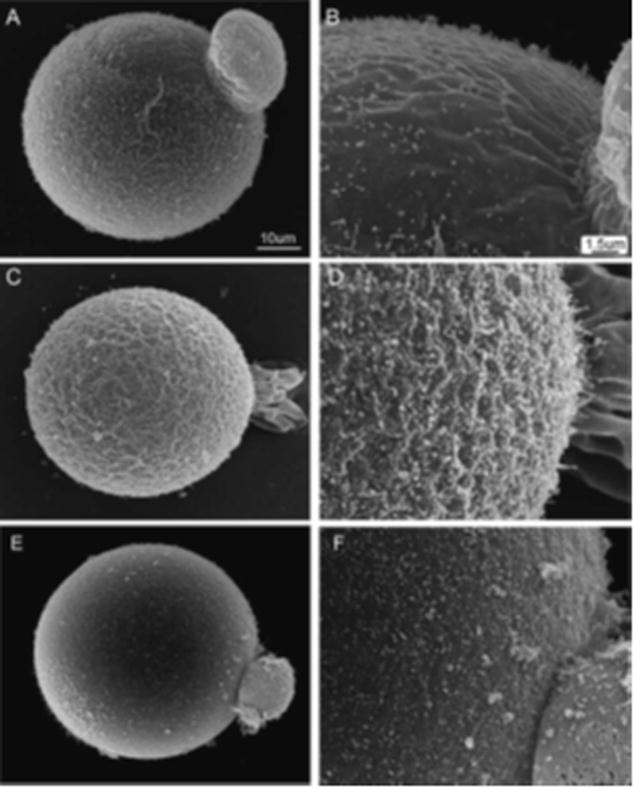
The observation that suppression of Fyn activity enabled oocytes to fuse with sperm in the area of the meiotic spindle indicated that the functional polarity of the oocyte may have been disrupted. One morphological feature that reflects the polarized nature of the oocyte cortex is the distribution of microvilli over the oocyte surface. The mature mammalian oocyte typically displays microvilli distributed densely over the oocyte surface with the exception of a microvilli-free zone overlying the meiotic spindle (Longo and Chen, 1984) (Fig. 3A,B). Recent evidence indicates that sperm–egg fusion occurs primarily at the tips of microvilli (Runge et al., 2007), and the absence of microvilli over the spindle could explain why sperm do not normally fuse with the oocyte in this area. In order to determine whether suppression of Fyn activity caused changes in the normal distribution of microvilli, we examined the effect of SKI-606 and Fyn knockout on oocyte surface morphology via scan- ning electron microscopy. Analysis of zona-free Fyn-null oocytes revealed that microvilli were present over the entire oocyte with no distinct microvilli-free zone (Fig. 3C,D) demonstrating that the morphological polarity of the oocyte was disrupted in Fyn-null oocytes. In order to test whether the normal pattern of microvilli in a mature oocyte could be disrupted by chemical suppression of Src-family PTK activity, mature oocytes from CF1 females were incubated with 25 mM SKI-606 for 1 hr and then processed for scanning electron microscopy. Exposure to SKI-606 caused redistribution of the microvilli obscuring the microvilli-free zone (Fig. 3E,F). This effect was reversible in the majority of oocytes (not shown). In summary, conditions that sup- pressed Fyn signaling in the oocyte caused disruption of the polarized distribution of microvilli leading to their inap- propriate appearance in the region of the cortex overlying the meiotic spindle.
Figure 3.
Suppression of Src-family PTK signaling disrupts the microvilli-free zone in mouse oocytes. The distribution of microvilli was determined by scanning electron microscope imaging of zona-free oocytes. Control CF1 oocytes exhibit a microvilli-free zone adjacent to the first polar body in the region overlying the meiotic spindle (panels A,B). Fyn-null oocytes did not exhibit such a region (panels C,D). When CF1 oocytes were treated with 25 mM SKI-606 for 1 hr, the microvilli-free zone disappeared as microvilli formed or migrated into the area of cortex immediately surrounding the polar body (panels E,F).
Fyn Activity Is Required for Establishment and Maintenance of the Cortical Granule-Free Zone in Oocytes
Previous work has documented that the cortical secretory granules are excluded from the oocyte cortex overlying the meiotic spindle as a result of exocytosis during oocyte maturation and through translocation in response to cortical positioning of the maternal chromatin (Hoodbhoy and Talbot, 2001; Deng et al., 2003). In order to determine whether suppression of Fyn activity caused changes in the normal distribution of cortical granules, we used fluorescein isothiocyanate (FITC)-LCA to detect cortical granules by confocal fluorescence microscopy. The normal unfertilized oocyte exhibited a large cortical granule-free zone over the MII chromosomes as seen in Figure 4A. However, a large proportion of oocytes from Fyn-null females contained a patchy distribution of cortical granules with no distinct cortical granule-free zone (Fig. 4B,C). To determine whether Fyn activity was required for maintenance of the cortical granule- free zone, oocytes from CF1 females were treated with DMSO as a control (Fig. 4D) or with 25 mM SKI-606 for 1 hr (Fig. 4E) then washed free of inhibitor and allowed to recover for 1 hr (Fig. 4F). Exposure to SKI-606 for 1 hr caused redistribution of the cortical granules obscuring the cortical granule-free zone (Fig. 4E). Once the inhibitor was removed, the cortical granules were again redistributed recreating a cortical granule-free zone (Fig. 4F). These results demonstrated that Fyn activity played a critical role in maintenance of the distribution of cortical secretory granules in the oocyte cortex.
Figure 4.
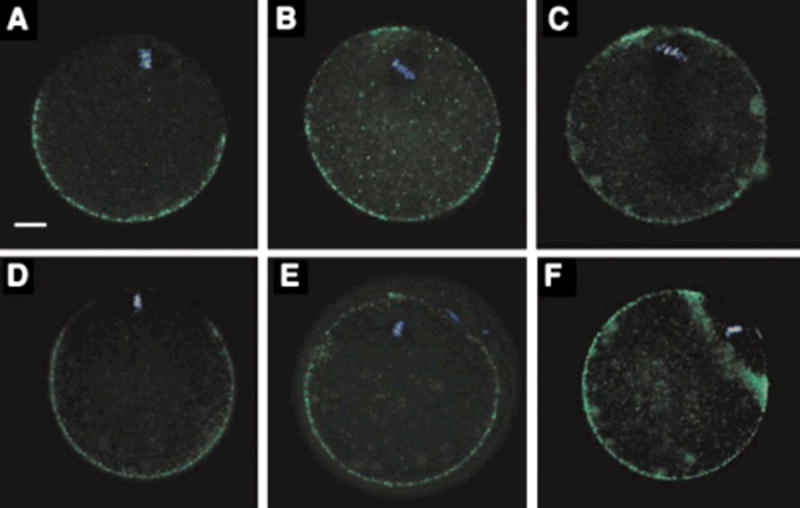
Suppression of Src-family PTK signaling disrupts the distribution of cortical granules. Oocytes from control CF1 females (panel A) or from Fyn-null females (panels B,C) were denuded of cumulus cells and fixed for confocal fluorescence analysis using FITC-labeled LCA to detect cortical granule contents. The control oocytes exhibit a normal cortical granule-free zone overlying the spindle; however, Fyn-null oocytes exhibit an uneven distribution of cortical granules with no cortical granule-free zone over the spindle. The effect of SKI-606 on the distribution of cortical granules was tested by treating CF1 oocytes with DMSO as a control (panel D), or with (25 mM SKI-606) for 1 hr (panel E). The reversibility of this treatment was tested by washing away the inhibitor and subsequently incubating the treated oocytes for an additional hour (panel F). Treatment with SKI-606 caused redistribution of cortical granules with the result that the cortical granule-free zone was disrupted. Subsequent removal of the inhibitor allowed the cortical granule-free zone to re-form as seen in panel F. Magnification is indicated by the bar which represents 10 mm.
Fyn Activity Is Required for Maintenance of the Cortical Actin Filamentous Network in Oocytes
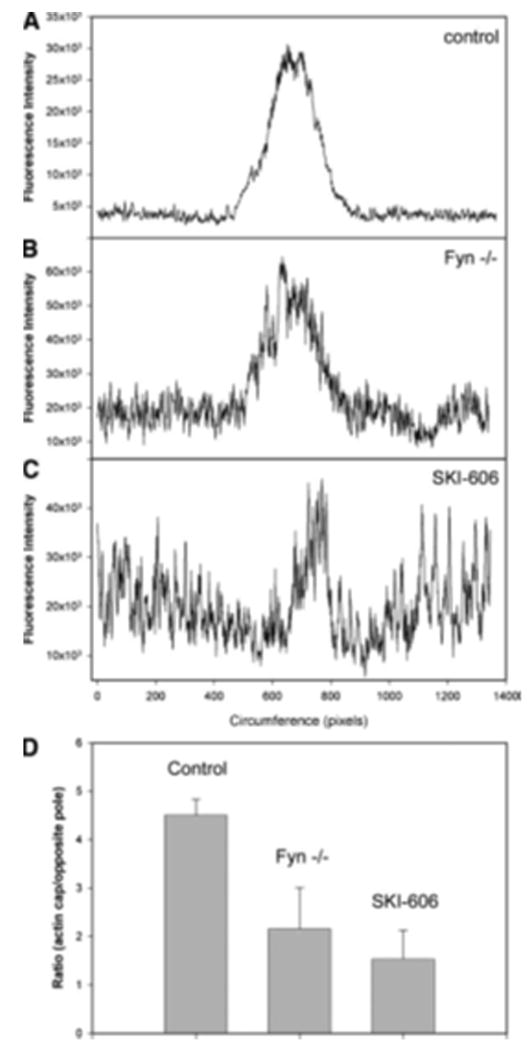
Mature mammalian oocytes normally exhibit a cortical filamentous actin meshwork, which underlies the plasma membrane. This network is normally thickened in the region overlying the meiotic spindle and represents another morphological feature that demonstrates the polarity of the mammalian oocyte. In order to determine whether suppression of Fyn activity caused changes in the normal distribution of filamentous actin, we examined the distribution of the cortical actin network in oocytes in which Fyn signaling was suppressed. Fixed samples of normal, Fyn-null, or SKI-606- treated oocytes were labeled with Oregon green-488-conjugated phalloidin to reveal the distribution of filamentous actin. EthD-2 was used to label chromatin. Confocal images were taken in Z-stack planes (30 per oocyte) providing overlapping optical sections approximately 2.5 mm thick. As seen in Figure 5A, the density of filamentous actin detected with this method was significantly polarized in control oocytes with the most intense fluorescence directly over the MII chromosomes. Oocytes from Fyn knockout females exhibited a less extensive actin cap, although the polarized nature of the actin layer was still evident (Fig. 5B). Treatment with SKI-606 altered the appearance and polarity of the actin meshwork as observed in Figure 5C where it is evident that treated oocytes exhibited an actin layer that was less com- pact in appearance with very little evidence of polarity. Quantitation of phalloidin fluorescence intensity was performed by a circumferential linescan analysis via Metamorph software beginning 1800 opposite the MII chromosomes and extending clockwise around the egg cortex to finish at 1800 opposite the chromosomes. This method provided a graphical presentation of the filamentous actin distribution as seen in Figure 6. The filamentous actin distribution in control oocytes was highly concentrated over the MII chromosomes and appeared as a symmetrical peak in the center of panel A. The filamentous actin distribution in Fyn-null oocytes was more irregular as seen by the large region to region variation in fluorescence intensity which gave the appearance of a “noisy” background (Fig. 6B).
Figure 5.
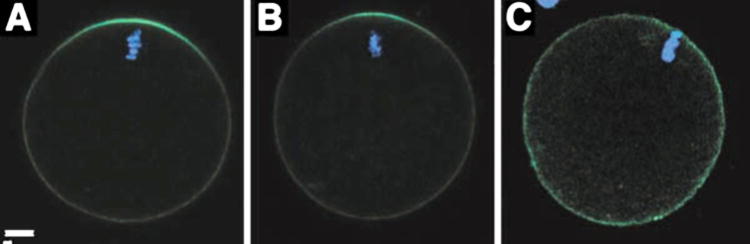
Suppression of Src-family PTK signaling disrupts the distribution of cortical actin filaments. CF1 oocytes treated with DMSO as a control (panel A), Fyn-null oocytes (panel B), or CF1 oocytes treated with 25 mM SKI-606 as in Figure 3, were fixed for confocal fluorescence analysis and stained with Oregon green-488-labeled phalloidin to detect filamentous actin. Ethidium homodimer was used to stain chromatin (blue). Magnification is indicated by the bar which represents 10 mm.
Figure 6.
Suppression of Src-family PTK signaling disrupts the integrity of the cortical actin cap. The relative amount of filamentous actin in the cortical actin layer of CF1 oocytes treated with DMSO as a control (A), Fyn-null oocytes (B), and CF1 oocytes treated with 25 mM SKI-606, was estimated by quantitation of the oregon green-phalloidin fluorescence intensity measured in the cortical actin layer. Confocal fluorescence images taken through the equator of the oocyte and including the MII chromosomes for reference were used for analysis. The linescan analysis tool of Metamorph 7.2 was used to trace a region of egg cortex approximately 5 mm thick beginning 180o opposite of the MII chromosomes, passing circumferentially around the egg, and finishing 180o opposite the MII chromosomes. Fluorescence intensity is presented graphically with circumferential distance on the X-axis and fluorescence intensity on the Y-axis. The position of the MII chromosomes was approximately in the center of graphs presented in panels A–C. The integrated fluorescence intensity within the actin cap region of each oocyte (n = 9 for each group) was compared to the fluorescence intensity of the opposite pole of each egg and the ratio is presented in panel D. Error bars represent standard deviation (n = 9). The fluorescence intensity of the actin cap region of control oocytes was significantly greater than that of Fyn-null oocytes and SKI-606-treated oocytes (P < 0.001).
Analysis of over 12 Fyn-null oocytes demonstrated that this variability was not electronic noise but rather actual differences in the pixel-to-pixel fluorescence intensity in the actin layer. In addition, the actin cap appeared to contain less actin relatively than controls based on the height of the central peak. SKI-606 treatment (Fig. 6C) severely disrupted the polarity of the actin layer with the result that the actin cap was often difficult to identify in this type of graphical presentation. Quantitation of the filamentous actin content (based on phalloidin fluorescence) of the actin cap region was per- formed on oocytes in which the MII chromosomes were located near the cortex and at the optical center of the oocyte. The integrated fluorescence intensity within the actin cap (identified at the central peak in graphs such as in Fig. 6A–C) was compared with an identical region of cortex from the opposite side of the oocyte. The ratio of phalloidin fluorescence in the actin cap relative to the opposite side of the oocyte is presented as a bar graph in Figure 6D. This analysis demonstrated that the filamentous actin content of the actin cap was significantly smaller in oocytes in which Fyn signaling was suppressed either by Fyn knockout or by SKI-606.
Fyn Signaling and the Positioning of MII Chromosomes Relative to the Oocyte Cortex
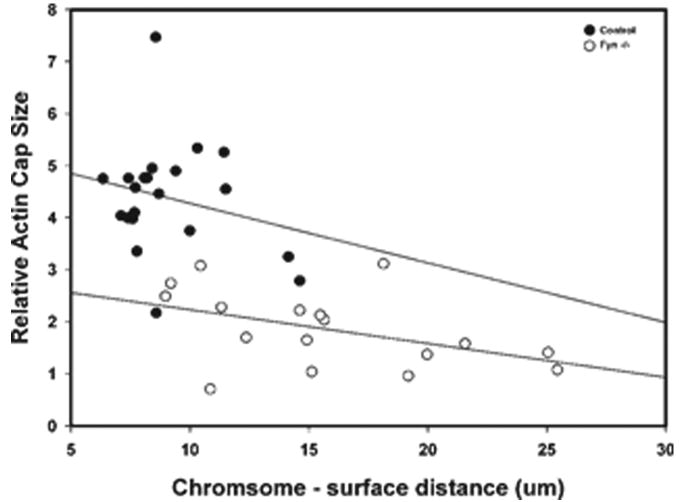
Recent studies have demonstrated that proximity of double-stranded DNA to the oocyte cortex induced many aspects of cortical polarity including actin cap formation (Deng et al., 2007). In order to investigate the possibility that the cortical polarity defects observed to result from suppression of Fyn signaling might reflect impaired docking of the MII chromosomes at the oocyte cortex, we characterized the distance of the chromosomes from the oocyte surface in normal and Fyn-suppressed oocytes. The results presented in Table 3 demonstrate that the MII chromosomes of oocytes from Fyn-null females were positioned at a greater distance from the oocyte surface than in control oocytes from the CF1 or B6/129 strains and were frequently misaligned with the cortex. Oocytes treated with SKI-606 exhibited more widely scattered chromosomes which made it difficult to identify the center of the chromosomal mass and to characterize the orientation of the metaphase plate relative to the cortex. Therefore, the SKI-606-treated oocytes were reported as “indeterminant.” The relationship between chromosome-cortex distance and the filamentous actin con- tent of the actin cap is presented graphically in Figure 7 where it can be seen that the filamentous actin content of the actin cap was inversely related to chromosome distance from the egg surface in both B6/129SF2/J oocytes and Fyn- null oocytes. The greater chromosome to egg surface distance typical in Fyn-null oocytes was correlated with a smaller filamentous actin cap.
Table 3. Effect of Src-Family PTK Suppression on Positioning of the MII Chromosomes.
| Sample | n | Distance Spindle (μm) | orientation % normal orientation |
|---|---|---|---|
| CF1 DMSO control | 8 | 11.4 ± 2 | 100% |
| B6/129SF/J control | 11 | 10.8 ± 7 | 91% |
| CF1 SKI-606 | 8 | 12.5 ± 5 | indeterminant |
| Fyn -/- | 8 | 19.0 ± 6* | 33% |
In order to determine the effect of Fyn kinase suppression on the position and orientation of the meiotic spindle relative to the egg cortex, oocytes from Fyn-null mice and CF1 oocytes treated with 25 mM SKI-606 in mKSOMaa for 1 hr were examined by confocal fluorescence microscopy following staining with Oregon green-phalloidin to stain the cortical actin layer and ethidium homodimer to stain chromosomes. Controls for the Fyn-null were from the parent strain B6/129SF2/J, and controls for SKI-606 treatment were CF1 oocytes treated with DMSO. Z-stack images were obtained and the distance from the center of the chromosomal mass to the oocyte cortex was measured within multiple optical sections to determine the minimum distance from the cortex.
indicates that the value was significantly different from controls (P < 0.012). The orientation of the chromosomes aligned on the metaphase plate was also characterized relative to the egg cortex. Metaphase plates that were arranged at 90 ± 30o to the oocyte surface were considered “normal.”
Figure 7.
Relationship between chromosome to surface distance and actin cap size. The relative actin cap size of individual control B6/129SF2/J oocytes (hollow circles) and Fyn-null oocytes (solid circles) was determined as in Figure 6 and is presented on the vertical axis. The distance of the center of the chromosomal mass from the oocyte surface is presented on the horizontal axis. Linear regression analysis was used to calculate the slope of each group of points.
Role of Fyn Signaling in the Distribution of Other Markers of Oocyte Polarity
In addition to the above morphological features that demonstrate the functional polarity of the oocyte cortex, several signaling proteins are known to be concentrated in certain regions of the mature oocyte. The partitioning defective gene product Par-3, for example, was found to be concentrated in a subdomain of the actin cap overlying the meiotic spindle during the process of oocyte maturation in mice (Duncan et al., 2005). Par-3 was found concentrated over the meiotic spindle in control oocytes (Fig. 8A) but was evenly dispersed in the Fyn-null oocytes (Fig. 8C) and SKI-606-treated oocytes (Fig. 8E) indicating that Fyn activity was involved in the polarized distribution of Par-3. Rho family members play an important role in actin dynamics (Witteck et al., 2003), are expressed in the oocyte cortex of a wide variety of organisms (Yoshida et al., 2003), and are required for translocation of cortical granules to the egg cortex (Covia'n-Nares et al., 2004), although not thought to be involved in permanently anchoring them in the cortex. Nor- mal mature oocytes displayed a concentration of Rho B in the oocyte cortex excluding the region over the meiotic spindle (Fig. 8B). This antibody also bound to the spindle region itself indicating that some population of Rho B may have associated with spindle components. Fyn-null and SKI-606-treated oocytes also exhibited cortical localization of Rho B (Fig. 8D,F). While labeling was less intense than in controls, the polarized pattern (less label over the spindle) was retained suggesting that Fyn signaling had little impact on the localization of this protein.
Figure 8.
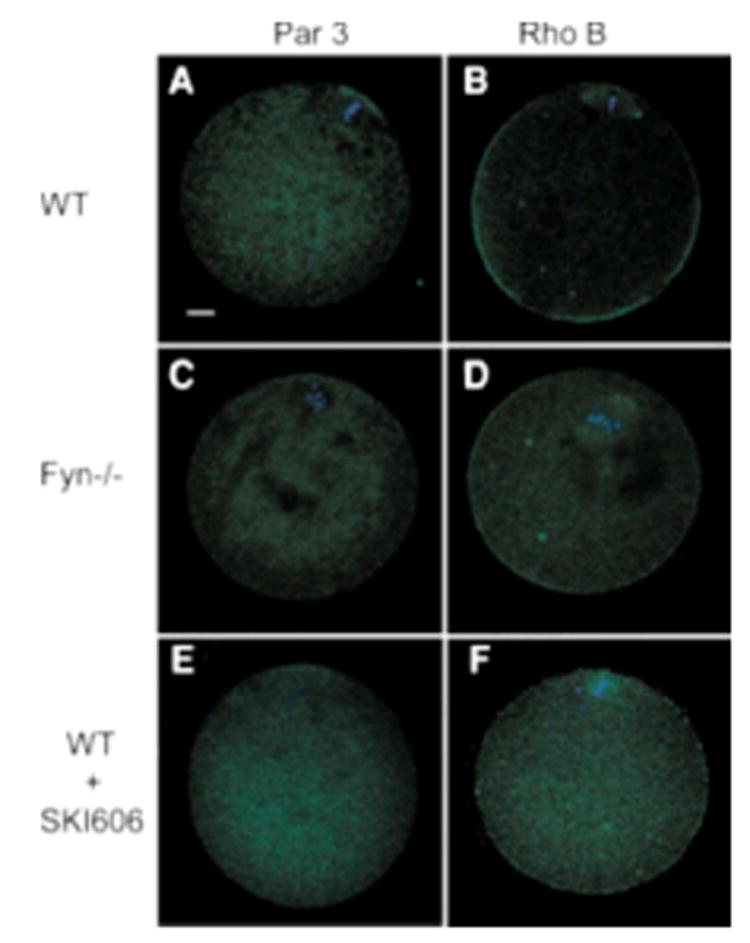
Suppression of Src-family PTK signaling disrupts the distribution of Par-3, but not Rho B. The distribution of known markers of oocyte polarity Par-3 and Rho B was determined by confocal immunofluorescence analysis of DMSO-treated CF1 oocytes (control, panels A,B), Fyn-null oocytes (panels C,D), or SKI-606-treated CF1 oocytes (panels E,F). Fixed oocytes were labeled with anti-Par-3 (A,C,E) or anti Rho B (B,D,F) followed by alexa 488-conjugated secondary antibody. Magnification is indicated by the bar which represents 10 mm.
Rescue of Oocyte Polarity in Fyn-Null Oocytes
In order to determine whether the observed defects in actin and cortical granule distribution present in Fyn-null oocytes were due to lack of Fyn kinase within the oocyte or to possible poor quality of oocytes due to endocrine or other problems in somatic cells of the Fyn-null mouse, we tested whether normal polarity could be restored by microinjection of c-Fyn cRNA into isolated germinal vesicle (GV) stage oocytes prior to maturation in vitro. GV stage oocytes were maintained at GV arrest by culture in the presence of dibutyryladenosine cyclic monophosphate sodium salt (dcAMP) and injected with cRNA encoding c-Fyn which, when translated, would provide active Fyn kinase to rescue the defects inherent in the Fyn-null oocyte. Controls included oocytes injected with the dominant-negative FynK299M mutant kinase (Sharma et al., 2005) which is structurally similar to the native kinase but catalytically inactive. Additional controls were treated identically but not injected with RNA. Following injection, the oocytes were maintained in GV arrest for a further 4–5 hr to allow translation of protein, then washed free of dbcAMP to allow maturation to proceed. The results presented in Figure 9 (bottom) demonstrate that the injected RNA was translated into Fyn or FynK299M kinase, both of which were detected with the Fyn-3 antibody. The un-injected Fyn-null oocytes or FynK299M mutant kinase-injected oocytes exhibited signs of loss of cortical polarity as evidenced by reduction or absence of the cortical granule-free zone and actin cap (Table 4 and Fig. 9). However, oocytes expressing active Fyn kinase translated from injected cRNA maintained a cortical granule-free zone and a normal appearing actin cap indicating that the absence of Fyn kinase within the Fyn-null oocyte was responsible for the loss of cortical polarity in Fyn-null oocytes.
Figure 9.
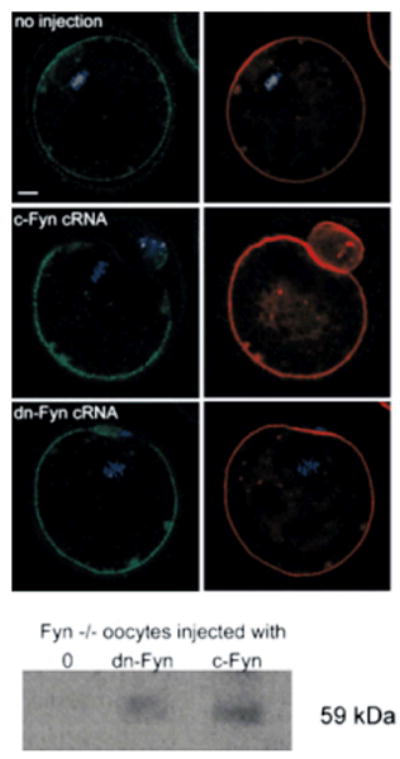
Rescue of Fyn-null oocytes by injection of Fyn cRNA. GV stage oocytes were collected from Fyn-null females following stimulation with PMSG and maintained in GV arrest by culture in the presence of db-cAMP as described in Materials and Methods Section. Oocytes were injected with cRNA encoding c-Fyn or the dominant-negative FynK299M mutant kinase, held in GV arrest for 4–5 hr to allow translation of the exogenous cRNA, then washed free of cAMP and matured for 17 hr before preparation for immunofluorescence. Controls were treated and cultured identically, though not injected with RNA. Samples were stained with FITC-labeled LCA to detect cortical granule contents (left panels labeled green), with alexa 568-phalloidin to stain actin filaments (right panels labeled red), and with to-pro-3 to label DNA (blue). Magnification is indicated by the bar which represents 10 mm. Following immunofluorescence analysis, fixed oocytes were recovered, washed in PBS containing 3 mg/ml PVP, and solubilized in SDS sample buffer containing 20 mM glycine and heated at 90o C for 5 min. Western blot analysis was performed and the blot was probed with anti Fyn-3 antibody (Santa Cruz Biotechnology, Inc.) to detect the presence of Fyn protein in the samples (bottom panel).
Table 4. Rescue of Oocyte Polarity by Injection of cRNA Encoding Fyn Kinase.
| Parameter | cRNA injected at GV stage | ||
|---|---|---|---|
|
| |||
| None (n=41) (%) | FynK299M (n=54) (%) | cFyn (n=55) (%) | |
| MII | 37 | 24 | 48 |
| Normal cortical granule-free zone | 27 | 62 | 82 |
| Normal actin cap | 7 | 34 | 77 |
GV stage oocytes were isolated from Fyn-null females and maintained at GV arrest by culture in the presence of db-cAMP. Cumulus cells were removed and the oocytes were injected with approximately 24 pg of cRNA encoding c-Fyn or the dominant-negative FynK299M mutant kinase as a control. Noninjected oocytes were treated identically but not injected with RNA. Following injection, the oocytes were maintained in GV arrest for a further 4–5 hr to allow translation of protein, then washed free of db-cAMP to allow maturation to proceed. Samples were fixed and prepared for confocal fluorescence microscopy to establish the extent to which meiosis was completed (MII stage). Those oocytes which had successfully reached MII were characterized as to the presence of a normal cortical granule-free zone and actin cap based on FITC-labeled LCA and alexa 568-phalloidin staining (see Fig. 8). The averaged results of two experiments are expressed as a percentage of the MII oocytes with normal cortical granule-free zone and actin cap.
Discussion
Previous studies have demonstrated that the mammalian oocyte responds to fertilization with localized PTK signaling events, which were detected as accumulation of P-Tyr- containing proteins in the oocyte cortex overlying the meiotic spindle and the incorporated sperm head. Suppression of Src-family PTK signaling via chemical inhibition or dominant negative constructs revealed a potential role for these kinases in anaphase completion and pronuclear congression (McGinnis et al., 2007). The objective of the present study was to test the role of Fyn kinase in the response of the oocyte to the early stages of the fertilization process such as sperm incorporation, meiosis resumption, meiosis completion, and pronuclear formation. Since Fyn kinase is very highly expressed in mammalian oocytes, the Fyn knockout oocyte was used as a model in which a large fraction of the oocyte Src-family PTK activity was suppressed. We have found that Fyn knockout oocytes overexpress Yes kinase in an apparent effort to compensate for the loss of Fyn (not shown), and we therefore employed an additional approach using the kinase inhibitor SKI-606 which is highly specific for Src-family protein kinases and would suppress all Src-family PTKs including Yes and Src. SKI-606 can block proliferation of cultured cells with an IC50 of 1.5–2.5 mM (Boschelli et al., 2001; Clark and Peterson, 2003) and blocked mitosis of mouse zygotes at 10 mM (McGinnis et al., 2007). The concentration used in this study was higher than we previously used because the experimental design required was ing out prior to insemination to avoid effects on the sperm. However, we confirmed the major findings obtained with SKI-606 in zona-free eggs at lower concentration. The chemical inhibitor was used here primarily to support the results obtained from the Fyn knockout oocytes and every effort was made not to rely solely on inhibitor data. Suppression of Src-family PTK activity via Fyn knockout or inhibitor treatment prior to fertilization had no significant effect on sperm incorporation or the block to polyspermy. These results, while negative, indicated that the processes of sperm–egg fusion, sperm head incorporation, and prevention of polyspermy did not absolutely require the action of Src-family PTKs.
Once sperm–oocyte fusion has occurred, signals delivered by the sperm trigger calcium signaling events in the ooplasm that cause release from anaphase arrest and allow MII to resume (reviewed in Swann et al., 2004; Parrington et al., 2007; Wortzman-Show et al., 2007; Grasa et al., 2008; Yoon et al., 2008). The present study used confocal micros- copy to detect potential effects of Fyn kinase inhibition on anaphase II resumption, meiosis completion, and pronuclear formation. The results revealed no significant requirement for Fyn signaling in anaphase resumption, but there was a significant negative effect on anaphase completion resembling earlier findings following intracytoplasmic sperm injection (ICSI) (Meng et al., 2006). This result differed from the situation in rat and parthenogenetically activated mouse oocytes which require Src-family PTK signaling for anaphase resumption (Sette et al., 2002; Tomashov-Matar et al., 2008) raising the possibility that the fertilizing sperm provides a more robust signal for anaphase resumption.
While suppression of Fyn activity did not affect anaphase resumption, it did cause defects in the chromosome-spindle organization during anaphase which ranged in severity from one or two displaced chromosomes to widely dispersed chromosomes as demonstrated in Figure 2. This defect correlated with a delay in the completion of meiosis as evidenced by pronuclear formation, possibly because more time was needed to overcome the spindle assembly check- point (Brunet et al., 2003). The present detailed analysis provided quantitative confirmation of our earlier observations (McGinnis et al., 2007) and demonstrated the clear requirement of Fyn kinase in maintaining normal chromosome-spindle organization during meiosis.
The detailed confocal analysis described in the present study also revealed that oocytes in which Src-family PTK activity had been suppressed exhibited loss of cortical polarity. The mammalian oocyte develops cortical polarity during oocyte maturation when the meiotic chromosomes move toward the oocyte cortex (Longo and Chen, 1984; Longo, 1987). As the chromosomes approach the cortex, one or more signaling events occur that induce thickening of the cortical actin layer to produce the actin cap, translocation of cortical secretory granules, and redistribution of microvilli forming a cortical granule and microvilli-free zone. Fyn-null oocytes failed to establish a normal actin cap as well as cortical granule and microvilli-free zone indicating that Fyn kinase was involved in establishing or maintaining polarization of the cortical cytoplasm. Expression of exogenous c- Fyn via cRNA injection of GV stage oocytes rescued oocyte polarity by re-establishing the actin cap and cortical granule- free zone after maturation in vitro. The Src-family kinase inhibitor SKI-606 could induce similar defects in normal oocytes suggesting that cortical actin, cortical granule, and microvillus distribution are dynamic and maintenance of the polarized distribution requires Fyn or other Src-family PTK activity.
The complete functional significance of the above defects in the filamentous actin cap, cortical granule, and microvilli-free zone is not readily apparent from these studies. However, one important consequence appears to be failure to exclude sperm–egg fusion in the vicinity of the meiotic apparatus. Normally, sperm–egg fusion rarely occurs in the microvilli-free zone, perhaps due to involvement of microvilli in the sperm–egg fusion process (Runge et al., 2007). This reduces the risk that sperm nuclear material may interfere with the meiotic spindle and maternal chromosomes during polar body emission. The surprising fact that Src-family PTK-suppressed oocytes allowed sperm–egg fusion in the region of egg cortex overlying the meiotic chromosomes demonstrated a potentially detrimental consequence of this loss of polarity following suppression of Fyn kinase activity. The potential mechanism by which loss of Fyn kinase signaling caused defects in cortical polarity are likely related to the reduced capacity of Fyn-suppressed oocytes to correctly position the meiotic spindle close to the oocyte sur- face. The results presented here clearly show a relationship between the distance of the meiotic chromosomes from the oocyte surface and the filamentous actin content of the actin cap. The Fyn-null oocytes failed to bring the meiotic chromosomes within the normal distance from the oocyte surface which could prevent normal signaling between the maternal chromatin and the egg cortex. Maternal chromatin is thought to signal the oocyte cortex through a mechanism involving the RanGTP gradient-induced MAPK localization which triggers myosin II/actin assembly (Klemke et al., 1997) and tethering of actin filaments to the PAR-3/aPKC polarity complex (Deng et al., 2007). The molecular basis for the inability of the Fyn-null oocyte to establish and maintain normal proximity of the MII chromosomes to the egg cortex, together with the tendency of Fyn-null oocytes to present with displaced chromosomes during metaphase and ana- phase of both MI (McGinnis et al., 2009) and MII (present results) may result from a common defect in chromosome- spindle signaling. For example, chromatin-associated microtubule motors have been proposed to play a role in bivalent congression (Brunet and Maro, 2005). Alternatively, factors necessary for maturation of kinetochores and sub- sequent stabilization of K-fibers may require action by Fyn or other Src-family PTKs. It is noteworthy that Fyn kinase was demonstrated to be tightly associated with the meiotic spindle in rat oocytes and to phosphorylate tubulin in vitro (Talmor-Cohen et al., 2004b); however, it could phosphorylate other proteins that may be involved in these processes. The possible role of Fyn kinase in the meiotic apparatus does not preclude additional functions in the cortical actin cytoskeleton since Fyn kinase is known, in other cell types, to be directly involved with actin cytoskeletal reorganization (Chen et al., 2006), including filamentous actin bundling (Xu et al., 2007) and actin cap formation (Samayawardhena et al., 2007). The dramatic cytoskeletal effects of deregu- lated Src kinase in the xenopus oocyte (Unger and Steele, 1992) further highlight the potential importance of this family of protein kinases in regulation of the oocyte cortical cytoskeleton.
The results of the present study have demonstrated a role of the Fyn kinase in maintenance and positioning of the meiotic apparatus in oocytes. The downstream consequences of insufficient Fyn activity on meiosis completion and oocyte polarity have also been detailed. Identification of the specific protein targets of Fyn in the oocyte cortex and meiotic apparatus will hopefully reveal the biochemical path- ways that are involved in these events which are important for effective fertilization and developmental competence.
Materials and Methods
Gamete Handling and In Vitro Fertilization
Cauda epididymal sperm from B6D2F1 mice (Harlan Sprague– Dawley, Indianapolis, IN) were released into modified tyrode's solution (mTyrodes) containing 4 mg/ml BSA (Sigma–Aldrich, St. Louis, MO). Capacitation was performed for 90 min in a swim up column of mTyrodes at 370 C in a humidified atmosphere of 5% CO2. Oocytes were obtained from female CF1 (Harlan Sprague–Dawley) (B6/129SF2/J) or B6/129S7-Fyntm1Sor/J strain mice (The Jackson Laboratory, Bar Harbor, ME) that were 6–7 weeks old. Superovu- lation was accomplished by injection of 7.5 IU pregnant mare serum gonadotropin (PMSG) (Sigma–Aldrich), followed 48 hr later by 7.5 IU of human chorionic gonadotropin (hCG) (Sigma–Aldrich). Cumulus oocyte complexes (COCs) were collected 15–16 hr post- hCG, washed in flushing and handling medium (FHM) N-(2- hydroxyethyl)piperazine-N′-[2-ethanesulfonic acid]) (HEPES)- buffered medium (Millipore Corporation, Phillipsburg, NJ) contain- ing 4 mg/ml BSA. Cumulus cells were removed from COCs by digestion with 0.3 mg/ml hyaluronidase (Sigma–Aldrich), and the cumulus-free oocytes were transferred to a dish containing 500 ml of pre-warmed KSOMaa (Millipore Corporation, NJ) containing 4 mg/ml BSA under equilibrated mineral oil, and cultured at 37o C in a humidified atmosphere of 5%CO2 in air. For IVF, 25–40 oocytes were transferred to an IVF cell culture dish (Corning Incorporated, Corning, NY) containing 500 ml of pre-warmed mKSOMaa medium supplemented with 4 mg/ml BSA under mineral oil. Insemination was begun by adding capacitated spermatozoa to a final concen- tration of 2×105/ml and fertilization was terminated after 4.5 hr incubation by washing the eggs in KSOMaa containing 1 mg/ml BSA. For in vitro maturation and expression of exogenous proteins, COCs were collected in FHM supplemented with 300 mM db-cAMP to prevent oocyte maturation. Cumulus cells were removed by brief exposure to 0.3 mg/ml hyaluronidase and gentle pipetting with a fine glass pipette. c-FYN cRNA and a dominant-negative (dn) Fyn cRNA were prepared as previously described (Sharma et al., 2005; McGinnis et al., 2007). Injections were performed on an inverted Nikon Eclipse TE2000-S with an Eppendorf FemtoJet injection system and 0.3 mm ID EggJek microinjection needles (MicroJek, Kansas City, KS). Oocytes were allotted into three groups, noninjected, or those injected with approximately 2–4 pg of either c-Fyn cRNA, dnFyn cRNA. Injected oocytes were transferred to Terasaki plates and cultured for 4–5 hr in KSOM-MAT1 (McGinnis et al., 2009) supplemented with 300 mM cAMP to maintain GV arrest and to allow for protein expression to occur. Oocytes were examined at the beginning and end of this culture and graded for presence of a visible GV. Following this culture, oocytes were washed free of db-cAMP and matured 17 hr in 400 ml KSOM- MAT in four-well culture plates. After maturation, oocytes were fixed for 10 min in 2% PFA then 30 min in MTSB-XF according to procedures previously published (McGinnis et al., 2007).
Pharmacological treatment of CF1 oocytes. Forty to fifty oo- cytes were transferred to 100 ml of KSOMaa containing 4 mg/ml BSA and 25 mM SKI-606 (Calbiochem) or DMSO control (1/400; v/v), then incubated for 1 hr at 37o C in a humidified atmosphere of 5% CO2. Post-treatment, the oocytes were rinsed in 100 ml of media, followed by insemination or fixation.
Confocal Fluorescence Microscopy
Cumulus-free oocytes or fertilized eggs were fixed in 2% para- formaldehyde, 1% picric acid in phosphate-buffered saline (PBS) (pH 7.4) for 1 hr at room temperature. Fixed oocytes were washed in blocking buffer (PBS containing 3 mg/ml BSA, 0.01% Tween-20, 0.1 M glycine). Oocytes were then permeablized with 0.1% Triton X-100 in PBS containing 3 mg/ml BSA for 5 min, then washed with blocking buffer. For detection of cortical granules, oocytes were incubated overnight at 4o C with 10 mg/ml lectin from Lens culinaris (lentil)-FITC conjugate (Sigma–Aldrich). Polymerized actin was detected by incubation with 66 nM Oregon green-488-phalloidin (Invitrogen Corporation, Carlsbad, CA) in blocking buffer containing 2 mM EthD-2 (Invitrogen Corporation). For immunofluorescence detection of Par-3 and RhoB, oocytes were incubated with anti-Par-3 (1:50; Millipore, Temecula, CA) (Deng et al., 2005) or rabbit anti-Rho B (1:100; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) (Kumakiri et al., 2003). Bound primary antibody was detected with alexa 488 goat anti-rabbit immunoglobulin G (IgG) (1:500; Invitro- gen Corporation), and DNA was stained by 2 mM EthD-2 in blocking buffer. Microscopy was performed with a Nikon TE2000-U micro- scope. Images were taken by excitation with 488 and 543 nm wavelength lasers and emission was detected through detectors 515/30 (green), 590/50 (red), and 650LP (blue).
Scanning Electronic Microscopy (SEM)
For comparison of the microvilli pattern, the zona pellucida (ZP) was dissolved by transient treatment of oocytes in acid tyrodes solution (pH 2.5) followed by immediately neutralizing the acid with FHM containing 4 mg/ml BSA. ZP-free oocytes were incubated in KSOMaa containing 1 mg/ml BSA then bound to poly-L-lysine (Sigma–Aldrich) coated coverslips, then fixed at 4o C for 1 hr by mixing with 1 ml of cold 2% glutaraldehyde in cacodylate buffer (pH 7.4) followed by 2% glutaraldehyde for overnight fixation. Coverslips containing the oocytes were rinsed for 5 min with 0.1 M sodium cacodylate buffer (pH 7.4) and then post-fixed in 1% buffered osmium tetroxide for 30 min. After rinsing twice in distilled water, samples were dehydrated in a graded series of ethanol, then critical point dried in liquid CO2. Coverslips were then mounted onto aluminum mounts using silver conductive paint. Samples were sputter coated with gold in a Pelco SC-6 coater. Finally, oocytes were examined in a Hitachi S-2700 microscope.
Acknowledgments
We are indebted to Lily Zhang for technical assistance and Barbara Fegley for assistance with electron microscopy. This work was supported by NICHD 14846 to W.H.K. and P20RR024214 from the National Center For Research Resources.
Abbreviations
- BSA
bovine serum albumin
- DMSO
dimethylsulfoxide
- MII
meiosis II
- db-cAMP
dibutyryladenosine-cyclic monophosphate sodium salt
- EthD-2
ethidium homodimer
- FHM
flushing and handling medium
- FITC
fluo- rescein isothiocyanate
- GV
germinal vesicle
- hCG
human chorionic gonadotro- pin
- ICSI
intracytoplasmic sperm injection
- KSOM
potassium simplex optimized medium
- IgG
immunoglobulin G
- HEPES
N-(2-hydroxyethyl)piperazine-N0 -[2- ethanesulfonic acid])
- PBS
phosphate-buffered saline
- PTK
protein tyrosine kinase
- PMSG
pregnant mare serum gonadotropin
- SH2
Src homology 2
- SH3
Src homology 3
References
- Angers-Loustau A, Côte' JF, Tremblay ML. Roles of protein tyrosine phosphatases in cell migration and adhesion. Biochem Cell Biol. 1999;77:493–505. [PubMed] [Google Scholar]
- Besterman B, Schultz RM. Regulation of mouse preimplan- tation development: Inhibitory effect of genistein, an inhibitor of tyrosine protein phosphorylation, on cleavage of one-cell embryos. J Exp Zool. 1990;256:44–53. doi: 10.1002/jez.1402560107. [DOI] [PubMed] [Google Scholar]
- Boschelli DH, Ye F, Wang YD, Dutia M, Johnson SL, Wu B, Miller K, Powell DW, Yaczko D, Young M, Tischler M, Arndt K, Discafani C, Etienne C, Gibbons J, Grod J, Lucas J, Weber JM, Boschelli F. Optimization of 4-phenylamino-3-quinolinecarbonitriles as potent inhibitors of Src kinase activity. J Med Chem. 2001;44:3965–3977. doi: 10.1021/jm0102250. [DOI] [PubMed] [Google Scholar]
- Bromann PA, Korkaya H, Courtneidge SA. The interplay between Src family kinases and receptor tyrosine kinases. On- cogene. 2004;23:7957–7968. doi: 10.1038/sj.onc.1208079. [DOI] [PubMed] [Google Scholar]
- Brunet S, Maro B. Cytoskeleton and cell cycle control during meiotic maturation of the mouse oocyte: Integrating time and space. Reproduction. 2005;130:801–811. doi: 10.1530/rep.1.00364. [DOI] [PubMed] [Google Scholar]
- Brunet S, Pahlavan G, Taylor S, Maro B. Functionality of the spindle checkpoint during the first meiotic division of mammalian oocytes. Reproduction. 2003;126:443–450. doi: 10.1530/rep.0.1260443. [DOI] [PubMed] [Google Scholar]
- Chen M, Chen SC, Pallen CJ. Integrin-induced tyrosine phosphorylation of protein-tyrosine phosphatase-alpha is re- quired for cytoskeletal reorganization and cell migration. J Biol Chem. 2006;281:11972–11980. doi: 10.1074/jbc.M600561200. [DOI] [PubMed] [Google Scholar]
- Clark DD, Peterson BR. Analysis of protein tyrosine kinase inhibitors in recombinant yeast lacking the ERG6 gene. Chem- biochem. 2003;4:101–107. doi: 10.1002/cbic.200390001. [DOI] [PubMed] [Google Scholar]
- Coluccia AM, Benati D, Dekhil H, De Filippo A, Lan C, Gambacorti- Passerini C. SKI-606 decreases growth and motility of colorectal cancer cells by preventing pp60(c-Src)-dependent tyrosine phosphorylation of beta-catenin and its nuclear signal- ing. Cancer Res. 2006;66:2279–2286. doi: 10.1158/0008-5472.CAN-05-2057. [DOI] [PubMed] [Google Scholar]
- Covia'n-Nares F, Mart'ýnez-Cadena G, Lo'pez-God'ýnez J, Voronina E, Wessel GM, Garc'ýa-Soto J. A Rho-signaling pathway mediates cortical granule translocation in the sea urchin oocyte. Mech Dev. 2004;121:225–235. doi: 10.1016/j.mod.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Deng MQ, Kishikawa H, Yanagimachi R, Kopf GS, Schultz RM, Williams CJ. Chromatin-mediated cortical granule redistri- bution is responsible for the formation of the cortical granule-free domain in mouse eggs. Dev Biol. 2003;257:166–176. doi: 10.1016/s0012-1606(03)00045-9. [DOI] [PubMed] [Google Scholar]
- Deng M, Williams CJ, Schultz RM. Role of MAP kinase and myosin light chain kinase in chromosome-induced development of mouse egg polarity. Dev Biol. 2005;278:358–366. doi: 10.1016/j.ydbio.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Deng M, Suraneni P, Schultz RM, Li R. The Ran GTPase mediates chromatin signaling to control cortical polarity during polar body extrusion in mouse oocytes. Dev Cell. 2007;12:301–308. doi: 10.1016/j.devcel.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Ducibella T, Fissore R. The roles of Ca2þ, downstream protein kinases, and oscillatory signaling in regulating fertilization and the activation of development. Dev Biol. 2008;315:257–279. doi: 10.1016/j.ydbio.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan FE, Moss SB, Schultz RM, Williams CJ. PAR-3 defines a central subdomain of the cortical actin cap in mouse eggs. Dev Biol. 2005;280:38–47. doi: 10.1016/j.ydbio.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Grasa P, Coward K, Young C, Parrington J. The pattern of localization of the putative oocyte activation factor, phospholi- pase C {zeta}, in uncapacitated, capacitated, and ionophore- treated human spermatozoa. Human Reprod. 2008 doi: 10.1093/humrep/den280. [DOI] [PubMed] [Google Scholar]; Haendeler J, Hoffmann J, Brandes RP, Zeiher AM, Dimmeler S. Hydrogen peroxide triggers nuclear export of telomerase reverse transcriptase via Src kinase family-dependent phosphor- ylation of tyrosine 707. Mol Cell Biol. 2003;23:4598–4610. doi: 10.1128/MCB.23.13.4598-4610.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoodbhoy T, Talbot P. Characterization, fate, and function of hamster cortical granule components. Mol Reprod Dev. 2001;58:223–235. doi: 10.1002/1098-2795(200102)58:2<223::AID-MRD12>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Jacquet P, Saint-Georges L, Barrio S, Baugnet-Mahieu L. Morphological effects of caffeine, okadaic acid and genistein in one-cell mouse embryos blocked in G2 by X-irradiation. Int J Radiat Biol. 1995;67:347–358. doi: 10.1080/09553009514550401. [DOI] [PubMed] [Google Scholar]
- Klemke RL, Cai S, Giannini AL, Gallagher PJ, de Lanerolle P, Cheresh DA. Regulation of cell motility by mitogen-acti- vated protein kinase. J Cell Biol. 1997;137:481–492. doi: 10.1083/jcb.137.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumakiri J, Oda S, Kinoshita K, Miyazaki S. Involvement of Rho family G protein in the cell signaling for sperm incorporation during fertilization of mouse eggs: Inhibition by Clostridium difficile toxin B. Dev Biol. 2003;260:522–535. doi: 10.1016/s0012-1606(03)00273-2. [DOI] [PubMed] [Google Scholar]
- Kurokawa M, Sato K, Smyth J, Wu H, Fukami K, Takenawa T, Fissore RA. Evidence that activation of Src family kinase is not required for fertilization-associated [Ca2þ] i oscillations in mouse eggs. Reproduction. 2004;127:441–454. doi: 10.1530/rep.1.00128. [DOI] [PubMed] [Google Scholar]
- Liu J, Maller JL. Calcium elevation at fertilization coordinates phosphorylation of XErp1/Emi2 by Pl×1 and CaMK II to release metaphase arrest by cytostatic factor. Curr Biol. 2005;15:1458–1468. doi: 10.1016/j.cub.2005.07.030. [DOI] [PubMed] [Google Scholar]; Longo FJ. Actin-plasma membrane associations in mouse eggs and oocytes. J Exp Zool. 1987;243:299–309. doi: 10.1002/jez.1402430215. [DOI] [PubMed] [Google Scholar]
- Longo FJ, Chen DY. Development of surface polarity in mouse eggs. Scan Electron Microsc. 1984:703–716. [PubMed] [Google Scholar]
- McGinnis LK, Albertini DF, Kinsey WH. Localized activation of Src-family protein kinases in the mouse egg. Dev Biol. 2007;306:241–254. doi: 10.1016/j.ydbio.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis LK, Kinsey WH, Albertini DF. The functions of Fyn kinase in the completion of meiosis in mouse oocytes. Dev Biol. 2009;327:280–287. doi: 10.1016/j.ydbio.2008.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlmann LM, Jaffe LA. SH2 domain-mediated activation of an SRC family kinase is not required to initiate Ca2þ release at fertilization in mouse eggs. Reproduction. 2005;129:557–564. doi: 10.1530/rep.1.00638. [DOI] [PubMed] [Google Scholar]
- Meng L, Luo JP, Li C, Kinsey WH. Role of SH2 domain- mediated PTK signaling in mouse zygotic development. Reproduction. 2006;132:413–421. doi: 10.1530/rep.1.01151. [DOI] [PubMed] [Google Scholar]
- Moore KL, Kinsey WH. Effects of protein tyrosine kinase inhibitors on egg activation and fertilization-dependent protein tyrosine kinase activity. Dev Biol. 1995;168:1–10. doi: 10.1006/dbio.1995.1056. [DOI] [PubMed] [Google Scholar]
- O'Neill FJ, Gillett J, Foltz KR. Distinct roles for multiple Src family kinases at fertilization. J Cell Sci. 2004;117:6227–6238. doi: 10.1242/jcs.01547. [DOI] [PubMed] [Google Scholar]
- Park RK, Izadi KD, Deo YM, Durden DL. Role of Src in the modulation of multiple adaptor proteins in FcaRI oxidant signaling. Blood. 1999;94:2112–2120. [PubMed] [Google Scholar]
- Parrington J, Davis LC, Galione A, Wessel G. Flipping the switch: How a sperm activates the egg at fertilization. Dev Dyn. 2007;236:2027–2038. doi: 10.1002/dvdy.21255. [DOI] [PubMed] [Google Scholar]
- Potireddy S, Vassena R, Patel BG, Latham KE. Analysis of polysomal mRNA populations of mouse oocytes and zygotes: Dynamic changes in maternal mRNA utilization and function. Dev Biol. 2006;298:155–166. doi: 10.1016/j.ydbio.2006.06.024. [DOI] [PubMed] [Google Scholar]
- Runft LL, Jaffe LA, Mehlmann LM. Egg activation at fertiliza- tion: Where it all begins. Dev Biol. 2002;245:237–254. doi: 10.1006/dbio.2002.0600. [DOI] [PubMed] [Google Scholar]
- Runge KE, Evans JE, He ZY, Gupta S, McDonald KL, Stahlberg H, Primakoff P, Myles DG. Oocyte CD9 is enriched on the microvillar membrane and required for normal microvillar shape and distribution. Dev Biol. 2007;304:317–325. doi: 10.1016/j.ydbio.2006.12.041. [DOI] [PubMed] [Google Scholar]
- Samayawardhena LA, Kapur R, Craig AW. Involvement of Fyn kinase in Kit and integrin-mediated Rac activation, cytoskel- etal reorganization, and chemotaxis of mast cells. Blood. 2007;109:3679–3686. doi: 10.1182/blood-2006-11-057315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sette C, Paronetto MP, Barchi M, Bevilacqua A, Geremia R, Rossi P. Tr-kit-induced resumption of the cell cycle in mouse eggs requires activation of a Src-like kinase. EMBO J. 2002;21:5386–5395. doi: 10.1093/emboj/cdf553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma D, Kinsey WH. Fertilization triggers localized activation of Src-family protein kinases in the zebrafish egg. Dev Biol. 2006;295:604–614. doi: 10.1016/j.ydbio.2006.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma D, Kinsey WH. Regionalized calcium signaling in zebrafish fertilization. Int J Dev Biol. 2008;52:561–570. doi: 10.1387/ijdb.072523ds. [DOI] [PubMed] [Google Scholar]
- Sharma D, Holets L, Zhang X, Kinsey WH. Role of Fyn kinase in signaling associated with epiboly during zebrafish develop- ment. Dev Biol. 2005;285:462–476. doi: 10.1016/j.ydbio.2005.07.018. [DOI] [PubMed] [Google Scholar]
- Stein PL, Lee HM, Rich HM, Soriano S. pp59fyn mutant mice display differential signaling in thymocytes and peripheral T cells. Cell. 1992;70:741–750. doi: 10.1016/0092-8674(92)90308-y. [DOI] [PubMed] [Google Scholar]
- Stein PL, Vogel H, Soriano P. Combined deficiencies of Src, Fyn, and Yes tyrosine kinases in mutant mice. Genes Dev. 1994;8:1999–2007. doi: 10.1101/gad.8.17.1999. [DOI] [PubMed] [Google Scholar]; Swann K, Larman MG, Saunders CM, Lai FA. The cytosolic sperm factor that triggers Ca2þ oscillations and egg activation in mammals is a novel phospholipase C: PLCzeta. Reproduction. 2004;127:431–439. doi: 10.1530/rep.1.00169. [DOI] [PubMed] [Google Scholar]
- Talmor A, Kinsey WH, Shalgi R. Expression and immuno- localization of p59c-fyn tyrosine kinase in rat eggs. Dev Biol. 1998;194:38–46. doi: 10.1006/dbio.1997.8816. [DOI] [PubMed] [Google Scholar]
- Talmor-Cohen A, Tomashov-Matar R, Eliyahu E, Shapiro R, Shalgi R. Are Src family kinases involved in cell cycle resumption in rat eggs? Reproduction. 2004a;127:455–463. doi: 10.1530/rep.1.00104. [DOI] [PubMed] [Google Scholar]
- Talmor-Cohen A, Tomashov-Matar R, Tsai WB, Kinsey WH, Shalgi R. Fyn kinase-tubulin interaction during meiosis of rat eggs. Reproduction. 2004b;128:387–393. doi: 10.1530/rep.1.00266. [DOI] [PubMed] [Google Scholar]; Tomashov-Matar R, Levi M, Shalgi R. The involvement of Src family kinases (SFKs) in the events leading to resumption of meiosis. Mol Cell Endocrinol. 2008;282:56–62. doi: 10.1016/j.mce.2007.11.016. [DOI] [PubMed] [Google Scholar]
- Unger TF, Steele RE. Biochemical and cytological changes associated with expression of deregulated pp60src in Xenopus oocytes. Mol Cell Biol. 1992;12:5485–5498. doi: 10.1128/mcb.12.12.5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witteck A, Yao Y, Fechir M, Forstermann U, Kleinert H. Rho protein-mediated changes in the structure of the actin cytoskele- ton regulate human inducible NO synthase gene expression. Exp Cell Res. 2003;287:106–115. doi: 10.1016/s0014-4827(03)00129-0. [DOI] [PubMed] [Google Scholar]
- Wortzman-Show GB, Kurokawa M, Fissore RA, Evans JP. Calcium and sperm components in the establishment of the membrane block to polyspermy: Studies of ICSI and activation with sperm factor. Mol Hum Reprod. 2007;13:557–565. doi: 10.1093/molehr/gam042. [DOI] [PubMed] [Google Scholar]
- Wright SJ, Schatten G. Protein tyrosine phosphorylation during sea urchin fertilization: Microtubule dynamics require tyrosine kinase activity. Cell Motil Cytoskeleton. 1995;30:1122–1135. doi: 10.1002/cm.970300204. [DOI] [PubMed] [Google Scholar]
- Xu D, Kishi H, Kawamichi H, Kajiya K, Takada Y, Kobayashi S. Involvement of Fyn tyrosine kinase in actin stress fiber formation in fibroblasts. FEBS Lett. 2007;581:5227–5233. doi: 10.1016/j.febslet.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Yoon SY, Jellerette T, Salicioni AM, Lee HC, Yoo MS, Coward K, Parrington J, Grow D, Cibelli JB, Visconti PE, Mager J, Fissore RA. Human sperm devoid of PLC, zeta 1 fail to induce Ca release and are unable to initiate the first step of embryo devel- opment. J Clin Invest. 2008;118:3671–3681. doi: 10.1172/JCI36942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Horiuchi Y, Sensui N, Morisawa M. Signaling pathway from [Ca2þ ]i transients to ooplasmic segregation in- volves small GTPase rho in the ascidian egg. Dev Growth Diff. 2003;45:275–281. doi: 10.1046/j.1524-4725.2003.695.x. [DOI] [PubMed] [Google Scholar]
- Zaidi SK, Sullivan AJ, Medina R, Ito Y, Van Wijnen AJ, Stein JL, Lian JB, Stein GS. Tyrosine phosphorylation controls Run×- mediated subnuclear targeting of YAP to repress transcription. EMBO J. 2004;23:790–799. doi: 10.1038/sj.emboj.7600073. [DOI] [PMC free article] [PubMed] [Google Scholar]